Abstract
The HMW1 and HMW2 proteins are highly immunogenic adhesins expressed by approximately 75% of nontypeable Haemophilus influenzae (NTHi) strains, and HMW1- and HMW2-specific antibodies can mediate opsonophagocytic killing of NTHi. In this study, we assessed the ability of HMW1- and HMW2-specific antibodies in sera from healthy adults and convalescent-phase sera from children with NTHi otitis media to mediate killing of homologous and heterologous NTHi. The serum samples were examined pre- and postadsorption on HMW1 and HMW2 affinity columns, and affinity-purified antibodies were assessed for ability to mediate killing of homologous and heterologous strains. Adult serum samples mediated the killing of six prototype NTHi strains at titers of <1:10 to 1:1,280. HMW1- and HMW2-adsorbed sera demonstrated unchanged to 8-fold decreased opsonophagocytic titers against the homologous strains. Each affinity-purified antibody preparation mediated the killing of the respective homologous strain at titers of <1:10 to 1:320 and of the five heterologous strains at titers of <1:10 to 1:320, with most preparations killing most heterologous strains to some degree. None of the acute-phase serum samples from children mediated killing, but each convalescent-phase serum sample mediated killing of the infecting strain at titers of 1:40 to 1:640. HMW1- and HMW2-adsorbed convalescent-phase serum samples demonstrated ≥4-fold decreases in titer. Three of four affinity-purified antibody preparations mediated killing of the infecting strain at titers of 1:20 to 1:320, but no killing of representative heterologous strains was observed. HMW1- and HMW2-specific antibodies capable of mediating opsonophagocytic killing are present in the serum from normal adults and develop in convalescent-phase sera of children with NTHi otitis media. Continued investigation of the HMW1 and HMW2 proteins as potential vaccine candidates for the prevention of NTHi disease is warranted.
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHi) comprises small Gram-negative bacteria that colonize the upper respiratory tract of humans beginning at a very early age (1). Although these organisms are normally commensals, when host defenses are compromised by underlying medical conditions, such as malnutrition, immunodeficiency, chronic lung disease, or acute viral infection, disease caused by NTHi may develop (2, 3). Among children in the developed world, NTHi is currently responsible for an estimated 40 to 50% of the cases of acute otitis media and an even higher percentage of cases of chronic and recurrent disease (4, 5). Among adults, particularly among patients with chronic obstructive pulmonary disease, NTHi is a major cause of illness, particularly during the acute exacerbations that often characterize this disease (6). A vaccine capable of preventing disease caused by these organisms would offer substantial benefit to adult and pediatric populations alike.
NTHi vaccine development efforts are ongoing in a number of laboratories. Published studies have suggested that NTHi outer membrane proteins are the principal targets of bactericidal and protective antibodies (3, 7, 8). Several protein antigens have been the subject of detailed investigations as potential vaccine candidates (9–11). The proteins known as P2 and P6 have been studied in great detail. Each is a target of human bactericidal antibody (12–14), and each has demonstrated partial protection against infection in animal models (15, 16). Another leading vaccine candidate, the so-called P5-fimbrin adhesin (17, 18), has also demonstrated protection in the chinchilla otitis model (19, 20). Other proteins still under active investigation as possible vaccine candidates include protein D (21), protein E (22), type IV pili (23, 24), and outer membrane protein (OMP) 26 (20, 25). Even lipooligosaccharide, in the form of detoxified conjugate preparations, has been investigated as a potential vaccine candidate (26–28). A recent human clinical trial, in which children were immunized with a protein D-pneumococcal polysaccharide conjugate vaccine, reported protection against pneumococcal and NTHi otitis media (29, 30), but protection against NTHi disease was quite modest and did not correlate with serum anti-protein D antibody levels. A follow-up study of the same vaccine in a younger population demonstrated only marginal protection against NTHi otitis media (31).
It remains unclear which, if any, of the many NTHi vaccine candidates under study is best suited for inclusion in a human-protective vaccine. The heterogeneity known to be present among NTHi strains is a challenge that must be overcome for any vaccine development effort to succeed (32–34). Some in the NTHi vaccine community have suggested that only highly conserved proteins should be investigated as potential vaccine candidates (35), but it is questionable whether any conserved proteins exist that are capable, by themselves, of inducing a broad-based protective immune response. Many in the field have speculated that only by formulating a vaccine with multiple protective antigens will we be successful in developing a vaccine capable of protecting young children and adults against NTHi disease (17).
In our earlier work, we demonstrated that the development of bactericidal antibodies in serum samples from children who recovered from acute NTHi otitis media was associated with the appearance of serum antibodies directed against highly immunogenic high-molecular-weight proteins (2). This work led subsequently to the identification and characterization of the HMW1 and HMW2 family of proteins (36). The HMW1 and HMW2 proteins were subsequently shown to be major adhesins of NTHi (37, 38) and targets of opsonophagocytic (39, 40) and protective antibodies (58). The HMW1- and HMW2-like proteins are expressed by approximately 75% of NTHi strains (36, 41). The 25% of NTHi strains that do not express the HMW1 and HMW2 proteins express other immunogenic high-molecular-weight proteins that are recognized by human convalescent-phase serum antibodies (2). Almost all HMW1- and HMW2-negative strains were subsequently shown to express a second distinct class of adhesin known as Hia (42). Nearly all NTHi strains that lack the HMW1 and HMW2 proteins contain an hia gene and express an Hia protein; conversely, strains that express the HMW1 and HMW2 proteins lack an hia gene (41, 44).
Previously, we demonstrated that human HMW1- and HMW2-specific antibodies present in a commercially available intravenous immunoglobulin preparation were capable of mediating opsonophagocytic killing of a few representative NTHi strains (39). Whether these data were also applicable to understanding the immune responses of individual human subjects and the functional activity of antibodies present in their sera was unclear from this earlier work. The objective of the current study was to examine the HMW1- and HMW2-specific antibody responses of individual adults and children and to define the functional activity of HMW1- and HMW2-specific antibodies present in their serum. In the work described here, we characterized serum samples from four healthy adults and from four young children who recently recovered from acute NTHi otitis media.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains 5, 12, 15, 16, 17, 18, 26, 28, 30, and 31 are NTHi strains that express HMW1- and HMW2-like proteins (2, 36, 40, 45). Each strain was isolated in pure culture from a middle ear fluid specimen from a child with acute otitis media and was identified as H. influenzae using standard methods. Each was classified as an NTHi strain based upon failure to agglutinate with a panel of typing antisera for H. influenzae types a to f (Burroughs Wellcome Co., Research Triangle Park, NC) and failure to demonstrate the presence of capsular genes by PCR analysis using the methods described by Satola and coworkers (46). All organisms were stored at −70°C in skim milk within two or three subpassages of initial clinical isolation. For laboratory manipulation, the strains were grown on chocolate agar or in brain heart infusion broth supplemented with hemin and NAD, as previously described (2).
Human serum samples.
Four healthy adult volunteers provided serum samples to study the antibodies in their sera. Each of these individuals was immunologically normal and had no history of serious infectious problems in the past. Four children were also selected for study based upon a history of acute NTHi otitis media during which NTHi was isolated in pure culture from a middle ear fluid specimen obtained by tympanocentesis. The children ranged from 3 to 18 months of age and were in generally good health without underlying medical problems. The number of otitis episodes documented by a physician in the 6 months before their serum samples were collected for this study ranged from zero to two. Acute-phase serum samples were collected at the time each child presented with otitis media, and convalescent-phase samples were collected 4 to 5 weeks later. Adult volunteers and children provided samples for study after informed consent was obtained either from the adults themselves or the parents of the children. All studies with human samples were performed under a protocol approved by the institutional review board of the St. Louis University School of Medicine, St. Louis, MO.
Detection of antibodies directed against surface-exposed outer membrane proteins by whole-cell radioimmunoprecipitation.
A whole-cell radioimmunoprecipitation assay was performed as described in our earlier work (2, 7). In brief, 5 to 10 colonies from an overnight growth of NTHi on a chocolate agar plate were used to inoculate 50 ml of supplemented brain heart infusion broth and grown to early log phase, at which time the cells were harvested by centrifugation and washed with phosphate-buffered saline (PBS). The outer membrane proteins of intact cells were extrinsically radiolabeled with 125I by the lactoperoxidase method. The intact labeled cells were washed three times with PBS and then resuspended at a concentration of approximately 1010 cells per ml (specific activity, ∼0.01 counts per minute [cpm] per CFU). Labeled cells (250 μl) were mixed with an equal volume of test serum, and the mixtures were rocked at 4°C for 90 min. Unbound antibodies were removed from the cells by washing them once with PBS. Antigen-antibody complexes were then released from the cells by incubation in solubilization buffer consisting of 10 mM Tris buffer (pH 7.8) containing 150 mM NaCl, 10 mM EDTA, 1% (vol/vol) Triton X-100, 0.2% (wt/vol) sodium deoxycholate, and 0.1% sodium dodecyl sulfate for 1 h at 37°C. The mixture was then centrifuged at 45,000 × g for 60 min at room temperature to remove insoluble debris. Next, the supernatant was incubated overnight at 4°C with 4 mg of protein A-Sepharose beads (Sigma) to bind soluble antigen-IgG antibody complexes. The protein A-Sepharose beads with bound antigen-antibody complexes were washed five times in solubilization buffer and then dissociated by boiling for 5 min in dissolution buffer consisting of 0.0625 M Tris buffer (pH 6.8) containing 2% sodium dodecyl sulfate (SDS), 10% glycerol, and 0.001% bromphenol blue tracking dye. The Sepharose beads were removed by centrifugation, and the antigen-antibody complexes were resolved by SDS-polyacrylamide gel electrophoresis, followed by autoradiography to identify radiolabeled outer membrane proteins that were bound by the IgG antibodies present in the test sera.
In radioimmunoprecipitation assays with murine HMW1- and HMW2-specific monoclonal antibodies, slight variations of the above-described protocol were used. For the whole-cell assay, after the monoclonal antibodies were incubated with the labeled whole cells, they were incubated with goat anti-mouse IgG/IgM prior to incubation with protein A-Sepharose. This additional step was included to enhance binding of the bound monoclonal antibodies to the protein A-Sepharose beads. For the presolubilized cell assay, the radiolabeled bacterial cells were first solubilized in solubilization buffer prior to incubation with the monoclonal antibodies. This allowed for increased binding of the monoclonal antibodies to their cognate epitopes on the HMW1 and HMW2 proteins. An incubation step with goat anti-mouse IgG/IgM also was performed prior to incubation of the samples with protein A-Sepharose beads. The four HMW1- and HMW-specific monoclonal antibodies used in this study, 1C3, 1E7, 4B2, and 5C7, are murine IgM antibodies that were generated using previously described methods (43). Each recognizes an epitope common to the HMW1 and HMW2 proteins of NTHi strain 12.
Generation and purification of HMW1 and HMW2 proteins.
The HMW1 and HMW2 high-molecular-weight proteins were purified from each NTHi strain using previously described methods (39). In brief, a frozen bacterial stock culture was streaked onto a chocolate agar plate and allowed to grow overnight at 37°C in an atmosphere of 5% CO2. The following day, each bacterial strain was grown in bulk to late-log phase in six 500-ml flasks of brain heart infusion broth supplemented with NAD and hemin. The bacteria were then pelleted and frozen overnight at −20°C. The next day, the bacterial pellets were resuspended in extraction solution (0.5 M NaCl, 0.01 M Na2-EDTA, 0.01 M Tris, and 50 μM 1,10-phenanthroline [pH 7.5]) on ice for 1 h, followed by centrifugation at 12,000 × g to remove the intact cells and cellular debris. The supernatants, containing the soluble HMW1 and HMW2 proteins, were then centrifuged at 100,000 × g for 1 h to remove membrane fragments and other residual debris, and the final supernatants were dialyzed overnight against 0.01 M Na phosphate (pH 6.0). The next day, the pH-equilibrated supernatants were passed over CM Sepharose columns that captured the HMW1 and HMW2 proteins. Bound proteins were eluted using a 0 to 0.5 M KCl gradient, and column fractions were analyzed on Coomassie blue-stained gels to identify fractions containing the HMW1 and HMW2 proteins. Fractions containing the proteins were then pooled and concentrated prior to passage over a Sepharose CL-6B (Sigma) gel filtration column. Column fractions containing the HMW1 and HMW2 proteins were identified on Coomassie blue-stained gels, pooled, and stored at −70°C prior to further use.
Preparation of HMW1 and HMW2 protein affinity columns.
To prepare the affinity columns, purified HMW1 and HMW2 proteins from each strain were coupled to cyanogen-bromide-activated Sepharose 4B (Sigma) using standard techniques. In brief, 10 to 20 mg of each purified protein preparation was dissolved in 5 ml of coupling buffer (0.1 M sodium bicarbonate, 0.5 M NaCl [pH 8.3]) and reacted overnight at 4°C with gentle rocking with 0.75 g of activated Sepharose 4B. Residual reactive groups were blocked by the addition of 1 ml of 3 M ethanolamine to the gel suspensions and incubated until the following day at 4°C. The gel-protein matrices were then washed extensively with alternating volumes of coupling buffer and acetate buffer (0.1 M sodium acetate, 0.5 M NaCl [pH 4.0]) to remove non-covalently bound HMW1 and HMW2 proteins. Finally, the columns were equilibrated with PBS–0.5% bovine serum albumin (BSA) prior to use for affinity chromatography with the serum samples. The total swollen gel volume was approximately 2 ml for each column. A control column was prepared using BSA as the coupled protein.
Affinity chromatography sera from children and adults.
To generate the HMW1- and HMW2-adsorbed fractions and affinity-purified HMW1- and HMW2-specific antibodies, serum samples were passed over the HMW1 and HMW2 protein columns of interest. Two milliliters of serum from each adult was passed over each of four different HMW1 and HMW2 affinity columns and the albumin control column to remove HMW1- and HMW2-specific antibodies. Because of volume limitations, only 0.5 ml was used for adsorption of the serum samples from the individual children. The serum samples from the children were passed only over the HMW1 and HMW2 column of the respective infecting NTHi strain. Strain-specific HMW1 and HMW2 antibodies were removed with >95% efficiency by each of the columns, as determined by an enzyme-linked immunosorbent assay (ELISA). The columns were washed with 10 column volumes of PBS–0.5% albumin, and affinity-purified HMW1- and HMW2-specific antibodies were eluted with two column volumes of 0.1 M glycine (pH 2.5). The affinity-purified HMW1 and HMW2 antibody fractions were immediately neutralized following elution with 1 M Tris (pH 8.0). Both the HMW1- and HMW2-adsorbed fractions and the affinity-purified HMW1- and HMW2-specific antibodies were dialyzed extensively against PBS at 4°C before further use. Prior to use in the opsonophagocytic assay, the HMW1- and HMW2-adsorbed fractions were concentrated to the volume of the serum sample originally passed over the column. The affinity-purified strain-specific HMW1 and HMW2 antibodies were concentrated such that their titers were equivalent to those of the respective unadsorbed sera, as determined by ELISA (see below).
ELISA methodology.
Antibodies specific for native HMW1 and HMW2 proteins were measured by ELISA using previously described methods (39). In brief, 96-well flat-bottomed enzyme immunoassay microtitration plates (Nunc PolySorp; Thermo Scientific) were coated overnight at 4°C with purified HMW1 and HMW2 proteins (10 μg per ml of total protein) in NaCO3 buffer (pH 9.6). The following day, the plates were blocked with PBS–0.5% bovine serum albumin (BSA) at room temperature for 1 h and washed with PBS–0.5% BSA–0.05% Tween 20 prior to addition of the various sera or serum fractions. The samples were serially diluted in PBS–0.5% BSA–0.05% Tween 20 starting at a 1:100 dilution and incubated on the plates for 1 h at room temperature. After additional washes, the wells were incubated with a 1:3,000 dilution of goat anti-human IgG antibody (Life Technologies, Gaithersburg, MD) for 1 h at room temperature. After additional washes, the wells were incubated with 200 μl of a 1 mg/ml solution of p-nitrophenyl phosphate in 10% diethanolamine buffer (pH 9.8). Absorbance at 405 nm was monitored using a Titertek Multiskan spectrophotometer (Flow Laboratories). The IgG antibody titer of a serum sample was defined as the maximum dilution giving a reading of at least twice the mean of six control wells on each plate that consisted of buffer alone.
Opsonophagocytic assay with nontypeable H. influenzae strains.
The growth conditions of the bacteria for the opsonophagocytic assay, the growth and differentiation of the HL-60 cells, and the specific details of the opsonophagocytic assay itself were as described previously (45). The percent killing at each serum dilution was calculated by determining the ratio of the bacterial colony count at each dilution to that of the respective complement control. Statistical analyses were performed using the NCSS 2000 software package (NCSS, Kaysville, UT). Opsonophagocytic titers were defined as the reciprocals of the serum or antibody dilutions that resulted in ≥50% killing of the bacterial inocula compared to growth in the complement controls.
RESULTS
WC-RIP assay to identify surface-exposed outer membrane proteins recognized by antibodies in adult serum samples.
The whole-cell radioimmunoprecipitation (WC-RIP) assay allows the identification of surface-exposed bacterial proteins recognized by IgG antibodies present in the serum samples being characterized (Fig. 1). In a previous work, we reported that sera from normal healthy adults immunoprecipitate an array of high-molecular-weight proteins when assayed against representative NTHi in the WC-RIP assay (2). Several lines of evidence suggest that in NTHi strains that express the HMW1 and HMW2 proteins, these proteins are the principal targets of the high-molecular-weight protein-directed antibody observed in the WC-RIP assay (36, 39). The functional activity of the HMW1- and HMW2-specific antibodies in individual human serum samples is unknown. Our hypothesis is that these antibodies are major contributors to the NTHi-specific opsonophagocytic activity that may develop in human serum. The purpose of the work described in this paper was to address this question directly by studying sera from representative adults and children in greater detail.
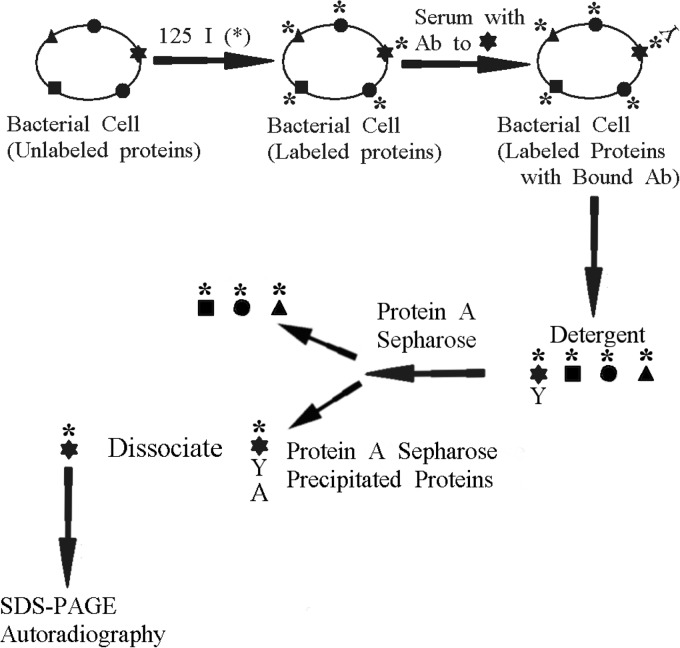
FIG 1.
Schematic diagram summarizing the steps involved in the whole-cell radioimmunoprecipitation assay. This assay allows the identification of the major surface-exposed bacterial proteins against which serum IgG antibody is directed.
First, serum samples from four healthy adult volunteers were examined in the WC-RIP assay to identify NTHi surface-exposed proteins recognized by antibodies present in each of the serum samples. As seen in Fig. 2, in a WC-RIP assay with prototype NTHi strain 12, each of the adult serum samples immunoprecipitated several high-molecular-weight Mr 100,000 to 150,000 protein bands in patterns very similar to one another (lanes 3 to 6). For comparison, shown in Fig. 2, lane 2 are the proteins immunoprecipitated by a high-titer rabbit immune serum sample prepared against purified HMW1 and HMW2 proteins from strain 12. The proteins immunoprecipitated by the HMW1 and HMW2 rabbit immune serum sample and by the four adult serum samples are nearly identical, suggesting that the human antibodies recognize the same HMW1 and HMW2 proteins as do the antibodies present in the rabbit immune serum. The explanation for the presence of several discrete immunoprecipitated bands in each lane lies in the fact that the NTHi target strain expresses both mature HMW1 and HMW2 proteins of slightly different molecular weights (36) and preprocessed forms of each protein (47), all of which might be recognized by HMW1- and HMW2-specific antibodies. Similar results, in terms of the immunodominance of similar high-molecular-weight proteins, were observed previously when adult serum samples were examined with other HMW1- and HMW2-expressing NTHi strains in the WC-RIP assay (2).
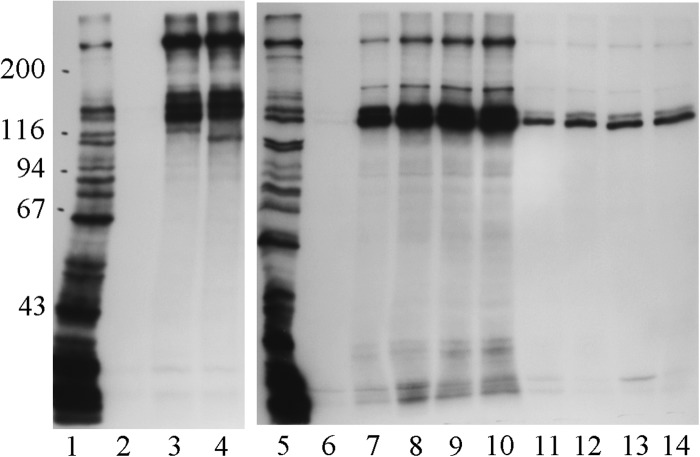
FIG 2.
Whole-cell radioimmunoprecipitation assay using 11% acrylamide gels with serum samples from a rabbit immunized with strain 12 HMW1 and HMW2 proteins and serum samples from four healthy adults. Each serum sample was tested against NTHi strain 12. Lane 1, radiolabeled proteins present in the NTHi strain 12 test strain; lane 2, radiolabeled proteins immunoprecipitated by the antibodies present in the serum sample from a rabbit immunized with strain 12 HMW1 and HMW2 proteins; lanes 3 to 6: radiolabeled proteins immunoprecipitated by the antibodies naturally present in the serum samples from the four healthy adults. Ordinate: molecular mass in kilodaltons.
Radioimmunoprecipitation assays with selected human sera and HMW1- and HMW2-specific monoclonal antibodies.
Radioimmunoprecipitation assays performed with HMW1- and HMW2-specific monoclonal antibodies provided additional evidence that the proteins immunoprecipitated by the adult sera consisted primarily of HMW1 and HMW2 proteins. Shown in the left panel of Fig. 3 are the results of a WC-RIP assay in which acute and convalescent-phase serum samples from the child infected with NTHi strain 12 and a serum sample from a representative adult were assayed against NTHi strain 12. This is the same adult serum sample that was examined in Fig. 2, lane 3. As can be seen, the convalescent-phase serum sample from the child and the serum sample from the adult immunoprecipitated several high-molecular-weight Mr 100,000 to 250,000 protein bands in patterns that were very similar to one another. In Fig. 3, the samples were separated on 7.5% acrylamide gels, as opposed to the 11% acrylamide gels used in Fig. 2. Use of the lower acrylamide concentration helps to separate the high-molecular-weight bands from one another.
FIG 3.
Radioimmunoprecipitation assays with NTHi strain 12 using 7.5% acrylamide gels. Left panel: acute and convalescent-phase serum samples from the child infected with NTHi strain 12 and serum from an unrelated adult assayed against whole bacterial cells. Right panel: tissue culture supernatants containing one of four HMW1- and HMW2-specific monoclonal antibodies assayed against presolubilized and whole cells. Lane 1, radiolabeled proteins present in the labeled bacterial cells; lanes 2 to 4, radiolabeled proteins immunoprecipitated by a child acute-phase serum sample, a child convalescent-phase serum sample, and an adult serum sample (same adult as shown in Fig. 2, lane 3), respectively; lane 5, radiolabeled proteins present in labeled bacterial cells; lanes 6 to 14, radiolabeled proteins immunoprecipitated by tissue culture medium alone (lane 6) and tissue culture supernatants from hybridomas expressing monoclonal antibody 1C3, 1E7, 4B2, or 5C7 assayed against presolubilized cells (lanes 7 to 10, respectively) or whole bacterial cells (lanes 11 to 14, respectively). Ordinate: molecular mass in kilodaltons.
Shown in the right panel of Fig. 3 are the results of a radioimmunoprecipitation assay in which four HMW1- and HMW2-specific monoclonal antibodies were assayed against either presolubilized or whole NTHi strain 12 cells. Each of the monoclonal antibodies immunoprecipitated four discrete Mr 100,000 to 250,000 protein bands in patterns identical to one another and nearly identical to the patterns produced by the convalescent-phase serum sample from the child and the adult serum sample seen in the left panel. The stronger signals produced by incubation with presolubilized cells likely resulted from greater accessibility of the epitopes on the HMW1 and HMW2 proteins recognized by the monoclonal antibodies when the proteins were free in solution. The demonstration that the monoclonal antibodies immunoprecipitated multiple discrete high-molecular-weight proteins in a pattern nearly identical to that produced by the human sera provides additional evidence that these bands likely represent different forms of preprocessed and mature HMW1 and HMW2 proteins.
Opsonophagocytic activity of HMW1- and HMW2-specific antibodies present in adult serum samples.
Having demonstrated the presence of high levels of high-molecular-weight protein-directed antibodies in the adult sera, we next sought to define the specific contribution of HMW1- and HMW2-directed antibodies to the in vitro functional activity of those sera. Our strategy was to first adsorb each of the adult serum samples with HMW1 and HMW2 proteins purified from a small panel of representative NTHi strains and then examine the original sera and HMW1- and HMW2-adsorbed serum fractions for their ability to mediate opsonophagocytic killing. In addition, we eluted affinity-purified HMW1- and HMW2-specific antibodies from each column and assessed those antibodies for their ability to mediate opsonophagocytic killing. Each of the adult serum samples was first assessed for the presence of HMW1- and HMW2-specific antibodies in a standard ELISA with purified HMW1 and HMW2 proteins from four representative NTHi strains, 5, 12, 16, and 17.
As shown in Table 1, each of the adult serum samples had substantial levels of antibody directed against the HMW1 and HMW2 proteins purified from each of the four strains. Once HMW1- and HMW2-specific antibodies were demonstrated to be present in each of the adult serum samples, we prepared the HMW1- and HMW2-adsorbed fractions and the affinity-purified HMW1- and HMW2-specific antibodies. Each adult serum sample was passed over strain 5, 12, 16, and 17 HMW1 and HMW2 columns and an albumin control column to generate four strain-specific HMW1- and HMW2-adsorbed fractions, four affinity-purified HMW1 and HMW2 antibody preparations, and respective albumin column control preparations. Prior to examination of the samples in the opsonophagocytic assay described below, each column-adsorbed sample was concentrated to the same volume as the original serum sample passed over the column, and each affinity-purified fraction was concentrated such that its HMW1 and HMW2 ELISA titer was equivalent to that of the original unadsorbed serum sample (Table 1).
TABLE 1.
HMW1- and HMW2-specific endpoint ELISA titers of serum samples from four healthy adults against HMW1 and HMW2 proteins from four prototype NTHi strains
| Adult no. | HMW1 and HMW2 protein titers by strain |
|||
|---|---|---|---|---|
| 5 | 12 | 16 | 17 | |
| 1 | 3,200 | 1,600 | 6,400 | 3,200 |
| 2 | 800 | 1,600 | 3,200 | 1,600 |
| 3 | 1,600 | 6,400 | 6,400 | 3,200 |
| 4 | 400 | 800 | 1,600 | 800 |
As seen in Table 2, the preadsorbed adult sera mediated killing of the four prototype NTHi strains over a wide range. Of note, these are the same four strains from which the HMW1 and HMW2 proteins used to prepare the affinity columns were purified. The preadsorbed sera from adults 2 and 3 mediated significant killing of most of the NTHi strains examined, whereas the preadsorbed sera from adults 1 and 4 mediated, at best, limited killing of any of the four strains. There was no obvious pattern noted in a comparison of the four adult serum samples in terms of which NTHi strains were more or less likely to be susceptible to opsonophagocytic killing. In addition, there was no obvious correlation between the measured HMW1 and HMW2 ELISA titers for an individual serum sample and the ability or inability of that sample to mediate killing (compare Tables 1 and 2).
TABLE 2.
Opsonophagocytic titers against the homologous NTHi strains of sera from four adults pre- and post-adsorption with HMW1 and HMW2 proteins or with serum albumina
| Serum prep | Titer by strain |
|||
|---|---|---|---|---|
| 5 | 12 | 16 | 17 | |
| Adult 1 | ||||
| Preadsorption | 20 | <10 | <10 | <10 |
| HMW-adsorbed | <10 | <10 | <10 | <10 |
| Alb-adsorbed | 20 | <10 | <10 | <10 |
| Adult 2 | ||||
| Preadsorption | 320 | 160 | <10 | 320 |
| HMW-adsorbed | 40 | 40 | <10 | 40 |
| Alb-adsorbed | 320 | 160 | <10 | 320 |
| Adult 3 | ||||
| Preadsorption | 10 | 80 | 1280 | 1280 |
| HMW-adsorbed | <10 | 20 | 640 | 640 |
| Alb-adsorbed | 10 | 80 | 1280 | 1280 |
| Adult 4 | ||||
| Preadsorption | <10 | <10 | 40 | <10 |
| HMW-adsorbed | <10 | <10 | <10 | <10 |
| Alb-adsorbed | <10 | <10 | 40 | <10 |
A single serum sample from each adult volunteer was adsorbed on each of five different affinity columns coupled with purified HMW1 and HMW2 proteins from strain 5, 12, 16, or 17, or with albumin (Alb) as a control. The preadsorbed sample, the HMW1- and HMW2 -adsorbed sample, and the albumin-adsorbed control sample were each assayed for their ability to mediate killing of the respective homologous NTHi strain.
The removal of strain-specific HMW1 and HMW2 antibodies from adult sera capable of mediating killing led to apparent decreases in their killing ability postadsorption (Table 2). However, the decreases in titer were rather modest, ranging from 2-fold to 8-fold, depending upon which sera and NTHi strains were being examined. All sera were also adsorbed on an albumin control column, and all of the albumin-adsorbed sera mediated killing at the same titers as those reported for the corresponding preadsorbed serum samples (Table 2).
Each of the affinity-purified HMW1 and HMW2 antibody preparations was also assessed for its ability to mediate opsonophagocytic killing. The NTHi target strains included the same four strains examined above with the preadsorbed and HMW-adsorbed sera and two additional NTHi strains that were also isolated from middle ear fluid specimens of children with acute otitis media. The hmw1A and hmw2A genes encoding the respective HMW1 and HMW2 proteins of four of these six strains (strains 5, 12, 15, and 16) have been sequenced (36, 59; our unpublished data). The deduced amino acid sequences of the eight corresponding HMW1 and HMW2 proteins are between 65 and 82% identical, a range that mirrors the variation present in the >30 HMW1 and HMW2 sequences currently available in public databases.
As shown in Table 3, each affinity-purified antibody preparation from each adult serum sample mediated killing of the respective homologous strain at titers ranging from <1:10 to 1:320. The anti-strain 5 HMW preparation from adult 4 was the only sample that did not mediate at least some killing of the respective homologous strain. Each affinity-purified antibody preparation also mediated killing of the five respective heterologous NTHi strains at titers of <1:10 to 1:320, with most affinity-purified antibody preparations killing most of the five heterologous strains to some degree. Even in cases in which the preadsorbed serum samples did not mediate killing of a given NTHi strain, affinity-purified HMW1- and HMW2-specific antibodies recovered from those same serum samples often mediated killing of those same strains (compare data in Tables 2 and 3). Antibodies eluted from the albumin control column did not mediate killing of any of the NTHi strains examined. It is worth noting that it may be difficult to elute high-affinity antibodies from solid-phase affinity columns. Thus, the results we observed in the opsonophagocytic assays with the affinity-purified HMW1- and HMW2-specific antibody preparations may not be reflective of the total HMW1- and HMW2-specific antibody activity present in those sera.
TABLE 3.
Opsonophagocytic titers against homologous and heterologous NTHi strains of HMW1- and HMW2-specific antibodies from samples from four adult volunteersa
| Antibody prep | Titer by strain |
|||||
|---|---|---|---|---|---|---|
| 5 | 12 | 15 | 16 | 17 | 18 | |
| Adult 1 | ||||||
| Anti-5 HMW | 40 | <10 | <10 | <10 | 40 | 80 |
| Anti-12 HMW | <10 | 160 | 40 | 20 | 40 | 320 |
| Anti-16 HMW | 10 | 20 | 20 | 80 | 320 | 10 |
| Anti-17 HMW | <10 | 20 | 10 | 320 | 320 | 10 |
| Antialbumin | <10 | <10 | <10 | <10 | <10 | <10 |
| Adult 2 | ||||||
| Anti-5 HMW | 20 | 40 | 10 | <10 | 80 | 10 |
| Anti-12 HMW | 10 | 80 | 80 | <10 | 80 | 10 |
| Anti-16 HMW | 10 | <10 | <10 | 160 | 80 | 40 |
| Anti-17 HMW | 10 | 20 | 20 | 80 | 80 | 40 |
| Antialbumin | <10 | <10 | <10 | <10 | <10 | <10 |
| Adult 3 | ||||||
| Anti-5 HMW | 20 | 40 | 40 | 80 | 160 | 320 |
| Anti-12 HMW | 20 | 80 | 320 | 160 | 80 | 320 |
| Anti-16 HMW | 10 | 320 | 40 | 80 | 320 | 320 |
| Anti-17 HMW | <10 | 320 | 40 | 320 | 320 | 320 |
| Antialbumin | <10 | <10 | <10 | <10 | <10 | <10 |
| Adult 4 | ||||||
| Anti-5 HMW | <10 | <10 | <10 | 40 | 20 | 20 |
| Anti-12 HMW | <10 | 20 | 20 | 40 | <10 | 20 |
| Anti-16 HMW | <10 | <10 | <10 | 80 | 160 | 160 |
| Anti-17 HMW | <10 | <10 | 10 | 80 | 160 | 80 |
| Antialbumin | <10 | <10 | <10 | <10 | <10 | <10 |
A single serum sample from each adult volunteer was adsorbed on each of five different affinity columns coupled with purified HMW1 and HMW2 proteins from strain 5, 12, 16, or 17, or with albumin as a control. Affinity-purified HMW1- and HMW2-specific or albumin-specific antibodies were eluted from each column and assayed for their ability to mediate opsonophagocytic killing of the six NTHi strains shown.
Whole-cell radioimmunoprecipitation assay to identify surface-exposed outer membrane proteins recognized by antibodies in serum samples from four children.
We next sought to investigate the contribution of HMW1- and HMW2-specific antibodies to the opsonophagocytic killing activity that develops in convalescent-phase sera from children with acute NTHi otitis media. The four children whose serum samples were selected for study each had an HMW1- and HMW2-expressing NTHi strain recovered in pure culture from a middle ear fluid specimen collected during an episode of acute otitis media. Furthermore, each child had acute- and convalescent-phase serum samples from that otitis episode available for study, with the convalescent-phase sample having been demonstrated to mediate opsonophagocytic killing of the respective infecting NTHi strain.
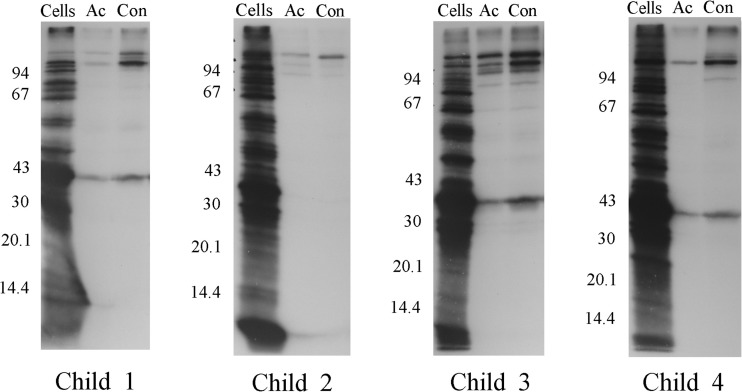
The acute- and convalescent-phase serum samples from the four children were first examined in the WC-RIP assay to identify NTHi surface-exposed proteins recognized by antibodies in each of the sera. As shown in Fig. 3, the acute-phase serum sample from each of the four children immunoprecipitated small quantities of a few proteins in the Mr 100,000 to 150,000 range and another protein in the Mr 39,000 to 40,000 range. The respective convalescent-phase samples demonstrated substantial increases in antibody activity directed predominantly against one or more of the Mr 100,000 to 150,000 proteins. As discussed above, our working hypothesis is that much of this high-molecular-weight protein-directed antibody is specific for the respective HMW1 and HMW2 proteins. The Mr 39,000 to 40,000 bands immunoprecipitated by several of the serum samples likely represent the P2 protein expressed by these strains, given the apparent molecular weight and known immunogenicity of this protein.
Opsonophagocytic activity of HMW1- and HMW2-specific antibodies present in serum samples from four children.
As was done with the adult serum samples, we next sought to define the contribution of the HMW1- and HMW2-specific antibodies to the functional opsonophagocytic activity of the convalescent-phase serum samples from the children. Each of the serum samples was first assessed for the presence of HMW1- and HMW2-specific antibodies in a standard ELISA with purified HMW1 and HMW2 proteins from their respective infecting NTHi strains. These were strains 26, 28, 30, and 31, recovered from middle ear fluid specimens from children 1 to 4, respectively (45). As shown in Table 4, three of the four children had no detectable HMW1- and HMW2-specific ELISA antibodies in their acute-phase serum samples, and one child had relatively low preexisting antibody levels. In contrast, each of the convalescent-phase serum samples demonstrated major boosts in HMW1- and HMW2-specific antibody activity compared to that of the respective acute specimens. Also included in Table 4 for comparison are the ELISA results when serum from adult 1 was assayed against the same HMW1 and HMW2 proteins as the respective acute and convalescent-phase serum samples. As can be seen, this adult serum sample had substantial antibody activity directed against the HMW1 and HMW2 proteins from all four strains, again demonstrating the common presence of naturally acquired antibodies in adult serum to many different HMW1 and HMW2 proteins.
TABLE 4.
HMW1- and HMW2-specific endpoint ELISA titers of serum samples from four children with acute NTHi otitis media against HMW1 and HMW2 proteins from their infecting strain
| Serum sample | Titer by child |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Acute phase | <200 | 200 | 400 | <200 |
| Convalescent phase | 800 | 3,200 | 6,400 | 1,600 |
| Adult 1 control | 3,200 | 3,200 | 3,200 | 1,600 |
To further characterize the functional activity of the HMW1- and HMW2-specific antibodies in the serum samples from the children, we next adsorbed each child's convalescent-phase serum sample on an HMW1 and HMW2 affinity column prepared with proteins purified from that child's infecting strain. Affinity-purified antibodies were also recovered for further study. The acute-phase, convalescent-phase, and convalescent-phase adsorbed sera and the affinity-purified antibody preparations were then examined in the opsonophagocytic assay.
As shown in Table 5, none of the acute-phase serum samples mediated opsonophagocytic killing of any of the respective infecting strains. In contrast, each convalescent-phase serum sample mediated killing of the corresponding infecting strain at titers of 1:40 to 1:640. Each of the HMW1- and HMW2 -adsorbed convalescent-phase serum samples demonstrated ≥4-fold decreases in killing activity compared to that of the respective unadsorbed convalescent-phase sample. Furthermore, three of the four affinity-purified anti-HMW1 and anti-HMW2 antibody preparations mediated substantial killing of the respective infecting strain at titers ranging from 1:20 to 1:320. The affinity-purified HMW1- and HMW2-specific antibodies recovered from the serum samples from the children did not mediate killing of any of six NTHi strains examined above with the adult sera, suggesting a much more restricted antibody repertoire in the convalescent-phase sera from children than that in the adult sera.
TABLE 5.
Opsonophagocytic titers against the infecting NTHi strain of sera and serum fractions from four children with NTHi otitis media
| Serum sample | Titer by child |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Acute phase | <1:10 | <1:10 | <1:10 | <1:10 |
| Convalescent phase | 1:640 | 1:40 | 1:640 | 1:40 |
| HMW-Ads Conva | <1:10 | <1:10 | 1:80 | 1:10 |
| Affinity-pure anti-HMW | 1:320 | 1:20 | 1:40 | <1:10 |
HMW-Ads Conv, HMW adsorbed convalescent-phase serum.
DISCUSSION
Characterization of the natural host immune response to colonization or infection with a potential pathogen may allow a prediction of the components of a protective immune response and the identification of potential protective antigens. In the case of a bacterial organism, such as NTHi, antibodies capable of interacting with surface-exposed antigens in the bacterial outer membrane are likely to be critical to host protection (3), but the specific antigens most important in a protective response remain unclear. In the work described here, we made use of a whole-cell radioimmunoprecipitation assay in an analysis of the serum antibody responses in children and adults. This assay allows the identification of specific surface-exposed bacterial proteins that are immunogenic in the course of natural colonization or infection and are recognized by and accessible to host antibodies. We demonstrated that sera from both individual children and adults primarily recognized high-molecular-weight proteins in the bacteria, sometimes immunoprecipitating several discrete protein bands. We presented indirect evidence that much if not most of this high-molecular-weight protein-directed antibody was specific for the HMW1 and HMW2 family of proteins.
A number of lower-molecular-weight bacterial surface proteins, including protein D, P2, P5, and P6, among others, remain under active investigation as potential vaccine candidates (48), and several of these proteins have been demonstrated to be naturally immunogenic in the course of NTHi colonization or disease (13, 25). In the WC-RIP studies described here, we noted occasional antibody activity against lower-molecular-weight proteins, one of which was likely the P2 protein, based upon the apparent molecular weight of the precipitated bands (Fig. 4). However, antibody activities against lower-molecular-weight proteins were quite variable from individual to individual and generally at much lower levels than those seen directed against the high-molecular-weight proteins. This is not to suggest that these other NTHi proteins would be any more or less valuable as vaccine candidates but simply to point out that their natural immunogenicity would appear to be less than that of the HMW1 and HMW2 proteins.
FIG 4.
Whole-cell radioimmunoprecipitation assay using 11% acrylamide gels with serum samples from four children with acute NTHi otitis media. Each serum sample was tested against the NTHi strain isolated from the middle ear fluid specimen of that same child. Cells, radiolabeled proteins present in the labeled cells of the NTHi strain isolated from the indicated child; Ac, radiolabeled proteins immunoprecipitated by antibody present in the acute-phase serum of the indicated child; Con, radiolabeled proteins immunoprecipitated by antibody present in the convalescent-phase serum sample of the indicated child. Ordinate: molecular mass in kilodaltons.
An in vitro opsonophagocytic assay was used throughout this work to assess the functional activity of antibodies directed against the HMW1 and HMW2 proteins. Much of the existing literature in the NTHi vaccine development field has made use of in vitro complement-dependent bactericidal assays to measure the functional activity of antibodies of interest (2, 13, 48, 49). However, as we reported previously (39), HMW1- and HMW2-specific antibodies are not capable of mediating killing of NTHi in standard complement-dependent bactericidal assays (2). The explanation for this finding is unclear but may relate to the structural characteristics of the HMW1 and HMW2 proteins. Using deep-etch transmission electron microscopy, Buscher and coworkers (50) reported that the HMW1 protein exists as a hair-like fiber that extends some distance from the bacterial surface, often in pairs. Binding of HMW1- and HMW2-specific antibodies to such an extended surface structure may be capable of activating complement but may not support the formation of a functional membrane attack complex on the bacterial surface proper where it might mediate bacteriolysis.
An immunogenic surface-exposed bacterial protein, such as those we identified in the WC-RIP assays, may or may not have a role to play in a protective host immune response. However, in our studies, we demonstrated directly that the affinity-purified HMW1- and HMW2-specific antibodies in individual human serum samples were functionally active. Specifically, we demonstrated that serum antibodies specific for the HMW1 and HMW2 proteins of four different NTHi strains could be purified from four different adult serum samples, and in almost every instance, those affinity-purified antibodies mediated efficient in vitro killing of the homologous NTHi strain (Tables 3). Furthermore, the HMW1- and HMW2-specific antibodies frequently were capable of mediating efficient killing of additional heterologous NTHi strains (Table 3). These naturally occurring HMW1- and HMW2-specific antibodies presumably resulted from exposure to NTHi either in the course of natural colonization of the nasopharynx or, less likely, in the course of actual NTHi infections. It is extremely unlikely that the four adult subjects would have been colonized with the NTHi from which the HMW1 and HMW2 proteins used to prepare the affinity columns were recovered, given the extensive population diversity known to be present among NTHi strains (32, 51, 52) and the fact that the isolates from which the proteins were purified were originally recovered from children who had acute otitis media 30 years ago (45). Thus, the functionally active antibodies recovered from the affinity columns almost certainly represent antibodies originally directed against immunogenic epitopes on the HMW1 and HMW2 proteins of other unrelated NTHi strains. Even so, these antibodies were cross-reactive with the HMW1 and HMW2 proteins of the NTHi strains that we tested and mediated opsonophagocytic killing of many of those strains.
Characterization of the serum samples from the children with acute NTHi otitis media yielded several interesting findings, a number of which contrasted with those yielded by samples from the healthy adults noted above. First, each of the acute-phase serum samples lacked or had very low levels of HMW1- and HMW2-specific antibodies (Table 4), likely reflecting the limited natural exposure of the children to diverse NTHi strains at a young age (1). Next, the HMW1- and HMW2-specific antibodies were major contributors to the opsonophagocytic activity that developed in each of the convalescent-phase serum samples. This was demonstrated both by the effect of removal of HMW1- and HMW2-specific antibodies on residual activity of the adsorbed sera and by the specific killing activity of the affinity-purified antibody preparations from three of the four children (Table 5). Finally, in contrast to the affinity-purified preparations recovered from the adult serum samples, the HMW1- and HMW2-specific antibodies from the serum samples from children had very limited to no ability to mediate killing of several heterologous strains that were examined (our unpublished data). This last finding is consistent with a model in which the HMW1- and HMW2-specific immune response in a child with a naive immune system is primarily directed against a few immunodominant strain-specific epitopes on the HMW1 and HMW2 proteins. In contrast, an adult with repeated exposures to NTHi bacteria over the course of a lifetime may develop a much broader antibody response to the HMW1 and HMW2 proteins, being able to recognize many more epitopes, some of which may be relatively immunorecessive and perhaps more widely shared among the HMW1 and HMW2 proteins of unrelated strains.
One final observation of note was the varied ability of preadsorbed serum samples from the four adults to mediate opsonophagocytic killing despite the presence of apparently high levels of HMW1- and HMW2-specific antibodies in each of those samples (Table 1 and Fig. 2). In particular, the serum samples from adults 1 and 4 had limited ability to mediate killing of any of the four prototype NTHi strains tested. Even so, affinity-purified antibodies recovered from those same serum samples were capable of mediating opsonophagocytic killing of multiple strains, sometimes at relatively high titers (compare Tables 2 and 3). The explanation for these somewhat incongruous results is not immediately clear. Earlier literature has described NTHi-specific IgA antibodies capable of inhibiting the bactericidal and opsonophagocytic activities of selected human sera (53). More-extensive literature in the Neisseria meningitidis field has described the ability of pathogen-specific IgA antibodies and lipoprotein-specific IgG antibodies to block complement-dependent bactericidal activity of human serum (54–57). Whether similar mechanisms might explain the varied abilities of human serum samples containing HMW1- and HMW2-specific antibodies to mediate opsonophagocytic killing is unknown at this time, but it would seem a fruitful area for future investigation.
In summary, we demonstrated the presence in representative adult sera of naturally acquired HMW1- and HMW2-specific antibodies capable of mediating relatively broad-based opsonophagocytic killing of representative unrelated NTHi strains. Furthermore, we demonstrated that HMW1- and HMW2-specific antibodies capable of mediating killing of the NTHi infecting strains commonly develop in convalescent-phase serum samples from children with acute NTHi otitis media and are major contributors to the killing activity that is observed. These data argue strongly for the continued investigation of the HMW1 and HMW2 proteins as potential vaccine candidates for the prevention of NTHi disease.
REFERENCES
- 1.Faden H, Duffy L, Williams A, Krystofik DA, Wolf J. 1995. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J Infect Dis 172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 2.Barenkamp SJ, Bodor FF. 1990. Development of serum bactericidal activity following nontypable Haemophilus influenzae acute otitis media. Pediatr Infect Dis J 9:333–339. doi: 10.1097/00006454-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gnehm HE, Pelton SI, Gulati S, Rice PA. 1985. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest 75:1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey JR, Pichichero ME. 2004. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J 23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 5.Pichichero ME, Casey JR, Hoberman A, Schwartz R. 2008. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003–2006. Clin Pediatr (Phila) 47:901–906. doi: 10.1177/0009922808319966. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Murphy TF. 2008. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 7.Barenkamp SJ. 1986. Protection by serum antibodies in experimental nontypable Haemophilus influenzae otitis media. Infect Immun 52:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karasic RB, Trumpp CE, Gnehm HE, Rice PA, Pelton SI. 1985. Modification of otitis media in chinchillas rechallenged with nontypable Haemophilus influenzae and serological response to outer membrane antigens. J Infect Dis 151:273–279. doi: 10.1093/infdis/151.2.273. [DOI] [PubMed] [Google Scholar]
- 9.Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun 67:2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green BA, Metcalf BJ, Quinn-Dey T, Kirkley DH, Quataert SA, Deich RA. 1990. A recombinant non-fatty acylated form of the Hi-PAL (P6) protein of Haemophilus influenzae elicits biologically active antibody against both nontypeable and type b H. influenzae. Infect Immun 58:3272–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green BA, Quinn-Dey T, Zlotnick GW. 1987. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun 55:2878–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy TF, Bartos LC. 1988. Purification and analysis with monoclonal antibodies of P2, the major outer membrane protein of nontypable Haemophilus influenzae. Infect Immun 56:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy TF, Bartos LC, Rice PA, Nelson MB, Dudas KC, Apicella MA. 1986. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Invest 78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neary JM, Murphy TF. 2006. Antibodies directed at a conserved motif in loop 6 of outer membrane protein P2 of nontypeable Haemophilus influenzae recognize multiple strains in immunoassays. FEMS Immunol Med Microbiol 46:251–261. doi: 10.1111/j.1574-695X.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 15.DeMaria TF, Murwin DM, Leake ER. 1996. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun 64:5187–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen EJ, Hart DA, McGehee JL, Toews GB. 1988. Immune enhancement of pulmonary clearance of nontypable Haemophilus influenzae. Infect Immun 56:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakaletz LO, Klein DL, Pelton SI, Barenkamp S, Denoel PA, Green B, Gu X-X, Heikkinen T, Hotomi M, Karma P, Kurono Y, Kyd J, Murphy TF, Ogra PL, Patel JA, Pettigrew MM, Veenhoven RH, Yamanaka N. 2010. Report of the vaccine panel: Ninth International Otitis Media Research Conference, p 94–119. In Lim DJ. (ed), Recent advances in otitis media. House Research Institute, St. Pete Beach, FL. [Google Scholar]
- 18.Bookwalter JE, Jurcisek JA, Gray-Owen SD, Fernandez S, McGillivary G, Bakaletz LO. 2008. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun 76:48–55. doi: 10.1128/IAI.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakaletz LO. 2001. Peptide and recombinant antigens for protection against bacterial middle ear infection. Vaccine 19:2323–2328. doi: 10.1016/S0264-410X(00)00522-3. [DOI] [PubMed] [Google Scholar]
- 20.Kyd JM, Cripps AW, Novotny LA, Bakaletz LO. 2003. Efficacy of the 26-kilodalton outer membrane protein and two P5 fimbrin-derived immunogens to induce clearance of nontypeable Haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx. Infect Immun 71:4691–4699. doi: 10.1128/IAI.71.8.4691-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsgren A, Riesbeck K, Janson H. 2008. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis 46:726–731. doi: 10.1086/527396. [DOI] [PubMed] [Google Scholar]
- 22.Singh B, Al-Jubair T, Thunnissen MM, Mörgelin M, Riesbeck K. 2013. The unique structure of Haemophilus influenzae protein E reveals multiple binding sites for host factors. Infect Immun 81:801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS Jr, Bakaletz LO. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol 65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 24.Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, Murphy TF, Bakaletz LO. 2009. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. 2010. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine 28:7184–7192. doi: 10.1016/j.vaccine.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu XX, Rudy SF, Chu C, McCullagh L, Kim HN, Chen J, Li J, Robbins JB, Van Waes C, Battey JF. 2003. Phase I study of a lipooligosaccharide-based conjugate vaccine against nontypeable Haemophilus influenzae. Vaccine 21:2107–2114. doi: 10.1016/S0264-410X(02)00768-5. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T, Hou Y, Jiao X, Gu XX. 2003. Intranasal immunization with a lipooligosaccharide-based conjugate vaccine from nontypeable Haemophilus influenzae enhances bacterial clearance in mouse nasopharynx. FEMS Immunol Med Microbiol 35:1–10. doi: 10.1111/j.1574-695X.2003.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu T, Chen J, Murphy TF, Green BA, Gu XX. 2005. Investigation of non-typeable Haemophilus influenzae outer membrane protein P6 as a new carrier for lipooligosaccharide conjugate vaccines. Vaccine 23:5177–5185. doi: 10.1016/j.vaccine.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, Schuerman L. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 30.Schuerman L, Borys D, Hoet B, Forsgren A, Prymula R. 2009. Prevention of otitis media: now a reality? Vaccine 27:5748–5754. doi: 10.1016/j.vaccine.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 31.Prymula R, Kriz P, Kaliskova E, Pascal T, Poolman J, Schuerman L. 2010. Effect of vaccination with pneumococcal capsular polysaccharides conjugated to Haemophilus influenzae-derived protein D on nasopharyngeal carriage of Streptococcus pneumoniae and H. influenzae in children under 2 years of age. Vaccine 28:71–78. [DOI] [PubMed] [Google Scholar]
- 32.Erwin AL, Sandstedt SA, Bonthuis PJ, Geelhood JL, Nelson KL, Unrath WC, Diggle MA, Theodore MJ, Pleatman CR, Mothershed EA, Sacchi CT, Mayer LW, Gilsdorf JR, Smith AL. 2008. Analysis of genetic relatedness of Haemophilus influenzae isolates by multilocus sequence typing. J Bacteriol 190:1473–1483. doi: 10.1128/JB.01207-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilsdorf JR. 1998. Antigenic diversity and gene polymorphisms in Haemophilus influenzae. Infect Immun 66:5053–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilsdorf JR, Marrs CF, Foxman B. 2004. Haemophilus influenzae: genetic variability and natural selection to identify virulence factors. Infect Immun 72:2457–2461. doi: 10.1128/IAI.72.5.2457-2461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy TF. 2005. Vaccine development for non-typeable Haemophilus influenzae and Moraxella catarrhalis: progress and challenges. Expert Rev Vaccines 4:843–853. doi: 10.1586/14760584.4.6.843. [DOI] [PubMed] [Google Scholar]
- 36.Barenkamp SJ, Leininger E. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun 60:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St. Geme JW III, Falkow S, Barenkamp SJ. 1993. High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci U S A 90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vuong J, Wang X, Theodore JM, Whitmon J, Gomez de Leon P, Mayer LW, Carlone GM, Romero-Steiner S. 2013. Absence of high molecular weight proteins 1 and/or 2 is associated with decreased adherence among non-typeable Haemophilus influenzae clinical isolates. J Med Microbiol 62:1649–1656. doi: 10.1099/jmm.0.058222-0. [DOI] [PubMed] [Google Scholar]
- 39.Winter LE, Barenkamp SJ. 2003. Human antibodies specific for the high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Infect Immun 71:6884–6891. doi: 10.1128/IAI.71.12.6884-6891.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter LE, Barenkamp SJ. 2006. Antibodies specific for the high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae are opsonophagocytic for both homologous and heterologous strains. Clin Vaccine Immunol 13:1333–1342. doi: 10.1128/CVI.00221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St. Geme JW III, Kumar VV, Cutter D, Barenkamp SJ. 1998. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun 66:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barenkamp SJ, St. Geme JW III. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol 19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 43.Barenkamp SJ, St. Geme JW III. 1996. Identification of surface-exposed B-cell epitopes on high molecular-weight adhesion proteins of nontypeable Haemophilus influenzae. Infect Immun 64:3032–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atack JM, Winter LE, Jurcisek JA, Bakaletz LO, Barenkamp SJ, Jennings MP. 2015. Selection and counterselection of Hia expression reveals a key role for phase-variable expression of Hia in infection caused by nontypeable Haemophilus influenzae. J Infect Dis 212:645–653. doi: 10.1093/infdis/jiv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winter LE, Barenkamp SJ. 2014. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable Haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol 21:613–621. doi: 10.1128/CVI.00772-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satola SW, Collins JT, Napier R, Farley MM. 2007. Capsule gene analysis of invasive Haemophilus influenzae: accuracy of serotyping and prevalence of IS1016 among nontypeable isolates. J Clin Microbiol 45:3230–3238. doi: 10.1128/JCM.00794-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grass S, St. Geme JW III. 2000. Maturation and secretion of the non-typable Haemophilus influenzae HMW1 adhesin: roles of the N-terminal and C-terminal domains. Mol Microbiol 36:55–67. doi: 10.1046/j.1365-2958.2000.01812.x. [DOI] [PubMed] [Google Scholar]
- 48.Murphy TF. 2015. Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin Vaccine Immunol 22:459–466. doi: 10.1128/CVI.00089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong JJ, Ogra PL. 1989. Otitis media in children. I. The systemic immune response to nontypable Hemophilus [sic] influenzae. J Infect Dis 160:999–1004. [DOI] [PubMed] [Google Scholar]
- 50.Buscher AZ, Grass S, Heuser J, Roth R, St. Geme JW III. 2006. Surface anchoring of a bacterial adhesin secreted by the two-partner secretion pathway. Mol Microbiol 61:470–483. doi: 10.1111/j.1365-2958.2006.05236.x. [DOI] [PubMed] [Google Scholar]
- 51.LaCross NC, Marrs CF, Gilsdorf JR. 2013. Population structure in nontypeable Haemophilus influenzae. Infect Genet Evol 14:125–136. doi: 10.1016/j.meegid.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musser JM, Barenkamp SJ, Granoff DM, Selander RK. 1986. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun 52:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musher DM, Goree A, Baughn RE, Birdsall HH. 1984. Immunoglobulin A from bronchopulmonary secretions blocks bactericidal and opsonizing effects of antibody to nontypable Haemophilus influenzae. Infect Immun 45:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffiss JM, Bertram MA. 1977. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis 136:733–739. [DOI] [PubMed] [Google Scholar]
- 55.Jarvis GA, Griffiss JM. 1991. Human IgA1 blockade of IgG-initiated lysis of Neisseria meningitidis is a function of antigen-binding fragment binding to the polysaccharide capsule. J Immunol 147:1962–1967. [PubMed] [Google Scholar]
- 56.Munkley A, Tinsley CR, Virji M, Heckels JE. 1991. Blocking of bactericidal killing of Neisseria meningitidis by antibodies directed against class 4 outer membrane protein. Microb Pathog 11:447–452. doi: 10.1016/0882-4010(91)90041-8. [DOI] [PubMed] [Google Scholar]
- 57.Ray TD, Lewis LA, Gulati S, Rice PA, Ram S. 2011. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H.8 and Laz lipoproteins. J Immunol 186:4881–4894. doi: 10.4049/jimmunol.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barenkamp SJ. 1996. Immunization with high-molecular-weight adhesion proteins of nontypable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun 64:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buscher AZ, Burmeister K, Barenkamp SJ, St. Geme JW III. 2004. Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J Bacteriol 186:4209–4217. doi: 10.1128/JB.186.13.4209-4217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]