Abstract
The adjuvant AS03 is stockpiled for future formulations with new and existing vaccines for the control of pandemic influenza virus. We previously reported the immunogenicity of an A/H5N1 vaccine extemporaneously mixed with the AS03 adjuvant for 42 days following vaccination. This report extends those findings to 1 year after vaccination.
TEXT
The immune-enhancing adjuvant AS03 is available as a component of the U.S. National Prepandemic Influenza Vaccine Stockpile (1). The adjuvant can be mixed at the point of use with a selected vaccine antigen to maximize protective antibody responses while minimizing the antigenic content (dose sparing). We recently published the safety and immunogenicity results of a multicenter, randomized, and double-blinded phase 1 trial of an influenza A/H5N1 virus vaccine mixed with the AS03 adjuvant. Antibody titers were measured through day 42 postvaccination (2). We report here the kinetics of the homologous and heterologous antibody responses at 6 months and 12 months after vaccination.
Healthy consenting adult volunteers were randomized 2:1 to receive either adjuvanted or nonadjuvanted vaccine and then randomized into 1 of 3 groups (1:1:1) to receive 2 intramuscular doses of vaccine containing 3.75, 7.5, or 15 μg of hemagglutinin (HA) spaced 21 days apart. The investigational subvirion inactivated monovalent vaccine was manufactured by Sanofi Pasteur, Inc. (Swiftwater, PA) from the clade 2.1.3.2 influenza A/H5N1 (A/Indonesia/05/2005) PR8-IBCDC-RG2 virus. The AS03 adjuvant was manufactured by GlaxoSmithKline Biologicals (Rixensart, Belgium). The U.S. Department of Health and Human Services (HHS) Biomedical Advanced Research and Development Authority (BARDA) provided the vaccine and adjuvant from the National Prepandemic Influenza Vaccine Stockpile. Serum samples were collected on days 0 (prevaccination), 8, and 21 (all before second vaccination) and on days 29 and 42. To assess the kinetics of the antibody response, serum samples were also collected at 6 months (day 201) and 12 months (day 386) postvaccination to assess hemagglutination inhibition (HAI) and microneutralization (MN) antibodies using standard methodologies (3, 4).
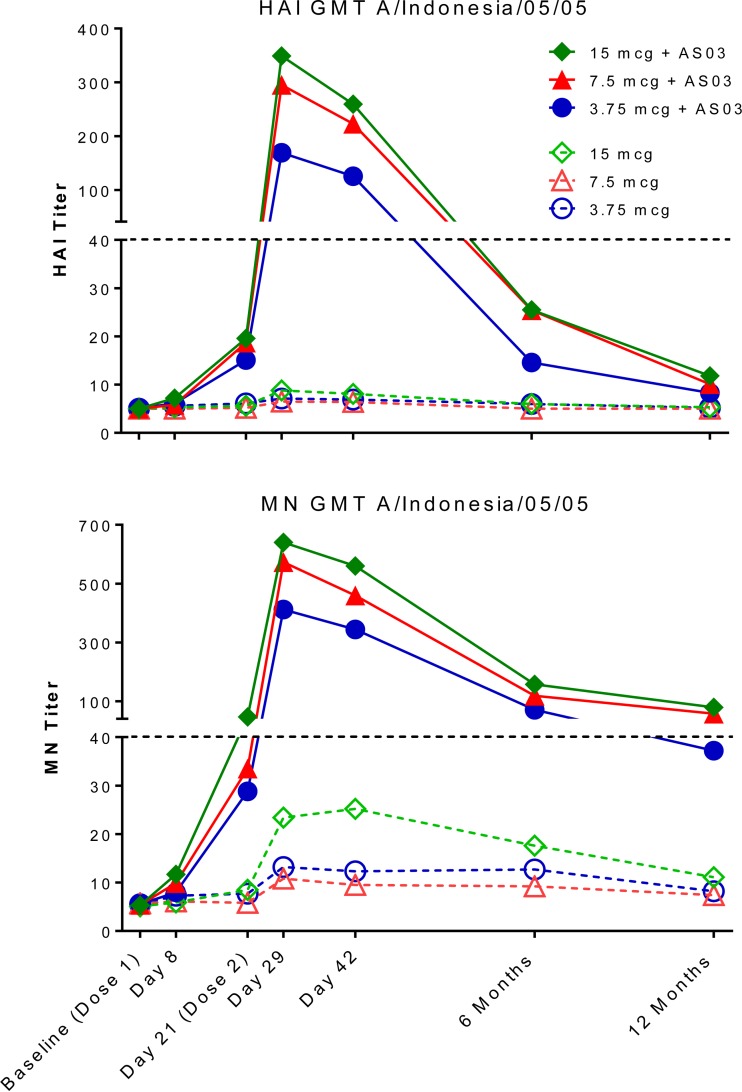
For all groups, the homologous virus (A/Indonesia/05/2005) serum HAI and MN antibody titers peaked on day 29 (day 8, postdose 2) and then declined precipitously. In the groups that received nonadjuvanted vaccine, the HAI and MN antibody titers at 6 months postvaccination had returned to baseline levels. Conversely, a majority of the AS03-adjuvanted vaccine recipients maintained MN antibodies at 12 months postvaccination, with the proportion of subjects with an MN titer of ≥1:40 to the homologous virus significantly increasing with increasing dose, at 58%, 79%, and 88% with 3.75-μg, 7.5-μg, and 15-μg doses of the AS03-adjuvanted vaccine, respectively (P = 0.0041, Cochran-Armitage trend test). In general, antibody titers as assessed by the MN assay were higher than antibody titers as assessed by the HAI assay, consistent with the observation that the MN assay is a more sensitive means of detecting anti-influenza antibody (4). Only a minority (<20%) of the AS03-adjuvanted vaccine recipients had HAI levels of ≥1:40 at 12 months postvaccination (Table 1 and Fig. 1). Previous trials of persons vaccinated with 2 doses of AS03-adjuvanted H5N1 A/Indonesia/05/2005 vaccine have also demonstrated near 50% or higher seroprotective HAI and MN levels of ≥1:40 at 6 months (5, 6) and 15 months (7) postvaccination. We use the term seroprotection; however, it should be noted that a titer of ≥1:40 of HAI or MN antibody is generally considered but has not been proven to be a predictor of protection against H5N1 viruses in humans, as has been shown for seasonal influenza viruses (8, 9).
TABLE 1.
HAI and MN responses to homologous and heterologous H5N1 virus strains with AS03-adjuvanted or nonadjuvanted vaccine
| H5 dose (μg) by virus strain | Adjuvant used | Antibody response (95% CI) by assay ata: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 mo postvaccination |
12 mo postvaccination |
||||||||||
| n | HAI |
MN |
n | HAI |
MN |
||||||
| GMT | % HAI ≥40 | GMT | % MN ≥40 | GMT | % HAI ≥40 | GMT | % MN ≥40 | ||||
| A/Indonesia/05/2005 | |||||||||||
| 3.75 | AS03 | 42 | 14.6 (10.8–19.7) | 24 (12–39) | 70.7 (57.1–87.5) | 93 (81–99) | 38 | 8.3 (6.6–10.6) | 8 (2–21) | 37.2 (28.6–48.4) | 58 (41–74) |
| 3.75 | None | 25 | 6.0 (5.1–7.0) | 0 (0–14) | 12.7 (9.4–17.1) | 16 (5–36) | 25 | 5.2 (5.0–5.5) | 0 (0–14) | 8.2 (6.7–10.1) | 0 (0–14) |
| 7.5 | AS03 | 45 | 25.4 (18.9–34.1) | 44 (30–60) | 118.5 (96.0–146.2) | 100 (92–100) | 39 | 10.1 (7.7–13.2) | 13 (4–27) | 57.6 (44.7–74.2) | 79 (64–91) |
| 7.5 | None | 26 | 5.0 (–) | 0 (0–13) | 9.2 (7.5–11.4) | 0 (0–13) | 24 | 5.0 (–) | 0 (0–14) | 7.4 (5.9–9.2) | 0 (0–14) |
| 15 | AS03 | 43 | 25.5 (18.6–35.0) | 53 (38–69) | 157.4 (123.5–200.7) | 98 (99–100) | 41 | 11.8 (8.9–15.7) | 17 (7–32) | 79.3 (61.4–102.5) | 88 (74–96) |
| 15 | None | 24 | 6.0 (4.9–7.5) | 4 (0–21) | 17.6 (11.6–26.6) | 21 (7–42) | 23 | 5.3 (4.7–6.0) | 0 (0–15) | 11.1 (7.8–15.9) | 9 (1–28) |
| A/Vietnam/1203/2004 | |||||||||||
| 3.75 | AS03 | 42 | 7.4 (5.8–9.4) | 7 (1–19) | 6.8 (6.1–7.5) | 0 (0–8) | 38 | 6.3 (5.1–7.9) | 3 (0–14) | 6.2 (5.6–6.8) | 0 (0–9) |
| 3.75 | None | 25 | 5.7 (4.8–6.6) | 0 (0–14) | 5.2 (5.0–5.5) | 0 (0–14) | 25 | 5.6 (4.8–6.5) | 0 (0–14) | 5.1 (4.9–5.2) | 0 (0–14) |
| 7.5 | AS03 | 45 | 10.2 (7.8–13.3) | 11 (4–24) | 8.9 (7.6–10.4) | 4 (1–15) | 39 | 7.3 (5.8–9.2) | 3 (0–13) | 7.0 (5.9–8.3) | 3 (0–13) |
| 7.5 | None | 26 | 6.3 (5.1–7.7) | 0 (0–13) | 5.1 (4.9–5.2) | 0 (0–13) | 24 | 5.4 (4.8–6.1) | 0 (0–14) | 5.1 (4.9–5.2) | 0 (0–14) |
| 15 | AS03 | 43 | 10.3 (7.7–13.9) | 9 (3–22) | 10.2 (8.5–12.1) | 2 (0–12) | 41 | 7.4 (5.7–9.7) | 7 (2–20) | 8.5 (7.2–10.1) | 0 (0–9) |
| 15 | None | 24 | 6.0 (5.0–7.3) | 0 (0–14) | 6.0 (5.0–7.2) | 0 (0–14) | 23 | 6.6 (5.0–8.7) | 4 (0–22) | 5.6 (4.8–6.6) | 0 (0–15) |
| A/Anhui/01/2005 | |||||||||||
| 3.75 | AS03 | 42 | 6.1 (5.3–7.1) | 0 (0–8) | 46.0 (39.6–53.5) | 76 (61–88) | 38 | 5.0 (5.0–5.1) | 0 (0–9) | 30.1 (24.3–37.4) | 50 (33–67) |
| 3.75 | None | 25 | 5.1 (4.9–5.2) | 0 (0–14) | 13.0 (10.2–16.6) | 4 (0–20) | 25 | 5.0 (–) | 0 (0–14) | 11.0 (9.1–13.3) | 0 (0–14) |
| 7.5 | AS03 | 45 | 6.8 (5.7–8.1) | 2 (0–12) | 64.5 (53.8–77.3) | 96 (85–99) | 39 | 5.4 (4.9–5.9) | 0 (0–9) | 38.3 (31.1–47.1) | 59 (42–74) |
| 7.5 | None | 26 | 5.0 (–) | 0 (0–13) | 11.6 (9.1–14.7) | 8 (1–25) | 24 | 5.0 (–) | 0 (0–14) | 8.5 (6.8–10.7) | 0 (0–14) |
| 15 | AS03 | 43 | 6.7 (5.6–8.0) | 2 (0–12) | 78.7 (64.6–95.9) | 95 (84–99) | 41 | 5.5 (4.9–6.2) | 0 (0–9) | 52.9 (42.5–65.7) | 73 (57–86) |
| 15 | None | 24 | 5.1 (4.8–5.5) | 0 (0–14) | 16.3 (11.6–23.0) | 17 (5–37) | 23 | 5.0 (–) | 0 (0–15) | 12.0 (8.9–16.2) | 9 (1–28) |
| A/turkey/Turkey/1/2005 | |||||||||||
| 3.75 | AS03 | 42 | 5.8 (4.9–7.0) | 5 (1–16) | 33.9 (28.8–40.0) | 50 (34–66) | 38 | 5.8 (4.7–7.1) | 5 (1–18) | 21.7 (17.2–27.3) | 34 (20–51) |
| 3.75 | None | 25 | 5.0 (–) | 0 (0–14) | 8.5 (6.7–10.7) | 0 (0–14) | 25 | 5.0 (–) | 0 (0–14) | 7.0 (5.9–8.2) | 0 (0–14) |
| 7.5 | AS03 | 45 | 10.7 (7.5–15.2) | 24 (13–40) | 50.8 (41.6–62.0) | 73 (58–85) | 39 | 6.4 (5.1–7.9) | 5 (1–17) | 30.6 (24.2–38.8) | 33 (19–50) |
| 7.5 | None | 26 | 5.7 (4.3–7.5) | 4 (0–20) | 7.4 (6.2–8.7) | 0 (0–13) | 24 | 5.0 (–) | 0 (0–14) | 5.8 (5.2–6.4) | 0 (0–14) |
| 15 | AS03 | 43 | 8.2 (6.3–10.8) | 9 (3–22) | 60.8 (49.6–74.6) | 84 (69–93) | 41 | 5.6 (4.8–6.5) | 5 (1–17) | 37.7 (29.8–47.7) | 59 (42–74) |
| 15 | None | 24 | 5.9 (4.2–8.1) | 4 (0–21) | 11.6 (8.2–16.2) | 13 (3–32) | 23 | 5.6 (4.5–6.9) | 4 (0–22) | 8.3 (6.4–10.9) | 4 (0–22) |
| A/Hubei/1/2010 | |||||||||||
| 3.75 | AS03 | 42 | 7.6 (5.8–10.6) | 14 (5–29) | 13.9 (11.5–16.8) | 7 (1–19) | 38 | 5.9 (4.6–7.5) | 5 (1–18) | 10.2 (8.4–12.4) | 5 (1–18) |
| 3.75 | None | 25 | 5.8 (4.4–7.8) | 4 (0–20) | 7.2 (5.9–8.8) | 0 (0–14) | 25 | 5.0 (–) | 0 (0–14) | 6.3 (5.5–7.3) | 0 (0–14) |
| 7.5 | AS03 | 45 | 7.0 (5.4–9.1) | 9 (2–21) | 18.2 (15.1–22.1) | 18 (8–32) | 39 | 6.0 (4.8–7.4) | 5 (1–17) | 11.4 (9.2–14.1) | 8 (2–21) |
| 7.5 | None | 26 | 5.0 (–) | 0 (0–13) | 6.7 (5.5–8.2) | 0 (0–13) | 24 | 5.0 (–) | 0 (0–14) | 6.5 (5.5–7.6) | 0 (0–13) |
| 15 | AS03 | 43 | 6.5 (5.1–8.4) | 7 (1–19) | 23.3 (18.4–29.5) | 21 (10–36) | 41 | 6.2 (4.8–8.1) | 5 (1–17) | 17.8 (13.3–23.8) | 20 (9–35) |
| 15 | None | 24 | 5.3 (4.7–6.0) | 0 (0–14) | 9.6 (6.7–13.6) | 8 (1–27) | 23 | 5.6 (4.8–6.5) | 0 (0–15) | 7.0 (5.2–9.3) | 4 (0–22) |
95% CI, 95% confidence interval. The presence of a single dash in parentheses indicates that there is no range for the 95% CI.
FIG 1.
HAI and MN geometric mean titers (GMT) to homologous (vaccine) virus. The point estimates for each time point and treatment arm are graphically represented; the 95% confidence intervals around each point estimate are detailed in Table 1.
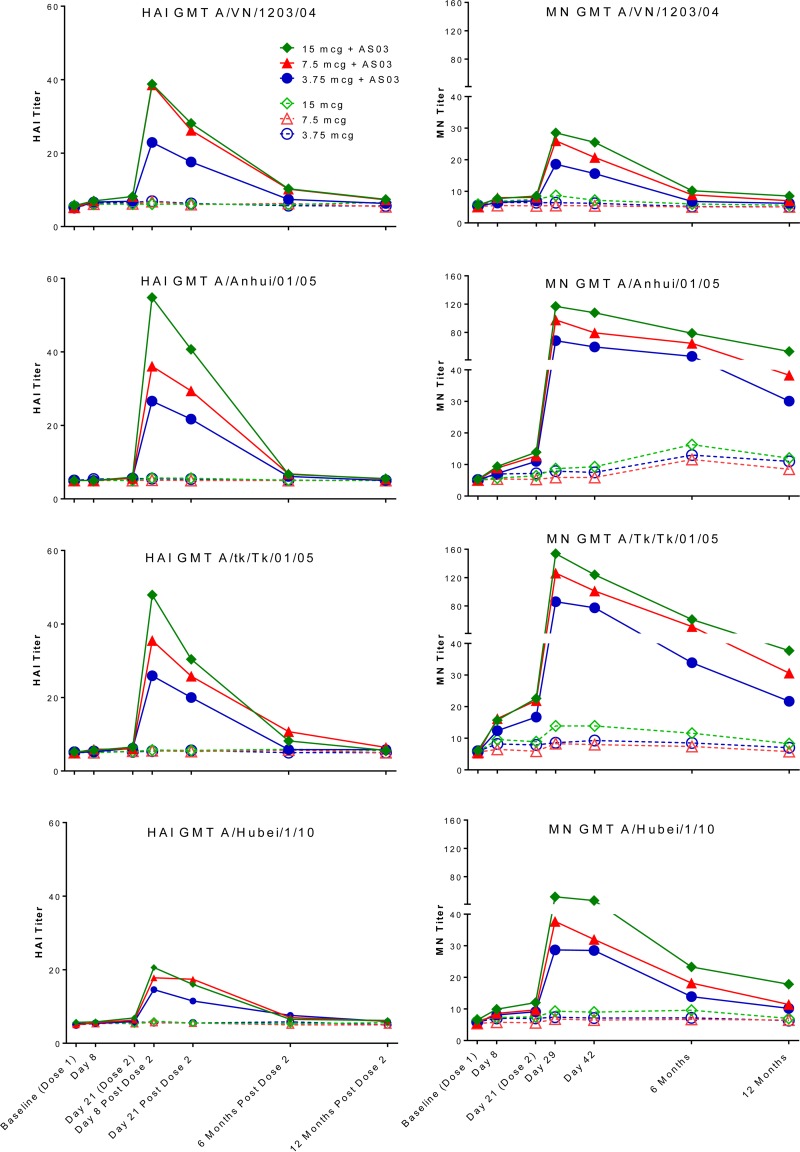
We previously showed that the HAI and MN responses to the heterologous (drifted viruses representing different clades) virus strains (A/Vietnam/1203/2004, A/Anhui/01/2005, A/turkey/Turkey/01/2005, and A/Hubei/1/2010) were lower than those to the homologous vaccine strain. The MN antibody to the A/Anhui/01/2005 strain at 12 months postvaccination remained elevated at ≥1:40 for the majority (50 to 73%) of the AS03-adjuvanted vaccine recipients. The response to A/turkey/Turkey/01/2005 also remained elevated, with 33 to 59% of the AS03-adjuvanted vaccine recipients maintaining a ≥1:40 level of MN antibody. However, the antibody responses to the A/Vietnam/1203/2004 and A/Hubei/1/2010 strains were close to baseline by 6 months postvaccination and remained low when evaluated at 1 year postvaccination. The HAI antibody levels against these two heterologous drifted viruses were near baseline by 6 months postvaccination, with the slope of decline of the HAI antibody steeper than the slope of decline in the MN antibody. Similar results were reported by Leroux-Roels and colleagues (10, 11) (Table 1 and Fig. 2).
FIG 2.
HAI and MN geometric mean titers (GMT) to heterologous virus strains. The point estimates for each time point and treatment arm are graphically represented; the 95% confidence intervals around each point estimate are detailed in Table 1.
A repeated-measures mixed-effects model (with dose group, adjuvant group, and time as fixed effects, both two- and three-way interactions as fixed effects, and a block diagonal unstructured covariance structure for the repeated observations within a subject) was fit to the homologous antibody data to further investigate the dose-response relationship over time. This model confirmed a statistically significant effect of the adjuvant (P < 0.0001) and of dose (P = 0.0002), demonstrating that there was a strong dose-response relationship between dosage of the AS03-adjuvanted vaccine and persistence of MN antibody to the homologous virus.
The data from this trial are intended to inform pandemic preparedness planning and the public health response to future pandemics regarding point-of-use mixing and matching of the AS03 adjuvant with vaccine antigens from different manufacturers. Such a strategy would allow for maximum flexibility, rapid and durable immunogenicity (i.e., protection), cross-reactivity to drifted viruses, and dose sparing of limited vaccine quantities in the face of an evolving pandemic. We did not evaluate the effect of homologous or heterologous booster doses and intervals, but such studies are also critical for pandemic planning.
In conclusion, the homologous seroprotective antibody responses as measured by the MN assay were persistent among a majority of the subjects receiving the AS03-adjuvanted vaccine, even 1 year after vaccination. Durable heterologous protection was also seen, but it was strain dependent. The HAI antibody level declined more steeply during the 1 year after vaccination, and these levels were not sufficient to predict protection, based on the protection extrapolated from seasonal influenza. If we make the assumption that MN antibodies are truly a better correlate of protection than HAI antibodies and further assume that achieving at least a 1:40 titer is a predictor of protection, we could anticipate that the majority of AS03-adjuvanted vaccine recipients would remain protected against the homologous virus at least 1 year after vaccination, even with a 3.75-μg antigen dosage (i.e., antigen-sparing dosage), while the full dose (15 μg) would protect a higher proportion (58% versus 88%) of recipients. On the other hand, the antibody responses were strain specific, and we cannot assume that protection would extend to the A/Vietnam/1203/204 and A/Hubei/01/2010 virus strains.
ACKNOWLEDGMENTS
We thank the staff at each of the clinical sites for the recruitment and care of the subjects. We thank the clinical and laboratory personnel who supported this study: Melissa Billington, Lisa Chrisley, and Mardi Reymann at UMB; Barbara Carste, Maya Dunstan, and Pat Starkovich at Group Health; Shanda Phillips, Belinda Gayle Johnson, Sandra Yoder, and Michael Rock at Vanderbilt; and Chanei Henry, Annette Nagel, Celsa Tajonera, and Diane Nino at Baylor. We also thank the members of the Safety Monitoring Committee (Margo L. Schilling, Charlene M. Prather, Bruce S. Ribner, Michael G. Spigarelli, and Kirsten E. Lyke); the staff at the EMMES Corporation for help in data management and analysis (Claire Stablein, Kuo Guo, and Fenhua He); Barbara Taggart, Valerie Johnsons, and the staff at Southern Research for the antibody sample analysis; and our colleagues at DMID (Linda Lambert, Sonnie Grossman, and Wendy Buchanan) and BARDA (James King, Michael O'Hara, Corrina Pavetto, Bai Yeh, Vittoria Cioce, and Lou Mocca) for their expertise.
This work was supported by NIAID Vaccine and Treatment Evaluation Unit (VTEU) contracts HHSN272200800001C (UMB), HHSN272200800004C (Group Health), HHSN27220080000C (Vanderbilt), and HHSN272200800002C (Baylor) and by the Statistical and Data Coordinating Center for Clinical Research in Infectious Diseases contract HHSN272200800013C (EMMES). Partial support was also provided through the University of Maryland General Clinical Research Center grant M01-RR-016500 and WHC grant K12-RR-023250. This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201200003I (to Battelle, with Southern Research as a subcontract).
The vaccine and adjuvant were provided by the U.S. HHS Biomedical Advanced Research and Development Authority (BARDA).
We declare no conflicts of interest.
REFERENCES
- 1.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. 2008. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis 8:650–658. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen WH, Jackson LA, Edwards KM, Keitel WA, Hill H, Noah DL, Creech CB, Patel SM, Mangal B, Kotloff KL. 2014. Safety, reactogenicity, and immunogenicity of inactivated monovalent influenza A(H5N1) virus vaccine administered with or without AS03 adjuvant. Open Forum Infect Dis 1:ofu091. doi: 10.1093/ofid/ofu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noah DL, Hill H, Hines D, White EL, Wolff MC. 2009. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol 16:558–566. doi: 10.1128/CVI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 37:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, Johnson C, Li P, Kenney R, Innis B, Fries L. 2010. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 201:1644–1653. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 6.Langley JM, Risi G, Caldwell M, Gilderman L, Berwald B, Fogarty C, Poling T, Riff D, Baron M, Frenette L, Sheldon E, Collins H, Shepard M, Dionne M, Brune D, Ferguson L, Vaughn D, Li P, Fries L. 2011. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis 203:1729–1738. doi: 10.1093/infdis/jir172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risi G, Frenette L, Langley JM, Li P, Riff D, Sheldon E, Vaughn DW, Fries L. 2011. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: results of a randomised single heterologous booster dose study at 15 months. Vaccine 29:6408–6418. doi: 10.1016/j.vaccine.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 8.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. 2010. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a Bayesian random-effects model. BMC Med Res Methodol 10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson D, Curry RL, Beare AS, Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 70:767–777. doi: 10.1017/S0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroux-Roels I, Bernhard R, Gérard P, Dramé M, Hanon E, Leroux-Roels G. 2008. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Dramé M, Gillard P, van der Most R, Van Mechelen M, Hanon E, Leroux-Roels G. 2010. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 28:849–857. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]