Abstract
Pertussis is a highly contagious respiratory illness caused by the bacterial pathogen Bordetella pertussis. Pertussis rates in the United States have escalated since the 1990s and reached a 50-year high of 48,000 cases in 2012. While this pertussis resurgence is not completely understood, we previously showed that the current acellular pertussis vaccines do not prevent colonization or transmission following challenge. In contrast, a whole-cell pertussis vaccine accelerated the rate of clearance compared to rates in unvaccinated animals and animals treated with the acellular vaccine. In order to understand if these results are generalizable, we used our baboon model to compare immunity from whole-cell vaccines from three different manufacturers that are approved outside the United States. We found that, compared to clearance rates with no vaccine and with an acellular pertussis vaccine, immunization with any of the three whole-cell vaccines significantly accelerated the clearance of B. pertussis following challenge. Whole-cell vaccination also significantly reduced the total nasopharyngeal B. pertussis burden, suggesting that these vaccines reduce the opportunity for pertussis transmission. Meanwhile, there was no difference in either the duration or in B. pertussis burden between unvaccinated and acellular-pertussis-vaccinated animals, while previously infected animals were not colonized following reinfection. We also determined that transcription of the gene encoding interleukin-17 (IL-17) was increased in whole-cell-vaccinated and previously infected animals but not in acellular-pertussis-vaccinated animals following challenge. Together with our previous findings, these data are consistent with a role for Th17 responses in the clearance of B. pertussis infection.
INTRODUCTION
Whooping cough is a highly contagious, acute respiratory illness caused by the bacterial pathogen Bordetella pertussis (1). In the prevaccine era, pertussis was rampant in the United States with annual reported cases ranging from 150,000 to 250,000 per year and with fatality rates approaching 10% (2). The introduction of combination diphtheria, tetanus, and whole-cell pertussis (DTwP) vaccines in the 1940s and a gradual increase in vaccine coverage led to a dramatic decrease in pertussis incidence with a nadir of 1,000 cases reported in 1976. Due to concerns over the reactogenicity of the whole-cell pertussis vaccine and the prospects of diminishing acceptance among parents, combination diphtheria, tetanus, and acellular pertussis (DTaP) vaccines were introduced in the United States in 1991 and replaced whole-cell vaccines for all pertussis vaccinations in 1997. Currently acellular vaccines are the only pertussis vaccines licensed in the United States and much of the developed world (3). However, despite 95% vaccine coverage in infants, the annual number of reported pertussis cases has been rising over the last 20 to 30 years in the United States (4, 5). The rate of pertussis resurgence increased dramatically following the introduction of acellular vaccines (6). With nearly 50,000 cases reported in the United States in 2012, the highest number since 1955, pertussis is the most common of the vaccine-preventable diseases (7). This resurgence is mirrored in other countries that exclusively use acellular pertussis vaccines, including Australia and Great Britain, though other countries that use acellular pertussis vaccines are not experiencing a similar resurgence (8–10).
While the pertussis resurgence is likely due to a multitude of reasons, a widely held hypothesis for the resurgence is that whole-cell pertussis vaccines provide better protection compared to that of acellular pertussis vaccines (11–15). The most convincing evidence for this hypothesis comes from a cohort study conducted in Australia, following that country's switch from DTwP to DTaP in early 1999. Since children born in 1998 were vaccinated with all DTwP doses, all DTaP doses, or a mixed series, Sheridan et al. were able to compare relative risk among closely age-matched cohorts during a pertussis outbreak from 2009 to 2011. Adolescents who received all acellular pertussis vaccine doses were 3.3-fold more likely to be diagnosed with pertussis compared to children vaccinated with only DTwP (13). Similar data have been observed among adolescents during outbreaks in Oregon and California (12, 14). While these data suggest that some whole-cell pertussis vaccines are more effective than acellular pertussis vaccines, care should be taken not to generalize these findings to all whole-cell pertussis vaccines. During comparative clinical trials in the 1990s, several licensed whole-cell pertussis vaccines were used as controls for experimental acellular pertussis vaccines. DTwP vaccines manufactured by Pasteur Mérieux, Behring, Wyeth-Lederle, and SmithKline Beecham had efficacies of 92% to 98%, but a DTwP vaccine from Connaught Laboratories had an extremely low efficacy of around 40% (16–22). These data suggest that it is possible for licensed whole-cell pertussis vaccines to pass recommended potency assays but still have low efficacy.
We previously showed in our nonhuman primate model that baboons vaccinated with a DTwP vaccine from one manufacturer cleared B. pertussis colonization faster than unvaccinated animals and DTaP-vaccinated animals (23). In order to understand if these results are generalizable, we used our baboon model to compare immunity from DTwP vaccines from three different manufacturers, which are approved outside the United States. We found that compared to no vaccine and acellular pertussis vaccine, immunization with any of the three DTwP vaccines significantly accelerated the clearance of B. pertussis following challenge. Similar to our previous data, there was no difference in the duration of colonization between unvaccinated and DTaP-vaccinated animals, while previously infected animals were not colonized following reinfection. We also determined that transcription of the gene encoding interleukin-17 (IL-17) was increased in DTwP-vaccinated and previously infected animals but not in DTaP-vaccinated animals following challenge. Together with our previous findings, these data are consistent with a role for Th17 responses in clearing B. pertussis infection (23, 24).
MATERIALS AND METHODS
Ethics statement.
All animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the CBER Animal Care and Use Committee and the principles outlined in the Guide for the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Resources, National Research Council.
Bacterial strains and media.
B. pertussis strain D420 was grown on Bordet-Gengou and Regan-Lowe plates prepared as described previously (25). Heat-killed B. pertussis was prepared by resuspending to an optical density at 600 nm (OD600) of 0.90 (5 × 109 CFU/ml) in phosphate-buffered saline (PBS) with a pH 7.4 and heating at 65°C for 30 min.
Vaccination, infection, and evaluation of baboons.
Baboons obtained from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center were inoculated with human doses of DTaP or DTwP administered intramuscularly (i.m.) at 2, 4, and 6 months of age. For DTaP, three animals were vaccinated with the vaccine Daptacel (Sanofi Pasteur Ltd., Toronto, Canada), which is licensed in the United States. For DTwP, two animals in each group were vaccinated with Triple Antigen (Serum Institute of India Ltd., Pune, India), DT-COQ (Sanofi Pasteur, Marcy l'Etoile, France), or Quinvaxem (Crucell-Janssen, Incheon, South Korea). All DTwP vaccines meet the WHO recommendations for potency. See Table 1 for a summary of the components of each vaccine. Unvaccinated animals were age matched. Previously infected animals (n = 3) were clear of B. pertussis infection for 1 to 2 months prior to reinfection. Direct challenge studies were performed as described previously (25, 26). The inoculum for each direct challenge was between 109 and 1010 CFU as determined by the measurement of optical density and was confirmed by serial dilution and plating to determine the number of CFU per milliliter of inoculum. Baboons were evaluated twice weekly as described previously. Peripheral blood was collected for enumeration of circulating white blood cells and serum separation as previously described (25). In addition, peripheral blood was collected into PAXgene Blood RNA tubes (Becton Dickinson, Franklin Lakes, NJ) to stabilize mRNA in blood cells. PAXgene tubes were frozen and stored at −20°C. Nasopharyngeal washes were performed as described previously, and the recovered washes were diluted and plated on Regan-Lowe plates to quantify B. pertussis cells (23). For each animal, we calculated the area under the curve from the nasopharyngeal colonization data over the course of the infection. Briefly, the B. pertussis CFU count in the nasopharyngeal wash was multiplied by the days between samplings.
TABLE 1.
Components of DTaP and DTwP vaccines used in this study
| Component | Amount of each component in: |
|||
|---|---|---|---|---|
| Daptacel | Triple Antigen | DT-COQ | Quinvaxem | |
| Diphtheria toxoida | 15 | 20–30 | ≥30 | ≥30 |
| Tetanus toxoida | 5 | 5–25 | ≥60 | ≥60 |
| Whole-cell B. pertussis (IU) | ≥4 | ≥4 | ≥4 | |
| Inactivated pertussis toxin (μg) | 10 | |||
| Filamentous hemagglutinin (μg) | 5 | |||
| Pertactin (μg) | 3 | |||
| Fimbriae types 2 and 3 (μg) | 5 | |||
| Other components | 10 μg Hib oligosaccharide conjugated to ∼25 μg of CRM 197, 10 μg of hepatitis B surface antigen | |||
| Aluminum (Al3+) content (mg) | 0.33 from aluminum phosphate | ≤1.25 from aluminum phosphate | 0.6 from aluminum hydroxide | 0.3 from aluminum phosphate |
Measured in limit of flocculation (Lf) for Daptacel and Triple Antigen and in international units for DT-COQ and Quinvaxem.
Detection of serum IgG antibodies to pertussis antigens.
Nunc MaxiSorp 96-well plates were coated overnight with 0.2 μg/ml pertussis toxin (PT), 2 μg/ml pertactin (PRN), or 0.2 μg/ml fimbriae 2/3 (FIM) in 100 mM carbonate buffer, pH 9.6. Additional plates were coated with 0.5 μg/ml filamentous hemagglutinin (FHA) in PBS, pH 7.4. All antigens were purchased from List Biologicals (Campbell, CA). For analyzing antibody responses to whole B. pertussis cells, plates were coated with heat-killed strain D420 prepared as described above. For coating, plates were incubated at 28°C for PRN, FIM, and FHA or at 37°C for PT and B. pertussis cells. After they were washed with PBS containing 0.05% Tween 20 (PBS-T), the plates were blocked for 30 min at room temperature with PBS-T containing 1% powdered skim milk (Bio-Rad, Hercules, CA). The plates were washed with PBS-T, and serum samples were added to duplicate wells in 3-fold serial dilutions (1:100 to 1:218,700 dilutions in PBS-T with 0.1% milk) and were incubated for 1 h at room temperature. The plates were washed with PBS-T and then incubated for 30 min at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-monkey IgG polyclonal antibody (catalog code AAI42P; AbD Serotec, Raleigh, NC) diluted 1:10,000 in PBS-T with 0.1% milk. SureBlue TMB microwell peroxidase substrate (KPL, Gaithersburg, MD) was used for detection, and the reaction was stopped with 1 N HCl. Absorbance was measured at 450 nm using a microplate reader (VersaMax; Molecular Devices, Sunnyvale, CA). Each plate contained a standard curve from the WHO international standard pertussis antiserum (NIBSC, Hertfordshire, England) used to assign international units for PT, FHA, and PRN, and relative units for FIM and B. pertussis cells.
Measurement of transcriptional activity by quantitative real-time PCR.
PAXgene tubes were thawed and allowed to equilibrate to room temperature for 3 h. RNA was purified from the blood cells using the PAXgene Blood RNA kit (Qiagen, Valencia, CA) and was converted to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). TaqMan gene expression assays (Applied Biosystems) for human IL-17A (Hs99999082_m1), rhesus gamma interferon (IFN-γ) (Rh02621721_m1), rhesus IL-4 (Rh02621716_m1), rhesus inducible T-cell co-stimulator (ICOS) (Rh02621771_m1), and human RPLP0 (Hs00420895_gH) were chosen based on 100% homology of the primers and probes to the baboon (Papio anubis) gene sequences (NCBI genome assembly accession number GCA_000264685.1) and were tested and found to detect baboon transcripts as expected. Gene expression was analyzed using TaqMan Fast Universal 2× PCR master mix (No AmpErase UNG). Each reaction mixture contained cDNA from 20 ng of RNA. Reactions were performed in duplicate and were run on the Applied Biosystems ViiA 7 real-time PCR system. Fold gene expression was calculated using the 2−ΔΔCT method, where CT is the cycle threshold (27).
Statistics.
All data are reported as mean ± standard error of the mean (SEM). Statistical analyses were performed by analysis of variance (ANOVA) with post hoc t test using JMP (version 10) software (SAS Institute Inc., Cary, NC, USA). CFU data were normalized by log transformation prior to analysis.
RESULTS
Serum antibody responses to B. pertussis antigens following vaccination.
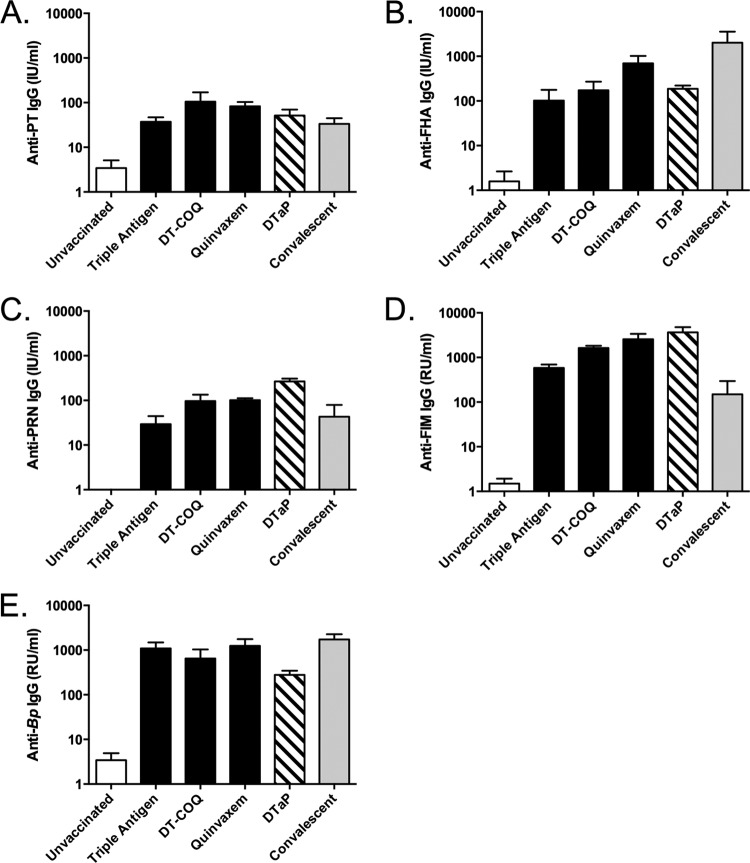
Baboons were vaccinated at 2, 4, and 6 months of age with one of three combination diphtheria, tetanus, and whole-cell pertussis (DTwP) vaccines (Table 1). All three DTwP vaccines are approved for use outside the United States and met the potency standards determined by the World Health Organization. As a comparator, age-matched baboons were vaccinated at 2, 4, and 6 months of age with a combination diphtheria, tetanus, acellular pertussis (DTaP) vaccine licensed in the United States. At 8 to 9 months of age, serum samples were collected from the vaccinated animals as well as from age-matched unvaccinated animals and convalescent animals (11 to 14 months of age) that were previously infected and allowed to clear the infection. For all animals, we analyzed serum IgG responses to the four pertussis antigens in the acellular vaccine (PT, PRN, FHA, FIM) as well as to whole-cell B. pertussis. As shown in Fig. 1, all of the vaccinated animals and convalescent animals had robust responses against all of the B. pertussis antigens and cells compared to unvaccinated animals.
FIG 1.
Vaccination and previous infection induce robust serum antibody responses. Serum samples were collected from unvaccinated animals (n = 4), DTaP-vaccinated animals (n = 3), convalescent animals (n = 3), and animals vaccinated with DTwP (Triple Antigen, DT-COQ, or Quinvaxem; n = 2 per group) 1 week prior to challenge. Antibody responses to the four vaccine antigens, PT (A), FHA (B), PRN (C), FIM (D), and to heat-killed B. pertussis (E) were measured by enzyme-linked immunosorbent assay (ELISA). International units (IU) or relative units (RU) in each sample were determined by comparing the responses to the WHO international standard pertussis antiserum on each plate. For each group, the mean response is presented with error bars representing the standard error (SE).
B. pertussis colonization and pertussis symptoms following challenge.
One month following the third vaccination, all groups of animals were challenged with B. pertussis strain D420, a recent clinical isolate that is genetically similar to current circulating strains (28). Twice weekly, samples were taken from the animals to evaluate B. pertussis colonization and pertussis disease. Since leukocytosis is the best marker for severe clinical pertussis in human infants, we determined complete blood counts at each time point. As shown in Fig. 2, leukocytosis was observed in unvaccinated animals, but there was no increase in circulating white blood cells in vaccinated or convalescent animals. In addition, while coughing was readily observed in unvaccinated animals, the vaccinated and convalescent animals were not observed coughing (data not shown). These data suggest that all vaccinated and convalescent animals were protected from pertussis disease following challenge.
FIG 2.
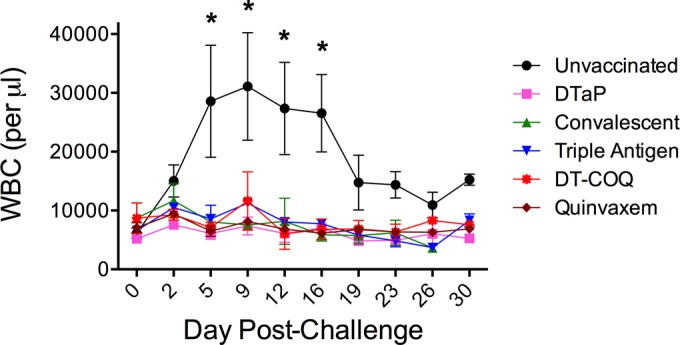
Vaccination and prior infection prevent leukocytosis. Unvaccinated animals (n = 4), DTaP-vaccinated animals (n = 3), convalescent animals (n = 3), and animals vaccinated with DTwP (Triple Antigen, DT-COQ, or Quinvaxem; n = 2 per group) were directly challenged with B. pertussis (n = 2 to 4 per group). The mean circulating white blood cell counts before and after challenge are shown for each group of animals. *, P < 0.05 versus preinfection from the same group. For each group, the mean count is presented at every time point with error bars representing the SE.
To assess colonization following challenge, nasopharyngeal washes were performed on each animal, and the recovered washes were diluted and plated to quantify viable B. pertussis organisms. Consistent with previous data, nasopharyngeal washes collected from unvaccinated animals contained between 107 and 108 CFU/ml for the first 2 weeks following challenge, after which colonization gradually decreased (Fig. 3A). Unvaccinated animals cleared the infection 30 days postchallenge on average (Fig. 3B). Convalescent animals, which possess a robust adaptive immune response, did not become colonized following challenge (Fig. 3A and B). Compared to that of the unvaccinated animals, the animals receiving DTaP had a minor decrease in colonization for the first 2 weeks. However, at subsequent time points there was no appreciable difference between DTaP-vaccinated and unvaccinated animals. For the three groups vaccinated with DTwP, all animals had initial colonization comparable to DTaP-vaccinated animals, but all three groups cleared the infection an average of 17.5 days following challenge, roughly half the time compared to DTaP-vaccinated animals (Fig. 3A and B).
FIG 3.
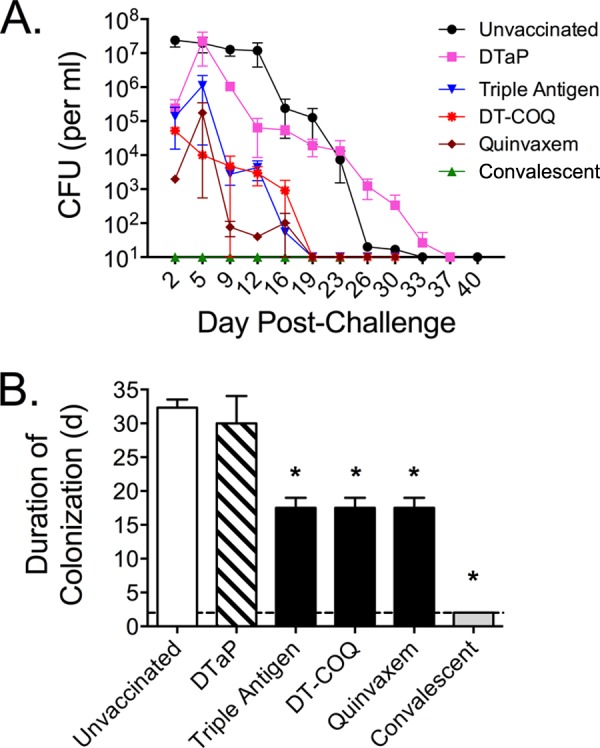
Effects of vaccination and previous infection on colonization. Unvaccinated animals (n = 4), DTaP-vaccinated animals (n = 3), convalescent animals (n = 3), and animals vaccinated with DTwP (Triple Antigen, DT-COQ, or Quinvaxem; n = 2 per group) were directly challenged with B. pertussis. (A) Colonization was monitored by quantifying B. pertussis CFU per milliliter in biweekly nasopharyngeal washes with a limit of detection of 10 CFU/ml. The mean colonization is presented for each group and time point with error bars representing the SE. (B) For each animal, the time to clearance is defined as the first day that no B. pertussis CFU were recovered from nasopharyngeal washes. The mean time to clearance is shown for each group (same sizes as above except n = 3 for the unvaccinated group since one unvaccinated animal was not followed to clearance). Since no B. pertussis organisms were recovered from the convalescent animals, the mean time to clearance was defined as the first day of sampling (day 2, indicated by the dashed line). *, P < 0.01 versus naive.
In order to estimate the impact of the different vaccines on bacterial shedding, we calculated the area under the curve for the nasopharyngeal colonization data presented above. These data provide a rough estimate of the total burden of nasopharyngeal B. pertussis over the course of the infection. As presented in Fig. 4, there was no statistical difference in total nasopharyngeal bacteria in the unvaccinated animals versus the DTaP-vaccinated animals (mean log10 values of 8.39 and 7.78, respectively). However, each DTwP vaccine significantly reduced the total amount of nasopharyngeal bacteria compared to that in the unvaccinated group. When the three DTwP groups were combined, the average DTwP-vaccinated animal showed a >1,000-fold reduction in bacteria compared to that of the unvaccinated animals (mean log10 value of 5.35).
FIG 4.
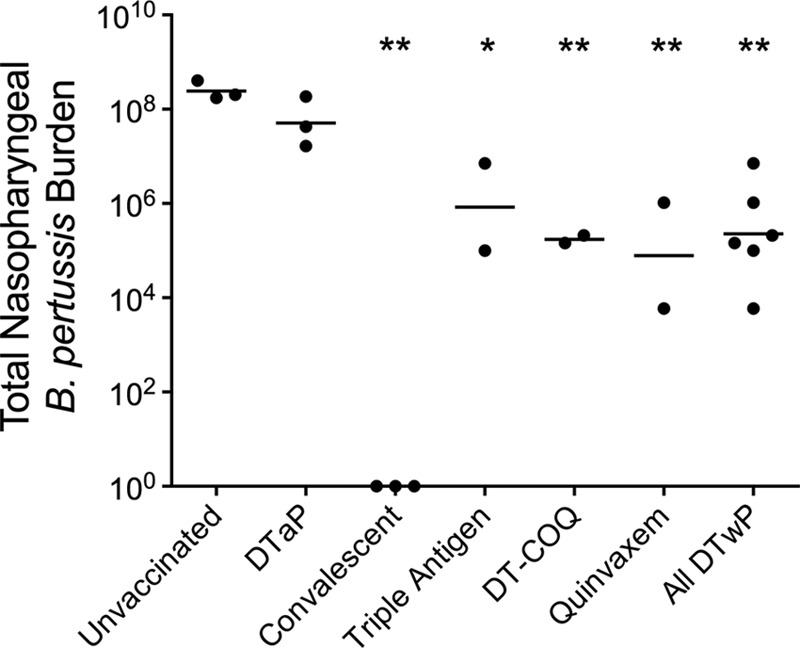
Whole-cell pertussis vaccines reduce total nasopharyngeal B. pertussis burden. The total nasopharyngeal burden over the course of the infection was estimated by calculating the area under curve of the serial nasopharyngeal wash counts for each animal. The data are shown for each unvaccinated animal (n = 3), DTaP-vaccinated animal (n = 3), convalescent animal (n = 3), and each animal vaccinated with DTwP (Triple Antigen, DT-COQ, or Quinvaxem; n = 2 per group), with a bar representing the mean value for each group. *, P < 0.05 versus naive; **, P < 0.01 versus naive.
Memory cytokine responses following B. pertussis challenge.
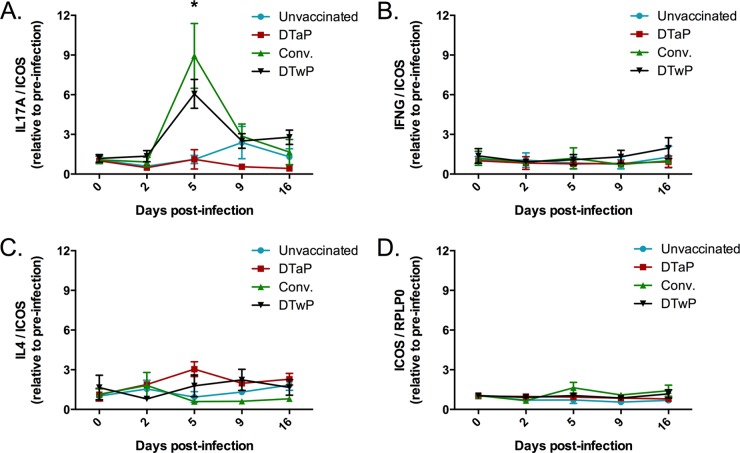
Previous data from the baboon model showed that clearance of B. pertussis following challenge was associated with the degree of Th17 memory responses (23). Peripheral blood mononuclear cells (PBMC) collected from convalescent animals prior to challenge displayed robust Th17 memory responses following restimulation with heat-killed B. pertussis. PBMC collected from DTwP-vaccinated animals had significant but milder Th17 memory responses while DTaP-vaccinated animals possessed Th2 memory but no Th17 response. In this study, we wanted to expand upon these findings by investigating memory cytokine responses stimulated in vivo following B. pertussis challenge. Blood samples were collected in PAXgene tubes to stabilize mRNA transcripts. Following RNA purification and conversion to cDNA, cytokine transcript levels were quantified by real-time PCR. We analyzed IL-17A, IFN-γ, and IL-4 transcript levels as markers for Th17, Th1, and Th2 responses, respectively. To account for potential differences in the numbers of circulating T cells, the cytokine transcript levels were normalized to ICOS, which is preferentially transcribed in T cells. To increase our sample sizes and since we did not observe any differences in protection between the different groups, all six DTwP-vaccinated animals were pooled for this analysis. As shown in Fig. 5, we detected an increase in IL-17A transcripts in convalescent and DTwP-vaccinated animals at 5 days postchallenge, while no increase in IL-17A was observed in DTaP-vaccinated or unvaccinated animals. At the time points studied, no increases were seen in IFN-γ or IL-4 transcripts in any of the groups. We also determined that there was no increase in ICOS transcription relative to the general housekeeping gene RPLP0 in any of the groups. These data suggest that B. pertussis infection stimulated an IL-17 memory response in convalescent and DTwP-vaccinated animals, consistent with our previous findings with the PBMC restimulation assay.
FIG 5.
Cytokine responses during infection. Blood was collected into PAXgene tubes to stabilize RNA at the indicated time points before and during the infection. RNA was converted to cDNA, and the transcript levels of the genes encoding IL-17A (A), IFN-γ (B), and IL-4 (C) were analyzed by real-time PCR. For each cytokine gene, the data are presented relative to ICOS transcript levels and are normalized to preinfection values. For each group, the mean response is presented with error bars representing the SE. (D) The ICOS transcript was similarly analyzed relative to the housekeeping gene RPLP0. For all graphs, n = 3 for unvaccinated and convalescent (Conv.), n = 2 for DTaP, n = 6 for DTwP. *, P ≤ 0.01 for convalescent versus unvaccinated and DTwP versus unvaccinated.
DISCUSSION
Infection of baboons (Papio anubis) with B. pertussis results in a disease that is very similar to severe clinical pertussis. Upon challenge with a recent clinical isolate of B. pertussis, baboons experience respiratory colonization for about 4 to 6 weeks, paroxysmal coughing, and leukocytosis (25, 29). In addition, infected baboons can transmit B. pertussis to unchallenged baboons by airborne transmission (26). We previously showed that vaccination of baboons with DTwP from one manufacturer accelerates the clearance of B. pertussis following challenge compared to that in unvaccinated animals or DTaP-vaccinated animals and that clearance was associated with induction of Th17 memory (23).
In the current study, we wanted to determine if these results are generalizable to other DTwP vaccines. Baboons were vaccinated at 2, 4, and 6 months of age with one of three DTwP vaccines or with a DTaP vaccine licensed in the United States. Each whole-cell vaccine met WHO potency recommendations. We did not detect major differences in the antibody responses to vaccination with the four vaccines. All three whole-cell vaccines and the acellular vaccine induced robust serum IgG responses to PT, PRN, FHA, FIM, and whole-cell B. pertussis cells. Following challenge, there was no difference in the duration of colonization between the 3 DTwP groups. All animals receiving a DTwP vaccine cleared the infection an average of 17.5 days following challenge with a recent clinical isolate. Unvaccinated animals and DTaP-vaccinated animals took about twice as long to clear the infection. We hypothesize that decreasing the duration and magnitude of B. pertussis colonization would decrease the amount of pathogen shed in respiratory droplets, thereby reducing the likelihood of transmission. As a surrogate for bacterial shedding and transmission, we calculated the total burden of nasopharyngeal B. pertussis over the course of the infection in each animal in this study. We found no difference in total nasopharyngeal burden between unvaccinated and DTaP-vaccinated animals. However, there was a >1,000-fold decrease in nasopharyngeal B. pertussis in DTwP-vaccinated animals compared to that in naive animals. These data suggest that even though DTwP vaccines do not prevent infection, they likely reduce the opportunity for B. pertussis transmission compared to DTaP vaccines or no vaccination. While there was no significant difference in B. pertussis burden between unvaccinated and DTaP-vaccinated animals, it is important to keep in mind that the paroxysmal coughing in the unvaccinated animals likely increases the likelihood of transmission compared to DTaP-vaccinated animals that are asymptomatic.
These results add to our previous findings that a single DTwP vaccination reduced the duration of colonization while DTaP vaccination did not prevent B. pertussis colonization or transmission to unvaccinated contacts (23). If true in humans, these data suggest that people vaccinated with acellular pertussis vaccines can become asymptomatically infected with B. pertussis and act as a reservoir for circulation within communities. This hypothesis is supported by a recent study by Althouse and Scarpino (30). The authors analyzed trends in reported pertussis cases and age-specific incidence rates from the United States and the United Kingdom and determined that in the two countries pertussis resurgence is best described by a model whereby acellular vaccines allow for greater asymptomatic transmission compared to that of whole-cell vaccines.
One possible explanation for the better protection afforded by the DTwP vaccines is that these vaccines induce antibody responses to a wider breadth of antigens compared to the DTaP vaccines that induce strong responses to a limited group of antigens. Since DTwP vaccines consist of whole bacteria, they elicit responses to many surface antigens not present in the DTaP vaccines. The antibody responses to these extra surface antigens may lead to inhibition of initial attachment of the bacteria to the respiratory epithelium. Additionally, we hypothesize that surface antigens in the DTwP vaccines may stimulate higher titers of opsonizing antibodies to aid in neutrophil-mediated clearance of the infection. Since DTwP vaccines, but not DTaP vaccines, induce Th17 immune responses that promote neutrophil recruitment, opsonizing antibodies would likely allow for more efficient clearance of B. pertussis in DTwP-vaccinated animals than that in animals receiving the DTaP vaccine.
Th17 cells are a component of the adaptive immune response that specializes in controlling extracellular pathogens such as B. pertussis at mucosal surfaces by inducing granulopoiesis and recruitment of neutrophils and macrophages to infected mucosal sites (31). In a previous study, we detected a significant increase in IL-17 in nasopharyngeal washes following B. pertussis infection of unvaccinated baboons. This response was followed by significant increases in downstream IL-17 effectors, including granulocyte colony-stimulating factor (GCSF), IL-8, monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1α (MIP-1α) (24). Later, we determined that whole-cell vaccination and pertussis infection induces Th17 and Th1 memory in baboons as determined by the presence of IL-17-secreting CD4+ memory T cells in peripheral blood samples. Acellular pertussis vaccination did not induce appreciable Th17 responses but skewed toward a mixed Th2 and Th1 phenotype (23, 24). In the present study, we expanded upon these findings by analyzing cytokine transcriptional responses in vivo during infection in peripheral blood samples. At 5 days following challenge, we detected increased IL-17A transcript levels in convalescent and whole-cell-vaccinated animals but not in unvaccinated or acellular-vaccinated animals. One apparent discrepancy with our previous data is that we did not detect evidence of an increased IFN-γ or IL-4 transcript, the markers for Th1 and Th2 responses, in any groups. One possible explanation for this is that Th1 and Th2 responses are not stimulated in vivo during a B. pertussis infection in baboons. However, we think a more likely explanation is that we failed to detect transient increases in IFN-γ and IL-4 transcripts prior to the first sample collection or between time points. Two recent clinical studies corroborate the notion that DTaP induces Th2 and Th1 responses in children and found no significant Th17 responses following vaccination (32, 33). However, there are currently no data on Th17 memory in DTwP-vaccinated and pertussis-infected children. Together with these scant clinical data and our previous findings, these data suggest that whole-cell pertussis vaccination and B. pertussis infection induce a Th17 memory response that aids in clearing B. pertussis infection. Meanwhile, acellular pertussis vaccination results in antibody-mediated prevention of pertussis symptoms but provides little protection against B. pertussis colonization.
In the short term, plans for addressing the resurgence of pertussis should include continued efforts to enhance immunization in children and adults. However, since acellular pertussis vaccines may not impact B. pertussis circulation, additional strategies are required to protect the most vulnerable members of the population, including infants too young to be vaccinated. These new data from the baboon model further highlight the Th17 response as a possible candidate for a correlate of protection against B. pertussis infection since greater Th17 responses are associated with the more protective immune responses elicited by B. pertussis infection and DTwP vaccination. Taken together, immunity from DTwP vaccination and pertussis infection may be more protective because they induce antibody responses to prevent symptomatic pertussis and Th17-mediated immunity to prevent B. pertussis colonization. The most pressing long-term goal in the pertussis research community is the development of a next-generation vaccine that combines the low reactogenicity of acellular pertussis vaccines with the ability of natural infection and, to a lesser extent, whole-cell vaccination to prevent colonization. Current strategies to reach this goal include using novel adjuvants to boost Th17 responses from acellular pertussis antigens, engineering a nonreactogenic whole-cell pertussis vaccine, or administring a live-attenuated strain of B. pertussis (34).
ACKNOWLEDGMENTS
We thank Sanofi Pasteur and Crucell-Janssen for generously providing vaccines for this study. We thank Lewis Shankle, Perry Altland, Faith Sentz, and Nicole Reuter for technical assistance. We thank John Dennis, Jill Ascher, Roman Wolf, and James Papin for technical assistance and critical discussions. We thank Drusilla Burns and Scott Stibitz for critical reading of the manuscript.
Funding Statement
This work was funded by the Food and Drug Administration and NIH/NIAID through interagency agreement number Y1-AI-1727-01. Baboons were obtained from the Oklahoma Baboon Research Resource. The Oklahoma Baboon Research Resource was supported by grant numbers P40RR012317 and 5R24RR016556-10 from the National Institutes of Health National Center for Research Resources.
REFERENCES
- 1.Melvin JA, Scheller EV, Miller JF, Cotter PA. 2014. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12:274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark TA. 2014. Changing pertussis epidemiology: everything old is new again. J Infect Dis 209:978–981. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 3.Libster R, Edwards KM. 2012. Re-emergence of pertussis: what are the solutions? Expert Rev Vaccines 11:1331–1346. doi: 10.1586/erv.12.118. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2014. Pertussis (whooping cough): surveillance and reporting. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/pertussis/surv-reporting.html Accessed 23 June 2015. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2014. Vaccination coverage in the United States. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/vaccines/imz-managers/coverage/imz-coverage.html Accessed 23 February 2015. [Google Scholar]
- 6.Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, Miller L, Selvage D, Lee A, Skoff TH, Kamiya H, Cassiday PK, Tondella ML, Clark TA. 2015. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 60:223–227. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- 7.CDC. 2013. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 62:669–682. [PubMed] [Google Scholar]
- 8.Australian Government Department of Health. 2014. National notifiable diseases surveillance system. Australian Government Department of Health, Canberra, Australia: http://www9.health.gov.au/cda/source/cda-index.cfm Accessed 23 June 2015. [Google Scholar]
- 9.Public Health England. 2014. Laboratory confirmed cases of pertussis infection by laboratory method, year and quarter, England: 2002–2013. Public Health England, London, England: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317133571358 Accessed 23 June 2015. [Google Scholar]
- 10.World Health Organization. 2014. SAGE pertussis working group background paper. World Health Organization, Geneva, Switzerland: www.who.int/entity/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf?ua=1 Accessed 19 June 2015. [Google Scholar]
- 11.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. 2013. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 56:1248–1254. doi: 10.1093/cid/cit046. [DOI] [PubMed] [Google Scholar]
- 12.Liko J, Robison SG, Cieslak PR. 2013. Priming with whole-cell versus acellular pertussis vaccine. N Engl J Med 368:581–582. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan SL, Ware RS, Grimwood K, Lambert SB. 2012. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308:454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 14.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. 2013. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131:e1716–e1722. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan SL, Frith K, Snelling TL, Grimwood K, McIntyre PB, Lambert SB. 2014. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: recent epidemiology. Expert Rev Vaccines 13:1081–1106. doi: 10.1586/14760584.2014.944167. [DOI] [PubMed] [Google Scholar]
- 16.Lambert LC. 2014. Pertussis vaccine trials in the 1990s. J Infect Dis 209(Suppl):S4–S9. doi: 10.1093/infdis/jit592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofi degli Atti ML, Giammanco A, Panei P, Blackwelder WC, Klein DL, Wassilak SG. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N Engl J Med 334:341–348. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 19.Stehr K, Cherry JD, Heininger U, Schmitt-Grohé S, uberall M, Laussucq S, Eckhardt T, Meyer M, Engelhardt R, Christenson P. 1998. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 101:1–11. doi: 10.1542/peds.101.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Simondon F, Preziosi MP, Yam A, Kane CT, Chabirand L, Iteman I, Sanden G, Mboup S, Hoffenbach A, Knudsen K, Guiso N, Wassilak S, Cadoz M. 1997. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 15:1606–1612. doi: 10.1016/S0264-410X(97)00100-X. [DOI] [PubMed] [Google Scholar]
- 21.Liese JG, Meschievitz CK, Harzer E, Froeschle J, Hosbach P, Hoppe JE, Porter F, Stojanov S, Niinivaara K, Walker AM, Belohradsky BH. 1997. Efficacy of a two-component acellular pertussis vaccine in infants. Pediatr Infect Dis J 16:1038–1044. doi: 10.1097/00006454-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt HJ, von König CH, Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H, Gahr M, Schult R, Folkens JU, Rauh W, Clemens R. 1996. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 275:37–41. doi: 10.1001/jama.1996.03530250041024. [DOI] [PubMed] [Google Scholar]
- 23.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warfel JM, Merkel TJ. 2013. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 6:787–796. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 25.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. 2012. Nonhuman primate model of pertussis. Infect Immun 80:1530–1536. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warfel JM, Beren J, Merkel TJ. 2012. Airborne transmission of Bordetella pertussis. J Infect Dis 206:902–906. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak K, Schmittgen T. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Boinett CJ, Harris SR, Langridge GC, Trainor EA, Merkel TJ, Parkhill J. 2015. Complete genome sequence of Bordetella pertussis D420. Genome Announc 3:e00657-15. doi: 10.1128/genomeA.00657-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warfel JM, Merkel TJ. 2014. The baboon model of pertussis: effective use and lessons for pertussis vaccines. Expert Rev Vaccines 13:1241–1252. doi: 10.1586/14760584.2014.946016. [DOI] [PubMed] [Google Scholar]
- 30.Althouse BM, Scarpino SV. 2015. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med 13:146. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolls JK, Khader SA. 2010. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev 21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schure RM, Hendrikx LH, de Rond LG, Oztürk K, Sanders EA, Berbers GA, Buisman AM. 2012. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol 19:1879–1886. doi: 10.1128/CVI.00277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schure RM, Hendrikx LH, de Rond LG, Oztürk K, Sanders EA, Berbers GA, Buisman AM. 2013. Differential T- and B-cell responses to pertussis in acellular vaccine-primed versus whole-cell vaccine-primed children 2 years after preschool acellular booster vaccination. Clin Vaccine Immunol 20:1388–1395. doi: 10.1128/CVI.00270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meade BD, Plotkin SA, Locht C. 2014. Possible options for new pertussis vaccines. J Infect Dis 209(Suppl):S24–S27. doi: 10.1093/infdis/jit531. [DOI] [PubMed] [Google Scholar]