Abstract
Vibrio cholerae and enterotoxigenic Escherichia coli (ETEC) are noninvasive mucosal pathogens that cause acute watery diarrhea in people in developing countries. Direct assessment of the mucosal immune responses to these pathogens is problematic. Surrogate markers of local mucosal responses in blood are increasingly being studied to determine the mucosal immune responses after infection. However, the volume of blood available in children and infants has limited this approach. We assessed whether an approach that first isolates β7-positive cells from a small volume of blood would allow measurement of the antigen-specific immune responses in patients with cholera and ETEC infection. β7 is a cell surface marker associated with mucosal homing. We isolated β7-expressing cells from blood on days 2, 7, and 30 and used an enzyme-linked immunosorbent spot (ELISPOT) assay to assess the gut-homing antibody-secreting cells (ASCs) specific to pathogen antigens. Patients with ETEC diarrhea showed a significant increase in toxin-specific gut-homing ASCs at day 7 compared to the levels at days 2 and 30 after onset of illness and to the levels in healthy controls. Similar elevations of responses to the ETEC colonization factors (CFs) CS6 and CFA/I were observed in patients infected with CS6- and CFA/I-positive ETEC strains. Antigen-specific gut-homing ASCs to the B subunit of cholera toxin and cholera-specific lipopolysaccharides (LPS) were also observed on day 7 after the onset of cholera using this approach. This study demonstrates that a simple ELISPOT assay can be used to study the mucosal immunity to specific antigens using a cell-sorting protocol to isolate mucosal homing cells, facilitating measurement of mucosal responses in children following infection or vaccination.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) and Vibrio cholerae O1 are two major bacterial causes of severe acute watery diarrhea in developing countries (1). The two pathogens are noninvasive mucosa-associated organisms that colonize the small intestine, resulting in losses of fluid and electrolytes due to the action of enterotoxins. Acute infectious diarrhea is the second most common cause of death in children living in developing countries, surpassed only by acute respiratory diseases, and accounts for approximately 20% of all childhood deaths (2). Cholera toxin (CT) and lipopolysaccharides (LPS) are well-characterized antigens and virulence factors of V. cholerae O1. For ETEC, the heat-labile (LT) and heat-stable (ST) enterotoxins, as well as colonization factors (CFs), are important virulence factors (3–5). The CFA/I antigen has been recognized as an important colonization factor of ETEC and has frequently been detected in ETEC isolated from patients with diarrhea. The colonization factor CS6 has been shown to promote binding of ETEC to rabbit and human enterocytes and has frequently been present on ETEC isolated from humans with diarrhea (6, 7).
During natural infection and after vaccination, protection from noninvasive pathogens results from induction of a relevant antibody response. These antibodies are usually quantified in body fluids using immunological assays, solid-phase immunoassays, or functional tests (8). However, because of the anatomic compartmentalization of the adaptive immune system, measurement of antibody responses themselves in blood may not predict local immunogenicity against noninvasive mucosal infections for which protection is often mediated by local responses at mucosal surfaces. This anatomical compartmentalization also constitutes a major challenge when one is attempting to measure immune responses in other mucosal secretions, as responses induced at one given mucosal site, e.g., the small intestine, are not always reflected in secretions from distant mucosal tissues (9, 10). Therefore, the best approach for assessing local mucosal immune responses to infection is not necessarily obvious. None of the techniques developed so far has gained general acceptance because of considerable variations in the content of mucosal secretions and practical difficulties in collecting these fluids or materials, especially in newborns and young infants. Furthermore, measurement of both the mucosal and systemic immune responses in neonates and young infants can be confounded by interference from maternal antibodies derived from breast milk or transudation of serum antibodies (IgG antibodies across the placenta). Specific antibody-secreting cells (ASCs) represent a subpopulation of immunoglobulin-secreting cells (ISCs) producing antibodies of known antigen specificity (e.g., against vaccine antigens). Previous work has shown that there is a transient migration of ASCs from intestinal tissue through the systemic circulation following mucosal stimulation (11). These cells are maturing and rehoming to intestinal tissue and bear mucosal homing markers such as β7 (12), and their presence in the peripheral circulation is claimed to peak at days 6 to 8 following illness or vaccination (13). These cells can be directly evaluated, and immunoglobulins produced by these cells can be detected as secreted products in the supernatant of in vitro-cultured peripheral blood mononuclear cells (PBMCs) (14, 15). However, these and related assays usually require at least 3 to 5 ml of blood. As such, sensitive and specific procedures are needed to determine the responses in PBMCs that reflect prior mucosal infection or vaccination using the smaller volumes of blood that are available from young children and infants. To address this, we carried out a study that used a technique developed by Saletti et al. (13) to enrich gut-homing cells from blood using the gut-homing surface marker β7, and we validated this approach by collecting only 1 ml of blood and assessing the gut-associated antigen-specific immune responses in patients with cholera and ETEC infection in Bangladesh.
MATERIALS AND METHODS
Study groups and recruitment.
We enrolled adult patients hospitalized at the Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), who presented with severe acute watery diarrhea and gave informed consent. Patients whose stool specimens were positive for V. cholerae O1 and negative for other enteric pathogens (Shigella, Salmonella, and ETEC) were enrolled as cholera patients, while patients whose stool specimens were positive for ETEC but negative for V. cholerae O1 and other enteric pathogens were enrolled as ETEC-infected patients. Screening of stool specimens for other common enteric pathogens to exclude patients with coinfections was done as previously described (16, 17). Cholera patients recruited for the study were all infected with the V. cholerae O1 Ogawa serotype. The strains in the ETEC-infected patients recruited for the study were analyzed for expression of specific colonization factors, including the CFA/I group or CS6, as well as LT, LT/ST, or ST alone, by dot blot and PCR, respectively (18, 19). Healthy adult individuals, who had no history of diarrhea, fever, or antibiotic use in the 2 weeks prior to enrollment, were recruited as control participants and were matched by age and socioeconomic status to the V. cholerae/ETEC-infected participants. This study was approved by the research review and the ethical review committees of the icddr,b.
Subjects and sample collection.
Heparinized venous blood samples were collected by venipuncture on days 2, 7, and 30 following the onset of hospitalization for V. cholerae-infected (n = 15) and ETEC-infected (n = 41: CFA/I group, n = 14; CS6, n = 8; CS7, n = 1; and CF negative, n = 18) adults and healthy controls (HC; n = 9). This study was conducted at the icddr,b in Bangladesh between May 2012 and June 2014.
Plasma separation.
One milliliter of blood was collected in sodium heparin tubes. After centrifugation at 600 × g for 10 min, plasma was separated and collected in Eppendorf tubes and stored at −20°C for plasma antibody analysis.
Preparation of beads coated with anti-human β7.
Sheep anti-rat IgG magnetic beads (Dynabeads; Invitrogen, Norway) were placed in a 9-ml culture tube (Pyrex, USA) containing 3 ml of cold fluorescence-activated cell sorter (FACS) buffer. For each blood specimen (1 ml), 25 μl of beads (107 beads) was used. The tube was placed in a magnet (Dynal MPC-6; Norway) for 2 min. The supernatant was aspirated without moving or detaching the tube from the magnet. The beads were washed with 3 ml of cold FACS buffer for 2 min, and this procedure was repeated twice. After removal of the tube from the magnet, beads were resuspended in FACS buffer in an initial volume of 25 μl/blood sample. Rat anti-human integrin β7 (BD Pharmingen, USA) was added, and the mixture was incubated for 30 min at 2 to 8°C with gentle tilting and rotation. Finally, the beads coated with anti-human β7 were added to mononuclear cells prepared as described below.
Cell preparation.
After separation of plasma, the remaining blood was transferred into a Falcon tube (BD, USA) and mixed with 30 ml lysing solution (1 M NH4Cl solution) for lysis of red blood cells. The mixture was incubated for 7 min at room temperature and then centrifuged. The supernatant was removed (non-β7 cells), and the pellet was resuspended immediately to the initial blood volume (1 ml) in FACS buffer. The cells were washed twice and were resuspended in 1 ml FACS buffer and transferred to freshly prepared beads coated with anti-human β7 in a glass tube as described above. The cells and beads were incubated at 4°C for 45 min on a horizontal shaker. The tubes were then placed into the magnet, and the supernatant was aspirated. Cells were resuspended in RPMI complete medium (Gibco, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT). After being counted, bead-separated mononuclear cells were plated onto the enzyme-linked immunosorbent spot assay (ELISPOT) plate for determination of the total immunoglobulin and the antigen-specific spots to ETEC and V. cholerae antigens. Depending on the retrieval of cells, 2,500 to 5,000 cells were used for determining the total immunoglobulin spots and 15,000 to 20,000 cells were used for detecting the antigen-specific spots; duplicate wells were used for the total immunoglobulin spots, while single wells were used for the antigen-specific spots. For the antigen-specific ASC enumerations, ELISPOT wells previously coated with specific antigens (i.e., LPS, cholera toxin B subunit [CTB], and CFA/I group colonization factor antigens) were prepared. Similarly, the total immunoglobulin-secreting cells (ISCs) irrespective of antigen specificity were enumerated in parallel wells coated with a mixture of affinity-purified goat antibodies to human Ig κ and λ light chains (17). The use of a horseradish peroxidase (HRP)-dependent two-color (3-amino-9-ethylcarbazole [AEC] and 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium [BCIP-NBT] substrate) ELISPOT procedure for detection has been previously described (13). The antigen-specific response was expressed as a percentage calculated as the number of antigen-specific gut-homing ASCs divided by the total gut-homing immunoglobulin-secreting cells of that same isotype; both the total ISC and the antigen-specific ASC numbers were adjusted to 106 prior to percent calculation.
Detection of CTB-specific antibodies in plasma.
The recombinant cholera toxin B subunit (rCTB)-specific IgA and IgG antibodies of patients infected with ETEC and V. cholerae were measured in plasma using a standardized enzyme-linked immunosorbent assay (ELISA) technique, as previously described (16, 20). As labile toxin B (LTB) has 80% nucleotide sequence homology and cross-reacts immunologically to CTB (21, 22), the plasma responses in the ETEC-infected patients were assessed using CTB as the antigen.
Statistical analysis.
For the analyses of the antibody-secreting cells at different time points following infection, we used the Wilcoxon signed-rank test. We used the Mann Whitney U test to compare the results between healthy participants and infected patients at different days after the onset of infection. The association between the ASC and plasma antibody responses was assessed using the Spearman correlation coefficient. We used GraphPad Prism 5.0 for statistical analyses and to generate the figures and considered a P value of <0.05 as significant.
RESULTS
Demographic characteristics of patients.
In this study, we enrolled 15 adult cholera patients (median age, 30 years; 26.7% female) and 41 adult ETEC-infected patients (median age, 33.5 years; 46.3% female). In addition, 9 age-matched adult healthy controls (HC) were recruited. Of the patients infected with ETEC, 44%, 39%, and 17% were infected with LT-, LT/ST-, or ST-expressing ETEC strains, respectively. The majority of the patients were infected with ETEC strains expressing either the CFA/I group (n = 14, 34%) or CS6 with or without CS5 (n = 8, 20%) as their colonization factor. Most of the ETEC strains expressing CFA/I group CFs or CS6 with or without CS5 produced either LT or both LT and ST (82% and 86%), respectively. The demographic, clinical, and microbiological characteristics of patients are presented in Table 1.
TABLE 1.
Demographic characteristics of ETEC-infected and cholera patients
| Characteristic | ETEC infected | V. cholerae infected |
|---|---|---|
| No. of study participants | 41 | 15 |
| Age (median [25th, 75th percentile]), yr | 33.5 (25, 40) | 30 (24, 37) |
| Female gender (no. [%]) | 19 (46.3) | 4 (26.7) |
| Duration of diarrhea (mean [SD]), h | 29.1 (25.3) | 24.1 (18.6) |
| Blood group (no. [%]) | ||
| A | 12 (29.3) | 3 (20) |
| B | 16 (39) | 7 (46.7) |
| O | 8 (19.5) | 4 (26.7) |
| AB | 5 (12.2) | 1 (6.7) |
| Toxin types of ETEC strains (no. [%]) | 41 | |
| LT/ST | 16 (39) | |
| LT | 18 (43.9) | |
| ST | 7 (17.1) | |
| Colonization factors of ETEC strains (no.) | 41 | |
| CFA/I group (CFA/I, CS17, CS4, CS14) | 5 (0, 0, 0, 5) | |
| CFA/I group (CS1+CS3+CS21, CS2+CS3, CFA/I+CS21) | 9 (4, 3, 2) | |
| CS6 (CS6 only, CS5+CS6) | 8 (2, 6) | |
| CS7 | 1 | |
| CF negative | 18 |
Total immunoglobulin responses.
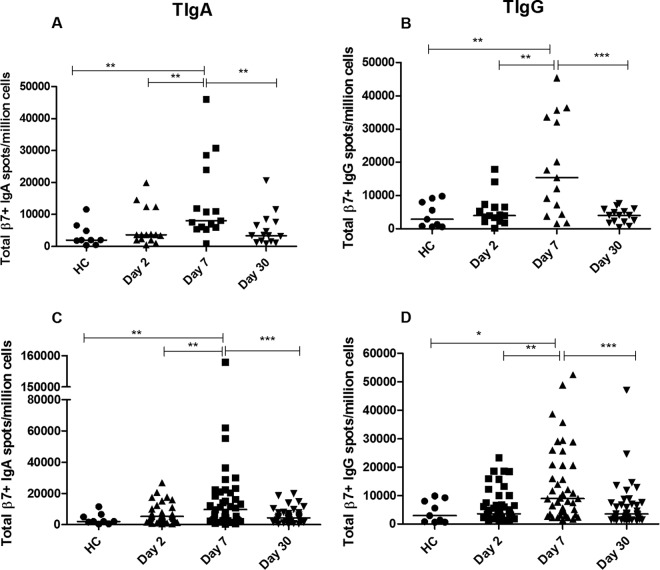
Total gut-homing immunoglobulin spots were enumerated per million gut-homing (β7+) cells at different days after diarrheal illness. Comparable levels of total gut-homing immunoglobulin-secreting cells (ISCs) were observed in cholera patients and ETEC-infected patients on different days (day 2, day 7, and day 30) after the onset of infection, and the levels in healthy controls were comparable to those on day 2 following infection (Fig. 1). In patients with cholera, we found a significant increase in total β7+ IgA ISCs at day 7 (Fig. 1A) compared to the numbers at day 2 (P = 0.005) and in healthy controls (P = 0.005), and the number of total β7+ IgA ISCs subsequently decreased at day 30 (P = 0.005). In the case of total gut-homing IgG ISCs, an increase was noted (Fig. 1B) at day 7 compared to the numbers at day 2 (P = 0.001) and in HC (P = 0.008), which then declined at day 30 (P = 0.0009). Similar to cholera patients, patients infected with ETEC also had significant increases in the numbers of total β7+ IgA-secreting cells at day 7 following infection (Fig. 1C) compared to the numbers at day 2 (P = 0.009) and in healthy controls (P = 0.008), and the numbers subsequently declined by day 30 (P = 0.0006) (Fig. 1C). The numbers of total β7+ IgG-secreting cells in patients infected with ETEC followed a pattern similar to that of total IgA ISCs (Fig. 1D).
FIG 1.
Total gut-homing immunoglobulin-secreting cell (ISC) responses in V. cholerae O1-infected (A, B) and ETEC-infected (C, D) patients (on day 2, day 7, and day 30) compared to those in healthy controls (HC). Total IgA (A) and total IgG (B) ISCs in patients with cholera and total IgA (C) and total IgG (D) ISCs in patients with ETEC infection. Each symbol represents an individual data point for an individual patient, and the horizontal lines indicate the median responses for each study group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. A P value of <0.05 was considered to be statistically significant.
CTB-specific responses.
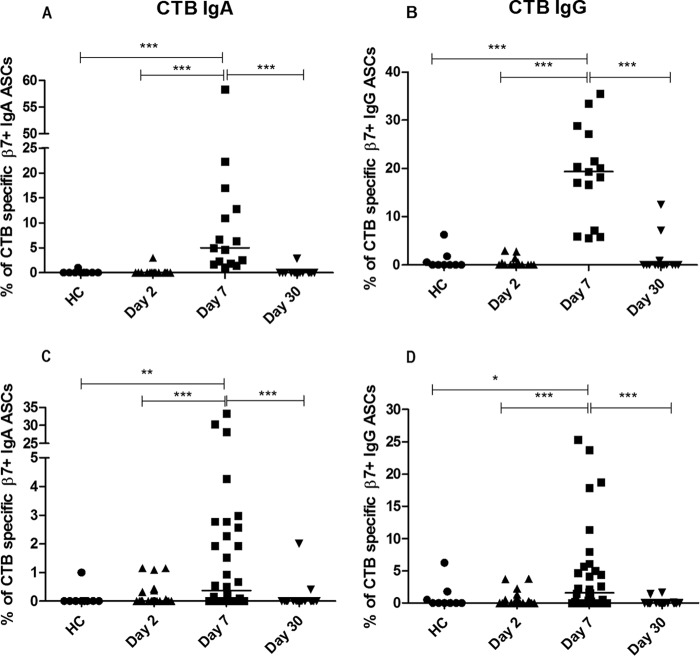
Antigen-specific immunospot assays were performed using CTB to assess the antigen-specific immune responses in both cholera and ETEC-infected patients. The CTB ELISPOT analyses showed increased percentages of CTB-specific gut-homing cells in patients with cholera, for both the IgA and IgG isotypes, at day 7 following infection compared to the levels at the other days following infection (days 2 and 30) and to the levels for healthy controls (Fig. 2A and B). For patients with ETEC infection, LT-producing strains induce cross-reactive antibody-secreting cell (ASC) responses to CTB. In the ETEC-infected group, we found that the frequency of CTB-specific gut-homing IgA ASCs was higher at day 7 (Fig. 2C) than at day 2 (P = 0.0004) and in healthy controls (P = 0.006), and the numbers of CTB-specific gut-homing ASCs waned at day 30 compared to those at day 7 (P < 0.0001). For IgG responses, a similar pattern was observed (Fig. 2D), the percentage being elevated at day 7 compared to those at day 2 (P = 0.0007) and in healthy controls (P = 0.04) and decreasing at day 30 (P = 0.0003). The median percentages of β7+ IgG ASCs in both cholera patients (19.3%) and ETEC-infected patients (1.6%) were 4-fold higher than the median percentages of β7+ IgA ASCs (4.6% and 0.4%, respectively) at day 7 for the CTB-specific responses (Table 2). Both β7+ CTB-specific IgA ASC responses (median 4.6%) and the IgG ASC responses (median 19.3%) in patients with cholera were 12-fold higher than the CTB-specific responses in patients with ETEC infection (median values of 0.4% and 1.6%, respectively) (Table 2). In patients infected with ETEC strains positive only for heat-stable toxin (ST), the ELISPOT assay showed almost no response specific to CTB.
FIG 2.
CTB-specific gut-homing antibody-secreting cell (ASC) responses in patients with cholera (A, B) and in patients infected with ETEC (C, D) compared to those in healthy controls (HC). Percentage of IgA (A) and IgG (B) ASCs in patients with cholera and of IgA (C) and IgG (D) ASCs in patients with ETEC infection. Each symbol represents an individual data point for that patient, and the horizontal lines indicate the median responses for each study group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. A P value of <0.05 was considered statistically significant.
TABLE 2.
Numbers of total β7+ ISCs and percentages of antigen-specific β7+ ASCs in cholera and ETEC infection
| Antigen | IgA |
IgG |
||||||
|---|---|---|---|---|---|---|---|---|
| HCa | Day 2 | Day 7 | Day 30 | HC | Day 2 | Day 7 | Day 30 | |
| Cholera | ||||||||
| Totalb | 2,000 | 3,700 | 8,100 | 3,375 | 2,900 | 4,000 | 15,400 | 4,050 |
| CTBc | 0 | 0 | 4.6 | 0 | 0 | 0 | 19.3 | 0 |
| LPSc | 0 | 0 | 30.2 | 0 | 0 | 0 | 11.6 | 0 |
| ETEC | ||||||||
| Total | 2,000 | 5,200 | 9,600 | 4,300 | 2,900 | 3,600 | 9,025 | 3,450 |
| CTBc | 0 | 0 | 0.4 | 0 | 0 | 0 | 1.6 | 0 |
| CFA/Ic | 0 | 0 | 0.8 | 0 | 0 | 0 | 2.3 | 0 |
| CS6c | 0 | NDd | 4.6 | 0 | 0 | ND | 11 | 0 |
HC, healthy controls.
Gut-homing ISC count (median, per million β7+ cells).
Data are percentage (median) of total ISCs.
ND, the assay was not done on that day.
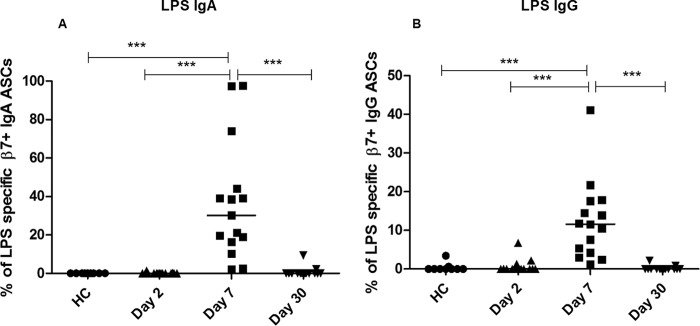
LPS-specific responses in cholera patients.
We also assessed V. cholerae O1 LPS-specific responses in patients following cholera (Fig. 3). We demonstrated higher levels of IgA gut-homing ASCs on day 7 following infection than on day 2 (P < 0.0001) and in healthy controls (P < 0.0001), with responses in patients decreasing by day 30 (P < 0.0001) (Fig. 3A). Similarly, IgG gut-homing, LPS-specific ASCs were significantly elevated on day 7 (P < 0.0001) compared to the levels on day 2 and day 30 and in healthy controls (Fig. 3B).
FIG 3.
LPS-specific gut-homing antibody-secreting cell (ASC) responses in patients with cholera compared to those in healthy controls (HC). Percentages of LPS-specific IgA (A) and IgG (B) ASCs. Each symbol represents an individual data point for that patient, and the horizontal lines indicate the median responses for each study group. ***, P < 0.001. A P value of <0.05 was considered statistically significant.
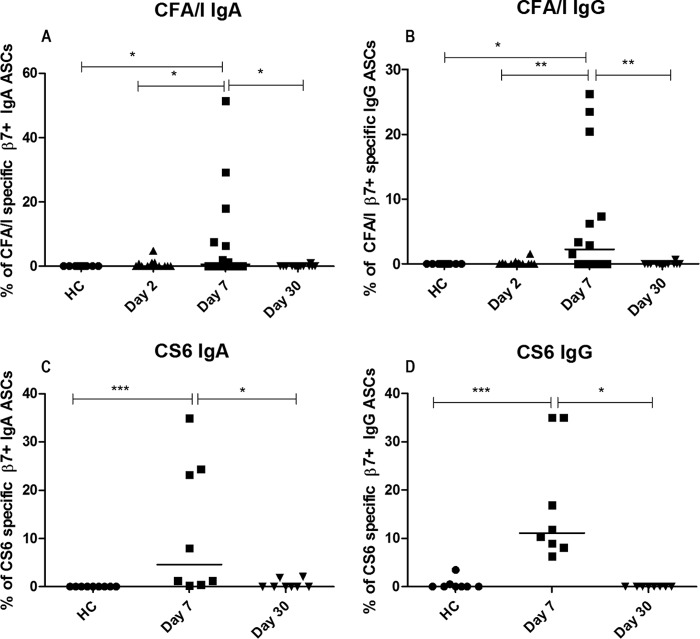
CF-specific responses to ETEC infection.
Colonization factors have a major role in ETEC infection, and immunity against colonization factors seems to plays a critical role in mediating protection against ETEC infection (23, 24). For this reason, we also assessed immune responses using a CFA/I-specific ELISPOT assay for patients infected with ETEC strains expressing the CFA/I group colonization factor antigens and a CS6-specific ELISPOT assay for patients infected with ETEC strains expressing the CS6 colonization factor alone or together with CS5. We found a significantly higher CF-specific response on day 7 than on day 2 or day 30 in patients infected with a CFA/I-positive ETEC strain and in healthy controls, respectively, for both CFA/I-specific IgA and IgG ASCs (Fig. 4A and B). We also detected significant β7+ immune responses to CS6 in patients infected with CS6-expressing ETEC strains. The frequencies of β7+ CS6-specific IgA and IgG ASCs were higher at day 7 than at day 30 and in healthy controls (Fig. 4C and D). We did not conduct the CS6-specific ELISPOT assay on day 2 as we had limited CS6 antigen and detection of this CF in stool isolates by the dot blot test required at least 2 days. Interestingly, patients with ETEC infections had stronger β7+ immune responses against the CS6 antigen (median of 4.6% of IgA ASCs and median of 11% for IgG responses on day 7) than against the CFA/I group (median values of 2.3% and 0.8% on day 7, respectively) (Table 2). No CFA/I-specific gut-homing ASC responses were observed in patients infected with CF-negative ETEC strains (n = 18).
FIG 4.
CFA/I-specific (A, B) and CS6-specific (C, D) gut-homing antibody-secreting cell (ASC) responses in patients with ETEC infection compared with those in healthy controls (HC). Percentage of CFA/I-specific IgA (A) and IgG (B) ASCs and CS6-specific IgA (C) and IgG (D) ASCs. Each of the symbols represents an individual data point for that patient, and the horizontal lines indicate the median responses for each study group. *, P < 0.05; **, P < 0.01; ***, P < 0.001. A P value of <0.05 was considered to be statistically significant.
Association of gut-homing ASCs with antibody responses in plasma.
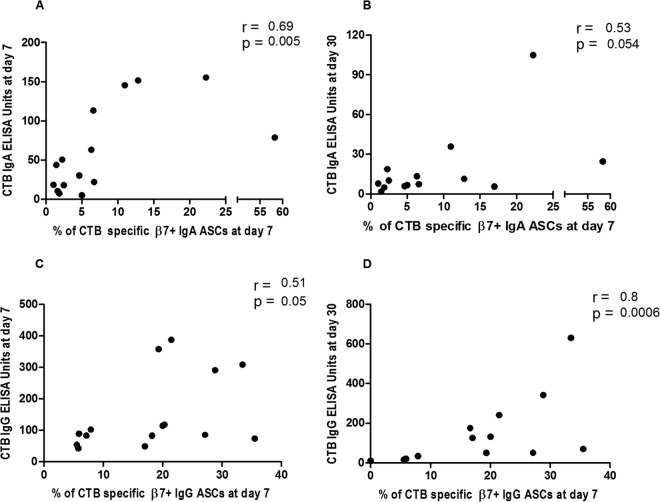
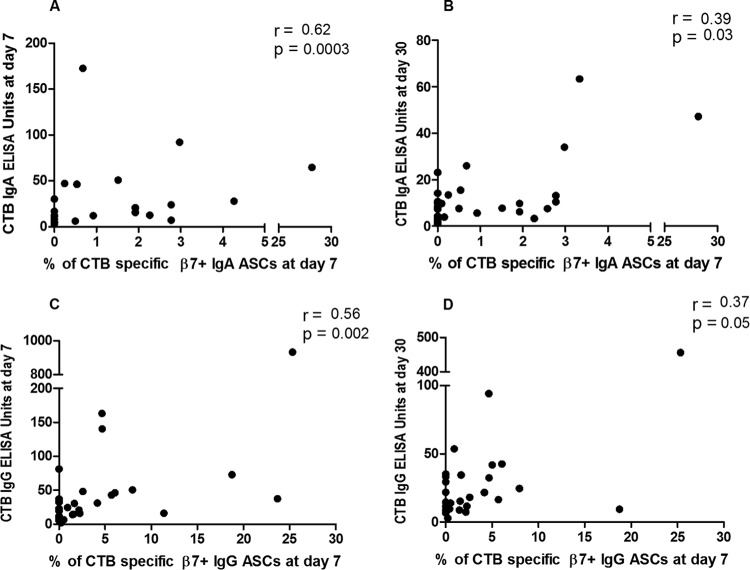
To determine the potential associations between gut-homing ASC responses and antibody responses in plasma, we compared CTB-specific IgA and IgG β7+ ASC responses on day 7 with the corresponding antibody responses in plasma on days 7 and 30 in patients infected with V. cholerae O1 (n = 15 at day 7 and n = 14 at day 30) and ETEC strains (n = 29) expressing LT or LT/ST. We found positive correlations between CTB-specific IgA and IgG β7+ ASC responses at day 7 and CTB-specific IgA and IgG antibody titers at both day 7 and day 30 in cholera patients (Spearman r = 0.51 to 0.8; P = 0.0006 to 0.05) (Fig. 5). Similarly, in patients infected with ETEC, CTB-specific gut-homing IgA and IgG ASCs at day 7 were positively correlated with CTB-specific IgA and IgG ELISA titers on days 7 and 30 (Spearman r = 0.37 to 0.62; P = 0.05 to 0.0003) (Fig. 6).
FIG 5.
Association of frequencies of gut-homing ASCs with plasma antibody titers against CTB in patients with cholera. Spearman correlation of CTB-specific gut-homing IgA ASCs at day 7 with CTB IgA antibody titers at day 7 (A), IgA ASCs at day 7 with CTB IgA antibody titers at day 30 (B), IgG ASCs at day 7 with CTB IgG antibody titers at day 7 (C), and IgG ASCs at day 7 with CTB IgG antibody titers at day 30 (D).
FIG 6.
Association of gut-homing ASCs with plasma antibody titers against CTB in patients with LT ETEC infection. Spearman correlation of CTB-specific gut-homing IgA ASCs at day 7 with CTB IgA antibody titers at day 7 (A), IgA ASCs at day 7 with CTB IgA antibody titers at day 30 (B), IgG ASCs at day 7 with CTB IgG antibody titers at day 7 (C), and IgG ASCs at day 7 with CTB IgG antibody titers at day 30 (D).
DISCUSSION
This study demonstrates that the immunomagnetic separation of β7-positive cells from a small volume of whole blood followed by detection of antigen-specific ASCs by the ELISPOT assay can be utilized to determine gut-homing, antigen-specific ASC responses following diarrheal infections from two major causes, cholera and ETEC. These results support further application of this simple technique for studying B cell responses against different bacterial antigens and for host cell surface markers on B cells in young children and even in infants, following infection or vaccination. The main goal of enumeration of gut-homing β7+ ASCs is to determine whether a participant responds to a specific antigen or vaccine and ideally whether the response measured is predictive of clinical efficacy; i.e., it constitutes a correlate of a specific antigen- or vaccine-induced immune protection. Although there is good correlation between plasma and ELISPOT data, no consensus exists as to whether a subject is a responder based on ASC ELISPOT data. In the past, most studies on human blood ASC responses have used an arbitrary cutoff to discriminate vaccine responders from nonresponders as well as from patients with natural infections (13). In addition, the unique benefit of measuring β7+ ASC responses is immunomagnetic enrichment of mucosally derived blood lymphocytes for the detection of human ASCs (circulating plasmablasts) specific to any vaccine antigen, which also can be performed in resource-poor settings.
Antibody-secreting cells are an essential component of adaptive immunity and immunological memory (25). Detection of peak ASC responses at an early time point after booster immunization can be used to assess vaccine efficacy and subsequent immunological memory (26). ASCs induced by mucosal immunization mainly express α4β7-integrin (27). In mice lacking β7, the plasma cell numbers in the intestinal lamina propria are reduced 10- to 30-fold (28). Gut-homing ASCs in the blood shortly after infection are considered a marker of a subsequent mucosal response (29, 30); e.g., 80% of circulating ASCs in cholera patients have been shown to express α4β7 (31). Immune responses at the gut surface play a critical role in mediating protection from cholera and ETEC infections, and previously infected patients may have an anamnestic immune response mediated by mucosal lymphocytes (24, 30, 32).
In this study, we confirmed that in cholera patients, total gut-homing IgA and IgG ASCs were elevated significantly on day 7 as expected. We also confirmed elevated gut-homing ASC responses on day 7 against lipopolysaccharide (LPS) and CTB, two important V. cholerae antigens (14, 30, 33, 34). Duodenal ASCs specific for CTB are more numerous than those in the blood after oral immunization with B subunit whole-cell (B-WC) cholera vaccine (9) and killed whole-cell (WC) bivalent cholera vaccine (35), even though in natural infections, no significant differences are found between duodenal and circulating ASC responses (30). A significant correlation (P = 0.005; Spearman r = 0.34 to 0.67) has been observed between the CTB-specific IgA and IgG isotypes of antibodies in both cholera and ETEC infection at early and late convalescence (data not shown).
LPS-specific immune responses play an important role in the protective immunity to cholera, and LPS is an attractive target for vaccine design (30, 36–38). As for ASCs specific to CTB, duodenal ASCs specific for LPS are also more numerous than those in the circulation after oral immunization with killed oral whole-cell (WC) bivalent cholera vaccine (35). In a previous flow cytometric-based study using CCR9 as a gut-homing marker, we found that almost 50% of the circulating gut-homing ASCs recognized either cholera toxin B subunit (CTB) or V. cholerae O1 LPS (39).
Although CTB is not an antigen of ETEC, CTB is homologous to LTB, and cross-reactive immune responses against CTB of almost the same magnitudes as those against LT in patients with ETEC infection have been identified (21, 22). As for cholera, CTB-specific ASC responses have been shown to increase in blood after oral immunization with killed whole-cell ETEC vaccine containing toxoid antigen (40, 41) as well as in patients with ETEC diarrhea (31). In this study, we found, as expected, that CTB-specific β7+ IgA and IgG ASCs were increased at day 7 following ETEC infection, although the median percentage of CTB-specific gut-homing ASCs (for both IgA and IgG ASCs) was about 12-fold lower in patients infected with ETEC than those with cholera.
ETEC can stimulate immune responses to both homologous and cross-reacting colonization factors (CFs) (17). For instance, patients infected with CFA/I-expressing ETEC have been shown to respond with significant IgA antibody responses to the cross-reactive CS1, CS2, CS4, and CS14 colonization factors, in addition to the homologous CFA/I antigen (42, 43), and patients infected with CS14-expressing ETEC have also responded immunologically to CFA/I (44). Interestingly, this is true also for CF-specific ASC and memory B cell responses and development of highly avid IgA and IgG antibody responses (17). In this study, we found that patients infected with ETEC strains expressing the CFA/I group antigens developed CFA/I-specific β7+ IgA and IgG ASCs responses on day 7. Previous studies have also shown development of mucosal and systemic immune responses against CS6 in humans infected with ETEC strains expressing CS6, with or without CS4 or CS5 (45), and oral immunization of mice with inactivated E. coli overexpressing CS6 induces substantial immune responses against this antigen (46). Patients infected with CS6-expressing ETEC develop not only CS6-specific IgG and IgA ASCs and antibody responses but also memory B cell responses and highly avid anti-CS6 IgA and IgG antibodies after onset of diarrhea (17). In this study, we confirmed that patients infected with ETEC strains expressing CS6 with or without CS5 also developed β7+ IgA and IgG ASC responses on day 7 after infection.
Antibody levels in the serum may correlate with the number of antigen-specific ASCs of that specificity and isotype (25). Uddin et al. reported significant correlations between circulating LPS-specific IgA ASCs on day 7 and IgA antibody levels to LPS in mucosal extracts; however, they found no correlation between ASCs and mucosal extract antibodies of the IgG isotype or for ASCs and antibody levels to CTB in mucosal extracts (30). One possibility for these results is that total ASCs, as opposed to gut-homing ASCs, were examined in this study. In this current study, we confirmed that β7+ ASCs of both the IgA and IgG isotypes specific to CTB significantly correlated with serum levels of IgA and IgG antibodies to CTB in patients with cholera and ETEC infections.
In the developing world, the volume of blood available for immunological studies, particularly in infants and children, is often a crucial limitation (47). The current approaches to determining circulating ASCs require a relatively large volume of blood (3 to 5 ml) (30) and a flow cytometry-based technique that is more expensive (39). Our current study suggests that these limitations can be overcome by enrichment of β7+ cells from as small a volume as 1 ml of blood, followed by an ELISPOT analysis. This should allow immunological assays of both B and T cells, possibly including cytokine production by specific T cells (48), to be conducted in individuals of all ages.
In summary, this study demonstrates that total as well as antigen-specific circulating gut-homing ASCs can be detected in a small blood volume and confirms that antigen-specific cells are elevated on day 7 following infection with both V. cholerae and ETEC. In addition, we found that the level of antigen-specific circulating ASCs on day 7 correlates with plasma antibody levels against CTB on day 7 and on day 30. The results suggest that oral vaccine studies might include determination of gut-homing ASCs to measure immune responses following vaccination, without requiring a substantial blood volume.
ACKNOWLEDGMENTS
This work was supported by the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), a Swedish Sida (SIDA) grant (INT-ICDDR,B-HN-01-AV), and a Swedish collaborative grant (SIDA-SWE2009-023), as well as by the Commission of the European Communities grant to STOPENTERICS (grant HEALTH-F3-2010-261472). This study was also supported by grants from the National Institutes of Health, including grants from the National Institute of Allergy and Infectious Diseases (AI106878 [E.T.R. and F.Q.], AI058935 [S.B.C., E.T.R., and F.Q.], and AI103055 [J.B.H. and F.Q.]), as well as the Fogarty International Center (training grant in vaccine development and public health TW005572 [T.R.B., R.R., and K.I.]).
The authors declare no competing financial interests.
Funding Statement
This work was supported by the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), a Swedish Sida (SIDA) grant (INT-ICDDR,B-HN-01-AV), and Swedish collaborative grant SIDA-SWE2009-023, as well as by the Commission of the European Communities grant to STOPENTERICS (grant HEALTH-F3-2010-261472). This study was also supported by grants from the National Institutes of Health, including grants from the National Institute of Allergy and Infectious Diseases (AI106878 [E.T.R. and F.Q.], AI058935 [S.B.C., E.T.R., and F.Q.], and AI103055 [J.B.H. and F.Q.]), and the Fogarty International Center (training grant in vaccine development and public health TW005572 [T.R.B., R.R., and K.I.]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Qadri F, Svennerholm AM, Faruque AS, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.Gaastra W, Svennerholm AM. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol 4:444–452. doi: 10.1016/0966-842X(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 4.Levine MM. 1990. Modern vaccines. Enteric infections. Lancet 335:958–961. [DOI] [PubMed] [Google Scholar]
- 5.Svennerholm AM, Holmgren J. 1995. Oral vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. Adv Exp Med Biol 371B:1623–1628. [PubMed] [Google Scholar]
- 6.Helander A, Hansson GC, Svennerholm AM. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb Pathog 23:335–346. doi: 10.1006/mpat.1997.0163. [DOI] [PubMed] [Google Scholar]
- 7.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 9.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson LA, Holmgren J, Czerkinsky C. 1991. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest 88:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun 65:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lycke N, Lindholm L, Holmgren J. 1985. Cholera antibody production in vitro by peripheral blood lymphocytes following oral immunization of humans and mice. Clin Exp Immunol 62:39–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Kantele A, Kantele JM, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher EC, Makela PH. 1997. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol 158:574–579. [PubMed] [Google Scholar]
- 13.Saletti G, Cuburu N, Yang JS, Dey A, Czerkinsky C. 2013. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat Protoc 8:1073–1087. doi: 10.1038/nprot.2013.058. [DOI] [PubMed] [Google Scholar]
- 14.Saxon A, Stevens RH. 1979. Stimulation and regulation of human IgE production in vitro using peripheral blood lymphocytes. Clin Immunol Immunopathol 14:474–488. doi: 10.1016/0090-1229(79)90100-4. [DOI] [PubMed] [Google Scholar]
- 15.Yarchoan R, Murphy BR, Strober W, Schneider HS, Nelson DL. 1981. Specific anti-influenza virus antibody production in vitro by human peripheral blood mononuclear cells. J Immunol 127:2588–2594. [PubMed] [Google Scholar]
- 16.Qadri F, Ahmed F, Karim MM, Wenneras C, Begum YA, Abdus Salam M, Albert MJ, McGhee JR. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin Diagn Lab Immunol 6:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam MM, Aktar A, Afrin S, Rahman MA, Aktar S, Uddin T, Al Mahbuba D, Chowdhury F, Khan AI, Bhuiyan TR, Begum YA, Ryan ET, Calderwood SB, Svennerholm AM, Qadri F. 2014. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl Trop Dis 8:e2822. doi: 10.1371/journal.pntd.0002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Svennerholm AM. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol 38:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodas C, Iniguez V, Qadri F, Wiklund G, Svennerholm AM, Sjoling A. 2009. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol 47:1218–1220. doi: 10.1128/JCM.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, Uddin T, Khan AI, Chowdhury F, Charles RC, Harris JB, Calderwood SB, Qadri F, Ryan ET. 2014. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis 8:e3076. doi: 10.1371/journal.pntd.0003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobias J, Svennerholm AM. 2012. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates. Appl Microbiol Biotechnol 93:2291–2300. doi: 10.1007/s00253-012-3930-6. [DOI] [PubMed] [Google Scholar]
- 22.Salmond RJ, Luross JA, Williams NA. 2002. Immune modulation by the cholera-like enterotoxins. Expert Rev Mol Med 4:1–16. [DOI] [PubMed] [Google Scholar]
- 23.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svennerholm AM, Lundgren A. 2012. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev Vaccines 11:495–507. doi: 10.1586/erv.12.12. [DOI] [PubMed] [Google Scholar]
- 25.Manz RA, Hauser AE, Hiepe F, Radbruch A. 2005. Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 26.Leach S, Lundgren A, Svennerholm AM. 2013. Different kinetics of circulating antibody-secreting cell responses after primary and booster oral immunizations: a tool for assessing immunological memory. Vaccine 31:3035–3038. doi: 10.1016/j.vaccine.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 27.Quiding-Järbrink M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher EC, Lazarovits AI, Holmgren J, Czerkinsky C. 1997. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest 99:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cyster JG. 2003. Homing of antibody secreting cells. Immunol Rev 194:48–60. doi: 10.1034/j.1600-065X.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 29.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun 65:3571–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin T, Harris JB, Bhuiyan TR, Shirin T, Uddin MI, Khan AI, Chowdhury F, LaRocque RC, Alam NH, Ryan ET, Calderwood SB, Qadri F. 2011. Mucosal immunologic responses in cholera patients in Bangladesh. Clin Vaccine Immunol 18:506–512. doi: 10.1128/CVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qadri F, Makela PH, Holmgren J, Albert MJ, Mannoor K, Kantele A, Saha D, Salam MA, Kantele JM. 1998. Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J Infect Dis 177:1594–1599. doi: 10.1086/515306. [DOI] [PubMed] [Google Scholar]
- 32.Alam MM, Riyadh MA, Fatema K, Rahman MA, Akhtar N, Ahmed T, Chowdhury MI, Chowdhury F, Calderwood SB, Harris JB, Ryan ET, Qadri F. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin Vaccine Immunol 18:844–850. doi: 10.1128/CVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svennerholm AM, Holmgren J. 1976. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun 13:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm AM, Holmgren J, Black R, Levine M, Merson M. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis 147:514–522. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 35.Shamsuzzaman S, Ahmed T, Mannoor K, Begum YA, Bardhan PK, Sack RB, Sack DA, Svennerholm AM, Holmgren J, Qadri F. 2009. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine 27:1386–1392. doi: 10.1016/j.vaccine.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovac P, Calderwood SB, Qadri F, Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol 19:1712–1721. doi: 10.1128/CVI.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losonsky GA, Yunyongying J, Lim V, Reymann M, Lim YL, Wasserman SS, Levine MM. 1996. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun 64:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman A, Rashu R, Bhuiyan TR, Chowdhury F, Khan AI, Islam K, LaRocque RC, Ryan ET, Wrammert J, Calderwood SB, Qadri F, Harris JB. 2013. Antibody-secreting cell responses after Vibrio cholerae O1 infection and oral cholera vaccination in adults in Bangladesh. Clin Vaccine Immunol 20:1592–1598. doi: 10.1128/CVI.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qadri F, Ahmed T, Ahmed F, Bradley Sack R, Sack DA, Svennerholm AM. 2003. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18-36 months of age. Vaccine 21:2394–2403. doi: 10.1016/S0264-410X(03)00077-X. [DOI] [PubMed] [Google Scholar]
- 41.Wennerås C, Svennerholm AM, Ahren C, Czerkinsky C. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect Immun 60:2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qadri F, Ahmed F, Ahmed T, Svennerholm AM. 2006. Homologous and cross-reactive immune responses to enterotoxigenic Escherichia coli colonization factors in Bangladeshi children. Infect Immun 74:4512–4518. doi: 10.1128/IAI.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudin A, Wiklund G, Wenneras C, Qadri F. 1997. Infection with colonization factor antigen I-expressing enterotoxigenic Escherichia coli boosts antibody responses against heterologous colonization factors in primed subjects. Epidemiol Infect 119:391–393. doi: 10.1017/S0950268897008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudin A, Svennerholm AM. 1994. Colonization factor antigens (CFAs) of enterotoxigenic Escherichia coli can prime and boost immune responses against heterologous CFAs. Microb Pathog 16:131–139. doi: 10.1006/mpat.1994.1014. [DOI] [PubMed] [Google Scholar]
- 45.Qadri F, Ahmed T, Ahmed F, Bhuiyan MS, Mostofa MG, Cassels FJ, Helander A, Svennerholm AM. 2007. Mucosal and systemic immune responses in patients with diarrhea due to CS6-expressing enterotoxigenic Escherichia coli. Infect Immun 75:2269–2274. doi: 10.1128/IAI.01856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Leung DT, Chowdhury F, Calderwood SB, Qadri F, Ryan ET. 2012. Immune responses to cholera in children. Expert Rev Anti Infect Ther 10:435–444. doi: 10.1586/eri.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. 1988. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods 110:29–36. [DOI] [PubMed] [Google Scholar]