Abstract
Enterotoxigenic Escherichia coli (ETEC) bacteria are the most common bacterial cause of diarrhea in children in resource-poor settings as well as in travelers. Although there are several approaches to develop an effective vaccine for ETEC, no licensed vaccines are currently available. A significant challenge to successful vaccine development is our poor understanding of the immune responses that correlate best with protection against ETEC illness. In this study, ETEC-specific mucosal immune responses were characterized and compared in subjects challenged with ETEC strain H10407 and in subjects rechallenged with the homologous organism. IgA responses to lipopolysaccharide (LPS), heat-labile toxin B subunit (LTB), and colonization factor antigen I (CFA/I) in antibody in lymphocyte supernatant (ALS), feces, lavage fluid, and saliva samples were evaluated. In all assay comparisons, ALS was the most sensitive indicator of a local immune response, but serum IgA was also a useful indirect marker of immune response to oral antigens. Volunteers challenged and then rechallenged with strain H10407 were protected from illness following rechallenge. Comparing mucosal antibody responses after primary and homologous rechallenge, protection against disease was reflected in reduced antibody responses to key ETEC antigens and in reduced fecal shedding of the H10407 challenge strain. Subjects challenged with strain H10407 mounted stronger antibody responses to LPS and LTB than subjects in the rechallenge group, while responses to CFA/I in the rechallenge group were higher than in the challenge group. We anticipate that this study will help provide an immunological benchmark for the evaluation of ETEC vaccines and immunization regimens in the future.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) bacteria are the most frequent cause of bacterial diarrhea in children in developing countries, resulting in approximately 200 million diarrheal episodes and 380,000 deaths annually (1–3). A more conservative estimate of about 170,000 deaths every year was recently suggested (4, 5). However, due to comparably complex laboratory methods for detection of ETEC, the true incidence and impact on infant and child health in the developing world are most likely underestimated (2, 6). In addition, ETEC is also the most common cause of traveler's diarrhea (7, 8). ETEC colonizes the surface of the small intestine. This colonization is facilitated by primary adhesins such as colonization factor antigens (CFA) and other secondary or accessory colonization factors such as EtpA and EatA (9). Once intestinal colonization has occurred, ETEC strains elaborate heat-labile toxins (LT) and/or heat-stable toxins (ST) that lead to secretory diarrhea (6, 8). Natural infection in areas of ETEC endemicity eventually results in the development of protective immunity as suggested by the decrease in age-specific rates of ETEC infections (10, 11). It has also been shown in animal studies and experimental human challenge studies that subjects infected with an ETEC strain are protected against illness when rechallenged with the homologous ETEC strain (12–14). However, the protective role of specific immune responses and the antigens that elicit these responses are not well understood.
Current approaches to development of vaccines against ETEC disease in human have included efforts to stimulate immunity to toxins and colonization factor antigens (CFA) to achieve a more optimal and synergistic local response at the intestinal mucosa (15–17). The gut mucosal immune system is a critical component of the body's defense against enteric pathogens, and this has been considered to be of prime importance for protection. Since ETEC bacteria cause noninvasive, gut-associated mucosal infections, the local IgA response is believed to play a major role in protective immunity, but other serum isotypes that leak on to the mucosal surface may also be involved in the protection. To date, the most logical approach to assess intestinal immune responses is to determine specific secretory IgA (sIgA) antibodies in intestinal secretions. Such secretions may be collected by the intestinal lavage procedure, in which the specimen includes antibodies produced in the entire gastrointestinal tract. Given that the lavage procedure is laborious and requires the patient's careful cooperation, a modified method to collect lavage fluid which is less labor-intensive and less time-consuming would be useful. Another approach is to measure IgA antibody responses in peripheral blood mononuclear cells (PBMCs) (antibody in lymphocyte supernatant [ALS] or enzyme-linked immunosorbent spot [ELISPOT] assays), stool, saliva, or breast milk, anticipating that these secretory specimens will reflect the same type of response that is occurring in the intestine (18). Finally, serum antibodies can also be measured to identify an immune response to orally administered antigens, even with the understanding that the serum response may not be fully reflective of local antibody responses seen in the intestine.
Clinical indicators of immune protection may include reductions in attack rates, reductions in the severity of diarrheal symptoms, or reductions in levels of bacterial shedding. Ideally, protection could completely inhibit infection, leading to “sterile immunity.” In assessing the different measures of immune responses, it is difficult to determine the relative importance of secretory IgA versus serum antibodies in the development of immune protection. As alluded to above, this uncertainty reflects incomplete knowledge about the most efficient means of inducing antigen-specific local immune responses in the intestine that are protective.
To evaluate different measures of the immune response to ETEC diarrhea, we measured immune responses in serum, ALS, fecal extracts, lavage fluid, and saliva while conducting a study in which volunteers were challenged or rechallenged with virulent ETEC strain H10407 serotype O78:H11. This ETEC strain produces both LT and ST and colonization factor CFA/I. In this study, we attempted to (i) evaluate the immune responses induced by oral ETEC strain H10407 challenge using various methods, (ii) compare the immune responses of volunteers challenged with strain H10407 to those seen when those volunteers were reinfected with the same strain, and (iii) compare ETEC antigen-specific antibody profiles in serum, ALS, fecal extracts, and saliva among naive and immune subjects following infection with strain H10407.
As indicated above, although prior challenge-rechallenge ETEC studies have been done, mucosal immune responses in those subjects were not characterized in any depth. The investigations outlined in this report attempted to take advantage of ongoing ETEC model refinement work (19) at the Johns Hopkins School of Public Health (JHSPH) to gain a better insight into the nature, kinetics, and magnitude of mucosal immune responses to ETEC lipopolysaccharide (LPS), heat-labile toxin B subunit (LTB), and CFA/I antigens in naive and immune subjects. This information will be of value to future ETEC vaccine development efforts since it will provide an immunological benchmark to help assess new candidate ETEC vaccines moving into early clinical development.
MATERIALS AND METHODS
Regulatory approval.
The protocol was conducted under BB-IND 12,243 at the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health. Approval to conduct the study was provided by the Western Institutional Review Board (Olympia, WA) for the Johns Hopkins Bloomberg School of Public Health, the Institutional Biosafety Committee of the Johns Hopkins Institutions, and PATH (19).
Study design.
The study was conducted in three cohorts, as described by Harro et al. in 2011 (19). All volunteers were challenged with ETEC strain H10407. In cohort 1, 20 subjects were fasted overnight and randomized 1:1:1:1 to receive virulent ETEC strain H10407 (2 × 108 CFU with bicarbonate, 2 × 108 CFU with CeraVacx buffer, 2 × 107 CFU with bicarbonate, or 2 × 107CFU with CeraVacx buffer). In cohort 2, 15 subjects were all given a dose of 2 × 107 CFU with bicarbonate buffer. Cohort 3 included 10 ETEC-naive volunteers and 10 volunteers who had been challenged with strain H10407 2 to 3 months earlier and were administered a dose of 2 × 107 CFU of strain H10407 with bicarbonate. In each challenge cohort, all subjects received the strain H10407 challenge; none of the subjects received a placebo challenge.
Sampling of specimens.
Blood, fecal, lavage fluid, and saliva samples were collected from volunteers for immunological evaluations of total IgA (excluding blood) and antigen-specific IgA and IgG (serum only) responses to CFA/I, LTB, and ETEC H10407 strain-specific LPS. Fecal, lavage fluid, and saliva samples were collected only in cohort 3 of the study.
Blood specimens.
Venous blood for the ALS assay was collected in BD Vacutainer cell preparation tubes (CPT) with heparin (Becton Dickinson, Franklin Lakes, NJ, USA) from volunteers on day 0 (before challenge) and days 7, 10, 28, and 84 (cohort 3 only) after challenge. Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation and resuspended at 1 × 107 viable lymphocytes per ml. PBMCs were then incubated at 37°C and 5% CO2 for 72 h with no antigenic stimulation. The supernatant fluid was cryopreserved and subsequently used in an enzyme-linked immunosorbent assay (ELISA) to measure the concentration of antibody released by the PBMCs. The ALS specimens were tested for antigen-specific IgA. An ALS response was defined as a ≥4-fold increase in antigen-specific IgA antibody titer over the baseline.
Pre- and postchallenge venous blood samples were collected the same days as the ALS samples and processed for serum as described previously (19). A serum response was defined as a ≥2.5-fold increase over the baseline.
Intestinal lavage.
Lavage fluid samples were collected only on day 10 following the challenge or rechallenge from volunteers in cohort 3. The volunteers, who had fasted overnight, drank 250 ml of GoLYTELY lavage solution every 10 min until a clear, watery stool appeared. The mean volume of lavage solution ingested by the volunteers was 4.9 liters. After intake of the lavage solution, the first “induced” stool (FIS) and the first “liquid” intestinal lavage fluid (FLS) samples were collected from each volunteer. Next, a 50-ml aliquot of each of the two samples was treated by the addition of soybean trypsin inhibitor (STI) (Sigma, MO) (final concentration, 100 μg/ml), EDTA (Merck, NJ) (final concentration, 0.05 M), and Pefablock (Roche, NJ) (final concentration, 0.35 mg/ml) and kept on ice. The two specimens were then centrifuged at 1,000 × g for 15 min, and the pellet was discarded. The supernatant was kept frozen at −70°C until assayed by ELISA.
Fecal samples.
Stool specimens from subjects were collected from cohort 3 on the day before challenge and 10, 28, and 84 days after challenge or rechallenge. Specimens were stored at −70°C after they were received. Since some specimens were brought to the clinic during outpatient follow-up visits, the durations between collection and freezing differed by up to 18 h. Antibodies were extracted by the following procedure. Four grams of thawed stool was mixed with 16 ml of a solution containing STI (Sigma, MO) (100 μg/ml), EDTA (Merck, NJ) (0.05 M), and Pefablock (Roche, NJ) (final concentration, 0.35 mg/ml) and dissolved in phosphate-buffered saline (PBS) (pH 7.2) supplemented with 0.05% Tween 20 (PBS-Tween). The mixture was left to stand on the bench at room temperature for 15 min with intermittent shaking. Next, the mixture was centrifuged at 12,000 × g for 30 min. Bovine serum albumin (BSA) (final concentration, 0.1% [wt/vol]) was added to the supernatant after the pellet was discarded. Aliquots of the supernatant were stored at −70°C until they were assayed by ELISA for total and specific IgA antibody contents.
Saliva samples.
Saliva samples were collected from cohort 3 on the day before and 7, 10, 28, and 84 days after challenge or rechallenge. Participants placed 1-by-4-cm absorbent oral swabs (Salimetrics, State College, PA) in three different areas of the mouth without any salivary stimulation. The first swab was placed under the tongue to absorb oral fluid produced in the sublingual salivary gland area; the two other swabs were placed between the upper right cheek and gum and the upper left cheek and gum in the rear of the mouth by the jaw hinge to gather oral fluid from the parotid salivary gland area. All oral swabs remained in their respective positions for 15 min. Swabs were collected in Salimetrics tubes and were kept on ice until processing. The tubes were centrifuged at 3,000 rpm for 15 min at 4°C to remove mucins. Next, 0.9 ml of each sample was mixed with 100 μl of enzyme inhibitor mixture containing STI (final concentration, 100 μg/ml), Pefablock (final concentration, 0.35 mg/ml), BSA (final concentration, 0.1%), and EDTA (final concentration, 0.05 M) and stored frozen at −70°C until assayed.
Determination of levels of specific antibodies and total IgA.
For the assessment of specific IgA titers, flat-bottom ELISA plates (Nunc, Roskilde, Denmark) were coated with purified CFA/I or LPS diluted in PBS. Samples were 3-fold serially diluted and tested in duplicate. A GM1-ELISA method was used for the determination of levels of LT-specific antibodies (19). GM1 and LTB antigens were purchased from Sigma (Sigma-Aldrich, St. Louis, MO), and CFA/I and LPS antigens were obtained from the laboratory of Ann Mari Svennerholm (University of Gothenburg, Gothenburg, Sweden). Secondary antibodies used were goat anti-human IgG or IgA conjugated to horseradish peroxidase (HRP) (KPL, Gaithersburg, MD). For each assay, the endpoint titer was calculated as the reciprocal dilution giving rise to an absorbance value of 0.4 above the background at 450 nm.
Antibody titers of lavage fluid, fecal, and saliva specimens were expressed as units per milligram of IgA, and these titers were calculated using the specific titer (in units per milliliter) divided by the total IgA contents (in micrograms per milliliter) multiplied by 1,000. The total IgA contents were determined by ELISA using a standard IgA product (Sigma, St. Louis, MO) with a known IgA concentration (1 mg/ml).
Comparison of different assay methods.
In this study, the ALS assay served as a “gold standard” for comparisons of the other immune assays. Sensitivity values and positive predictive values (PPV) were calculated as (true positive)/(true positive plus false negative) and (true positive)/(true positive plus false positive), respectively. A “true positive” result represents a subject who had responses in both the ALS assay and the other assay used; a “false negative” result represents a subject who had an ALS response but was a nonresponder by the other assay method being evaluated; a “false positive” result represents a subject who had a response in the other assay but not in the ALS assay; and a “true negative” result represents a subject who did not have a response in either the ALS assay or the other assay.
Statistical analyses.
Chi-square and t tests were used to determine differences between groups as appropriate for categorical and continuous variables. Results of statistical analyses were considered significant only if P was less than 0.05. We used GraphPad Prism (GraphPad, CA) software to analyze the results.
RESULTS
Reactogenicity and shedding.
The reactogenicity and shedding of ETEC strains in the subjects challenged or rechallenged with ETEC were described in detail in our previous study (19). In short, naive subjects had an attack rate of about 70% in each group. Among the rechallenged subjects, only one volunteer developed mild diarrhea. After challenge, the naive subjects shed ETEC strain H10407 in their stool, with geometric mean maximum concentrations of approximately 2 × 108 CFU per gram in those receiving the 108 CFU dose and 8 × 107 CFU per gram in those receiving the 107 CFU dose. However, those who were rechallenged with the same dose shed ETEC with the geometric mean maximum concentration of approximately 3 × 105 CFU per gram, approximately 2 logs lower than in naive subjects (P < 0.02 versus naive subjects) challenged with strain H10407 and consistent with the protection against disease seen in the rechallenged subjects.
For ease of analysis and to identify differences between the naive and rechallenge subjects, we combined all the ETEC-naive recipients from all cohorts for a total of 44 individuals (1 subject was lost to follow-up) and compared them with the 10 rechallenge subjects in cohort 3 to analyze immune responses in serum and ALS. We evaluated the dose and buffer effects in a separate analysis where we compared the four groups of cohort 1 separately, and there were no significant differences in their postchallenge immune responses in ALS and serum (19). Fecal, lavage fluid, and saliva samples were collected only from cohort 3; thus, for these assays, we compared the 10 naive recipients of strain H10407 in cohort 3 to the 10 rechallenged subjects. Volunteers were called “naive” if they were challenged only once with ETEC strain H10407 and called “rechallenged” if they were reinfected with strain H10407.
ALS.
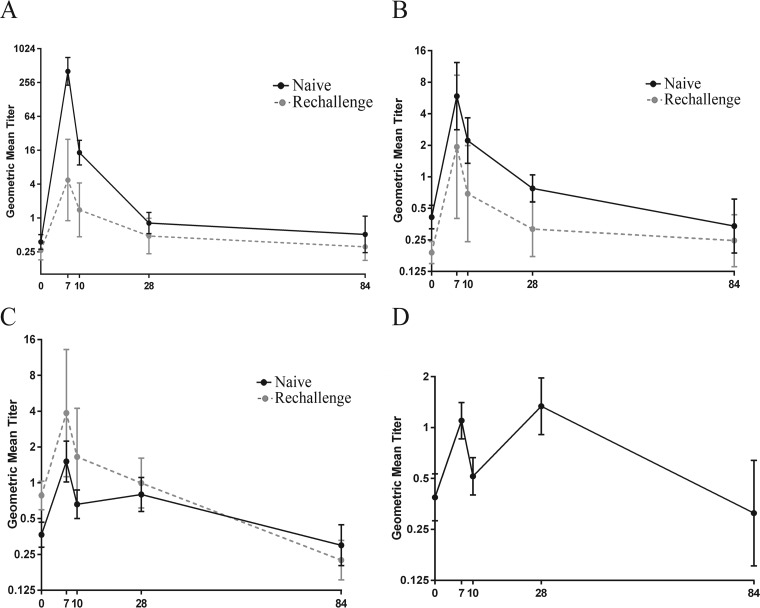
Among naive volunteers, IgA responses to LPS, LTB, and CFA/I in ALS increased significantly on day 7 but declined to baseline levels shortly thereafter (Fig. 1A, B, and C, respectively). The highest response frequency seen among challenged subjects was to the O78 LPS antigen, with 96% (42 of 44) of the naive volunteers mounting a ≥4-fold rise in anti-LPS IgA (P < 0.01) postinfection. In contrast, only 70% (7 of 10) of rechallenged volunteers responded to LPS IgA with an increase in titer following the rechallenge (see Table 2). The increase in the anti-O78 ALS titers on day 7 postchallenge for naive subjects was 1,093-fold higher on day 7 (geometric mean titer [GMT] of 404.47) than the baseline (GMT of 0.37) (P < 0.0001) (Fig. 1A and Table 1). In contrast, the magnitudes of the O78 responses were much more modest or blunted among rechallenge subjects, with only an 18-fold rise over the baseline. In the rechallenged group, the GMTs of baseline and day 7 samples were 0.26 and 4.74, respectively (Fig. 1A and Table 1).
FIG 1.
(A to C) ALS IgA geometric mean titers (95% confidence intervals) of antibody responses to LPS (A), LTB (B), and CFA/I (C) on the day before challenge (day 0) and on days 7, 10, 28, and 84 following challenge or rechallenge with ETEC strain H10407. (D) IgA responses to CFA/I expressed in ALS geometric mean titers (95% confidence intervals) in naive subjects who showed bimodal responses after challenge with ETEC strain H10407. The titers are in log2 scale and presented as antilogs.
TABLE 2.
| Antigen | No. (%) of subjects showing an IgA response |
|
|---|---|---|
| Naive (n = 44) | Rechallenge (n = 10) | |
| LPS | 42 (96) | 7 (70) |
| LTB | 35 (80) | 6 (60) |
| CFA/I | 21 (48) | 6 (60) |
A 4-fold or greater rise in titer from baseline was considered a response.
TABLE 1.
Baseline titers and fold changes for IgA and IgG response to LPS, LTB, and CFA/I in serum ALS, fecal, and saliva samplesa
| Antigen and antibody | Baseline titer |
Fold increase of titer on day: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 |
10 |
28 |
84 |
||||||||
| N | RC | RC/N | N | RC | N | RC | N | RC | N | RC | |
| LPS | |||||||||||
| Serum IgA | 13.76 | 58.81 | 4.28** | 18.91** | 1.40 | 59.63** | 1.61 | 11.46** | 0.94 | 3.92* | 0.65 |
| Serum IgG | 146.7 | 969.6 | 6.61** | 2.96** | 1.02 | 7.85** | 1.05 | 5.10** | 0.96 | 6.35** | 0.87 |
| ALS IgA | 0.37 | 0.26 | 0.70 | 1,093** | 18.23 | 38.66** | 5.24** | 2.17* | 1.81 | 1.37 | 1.17 |
| Fecal IgA | 20.41 | 425.9 | 20.9* | ND | ND | 86.63* | 4.42 | 21.34** | 3.59 | 6.11 | 1.53 |
| Parotid IgA | 697.6 | 659.5 | 0.95 | 1.99 | 1.15 | 3.02 | 0.95 | 1.31 | 1.46 | 0.77 | 1.17 |
| Sublingual IgA | 794.5 | 670.4 | 0.76 | 1.5 | 1.16 | 4.53 | 2.0 | 1.9 | 2.13 | 1.73 | 1.86 |
| LTB | |||||||||||
| Serum IgA | 237.3 | 399.4 | 1.68* | 1.66* | 1.22 | 2.51** | 1.40 | 2.10** | 1.10 | 1.27 | 1.14 |
| Serum IgG | 632.5 | 758.2 | 1.20 | 1.15 | 1.02 | 1.67 | 1.04 | 2.32** | 0.98 | 0.89 | 0.83 |
| ALS IgA | 0.41 | 0.19 | 0.46 | 14.28** | 10.20** | 5.37** | 3.65 | 1.88** | 1.68 | 0.06 | 1.30 |
| Fecal IgA | 21.03 | 73.97 | 3.52 | ND | ND | 2.37 | 2.95 | 3.63 | 2.43 | 3.74 | 1.12 |
| Parotid IgA | 4,402 | 6,332 | 1.44 | 0.96 | 0.32 | 1.62 | 1.06 | 0.69 | 1.24 | 0.49 | 0.25 |
| Sublingual IgA | 7,628 | 5,275 | 0.69 | 0.93 | 1.48 | 0.95 | 1.94 | 1.0 | 1.65 | 0.40 | 1.40 |
| CFA/I | |||||||||||
| Serum IgA | 168.4 | 248.1 | 1.47 | 1.17 | 1.90 | 1.47 | 2.38* | 2.08** | 1.30 | 2.02* | 1.21 |
| Serum IgG | 366.5 | 251.9 | 0.69 | 1.10 | 1.90 | 1.37 | 2.38* | 1.66 | 1.30 | 0.94 | 1.21 |
| ALS IgA | 0.36 | 0.78 | 2.17 | 4.14** | 4.93** | 1.81 | 2.11 | 2.17** | 1.27 | 0.82 | 0.29 |
| Fecal IgA | 12.43 | 9.30 | 0.75 | ND | ND | 0.42 | 1.61 | 0.55 | 0.66 | 1.94 | 0.94 |
N, naive subjects; RC, rechallenged subjects; RC/N, fold increase of baseline titers in rechallenged compared to naive subjects; ND, not done. All the fold increases are compared to the baseline (the day before challenge in naive subjects and the day before rechallenge in rechallenged subjects). *, P < 0.05; **, P < 0.005.
The frequency of ALS responses to LTB was higher in the naive subjects than in the rechallenged individuals. The majority (80% [35 of 44]) of naive subjects responded to LTB, compared to 60% (6 of 10) of rechallenged subjects (Table 2 and Fig. 1B). The increase in GMT on day 7 (GMT of 5.89) compared to the baseline (GMT of 0.41) was greater in naive volunteers (14.28-fold [P = 0.0071]) than in rechallenged volunteers (10.20-fold [P = 0.0002]), for whom the GMTs at the baseline and on day 7 were 0.19 and 1.93, respectively (Fig. 1B and Table 1).
Interestingly, the kinetics of the IgA response to CFA/I in the ALS assay were different from those observed for the LPS and LTB antigens (Fig. 1C). The proportions of responders were similar in the naive and rechallenged groups (48% and 60%, respectively) (Table 2). However, the GMT in the rechallenged group (3.86) on day 7 was 2.6-fold higher than that in the naive group (1.51) (Table 1 and Fig. 1C).
Notably, for CFA/I IgA, 50% (22 of 44) of the subjects in the naive group showed a bimodal response curve. The titers increased on day 7 (any fold increase over the baseline), decreased on day 10, increased again on day 21, and then decreased again, giving a bimodal response curve (Fig. 1D). A total of 64% (14 of 22) of these subjects who showed a bimodal response had a lower-than-4-fold increase at day 7 after challenge.
Serum.
For serum, IgA and IgG responses to LPS and LTB were seen more frequently in the naive group than in the rechallenged group, as shown in Table 3. In the naive subjects, 96% (42 of 44) and 64% (28 of 44) had an IgA response to LPS and LTB, respectively. In contrast, only 30% (3 of 10) and 20% (2 of 10) of the rechallenged subjects had an IgA response to LPS and LTB, respectively. The IgG anti-LPS and anti-LTB responses followed a similar pattern, also being significantly more frequent among naive subjects. A total of 73% (32 of 44) and 41% (18 of 44) of the naive subjects responded to these two antigens, respectively, whereas no subjects in the rechallenged group mounted IgG responses to either antigen (Table 3). The highest titers for LPS IgA (GMT of 820.10 in naive subjects [P < 0.0001] and GMT of 94.93 in rechallenged subjects [P = 0.0046]) and IgG (GMT of 1,151.74 for naive subjects [P < 0.0001] and GMT of 1,018.05 in rechallenged subjects) and LTB IgA (GMT of 594.69 in naive subjects [P = 0.0046] and GMT of 558.65 in rechallenged subjects) were seen on day 10 after challenge or rechallenge. For LTB IgG, the highest titer (1,469.15) (P = 0.0117) in the naive group was seen on day 28, whereas the highest titer (789.97) in the rechallenged group was seen on day 10 (Table 1). The baseline GMTs of the rechallenged subjects were significantly higher than those of the naive individuals for IgA and IgG responses to LPS and IgA responses to LTB (P = 0.006 and 0.0005 and 0.0336, respectively). Responses to CFA/I were less common in both groups, but the frequency of responders was higher among naive subjects. Among naive subjects, 50% (22 of 44) and 27% (12 of 44) had IgA and IgG anti-CFA/I responses, respectively, while 30% (3 of 10) and 20% (2 of 10) of the rechallenged volunteers had responses (Table. 3). Interestingly, in contrast to the LPS and LTB results, there was a higher increase in titer against anti-CFA/I in the rechallenged group than in the naive group. Notably, the highest titers (589.90 for IgA [P = 0.0453] and 598.92 for IgG [P = 0.0454]) in rechallenged subjects were observed on day 10 whereas the responses in naive subjects peaked later, on day 28 (350.05 for IgA [P = 0.0002] and 610.06 for IgG).
TABLE 3.
| Antigen | No. (%) of subjects showing a response to: |
|||
|---|---|---|---|---|
| IgA |
IgG |
|||
| Naive (n = 44) | Rechallenge (n = 10) | Naive (n = 44) | Rechallenge (n = 10) | |
| LPS | 42 (96) | 3 (30) | 32 (73) | 0 (0) |
| LTB | 28 (64) | 2 (20) | 18 (41) | 0 (0) |
| CFA/I | 22 (50) | 3 (30) | 12 (27) | 2 (20) |
A 2.5-fold or greater rise in titer from baseline was considered a response.
Fecal IgA.
IgA antibody responses to LPS O78, LTB, and CFA/I were determined in fecal extracts in cohort 3. Specimens with total IgA contents of <10 μg/ml were excluded from analyses, since those specimens were likely degraded (20). On the basis of the exclusion criteria and due to the unavailability of some samples for this assay, samples from only 5 to 7 of the 10 subjects in each group could be evaluated.
For anti-LPS IgA, all 5 of the naive subjects responded to LPS whereas 71% (5 of 7) of the rechallenged subjects responded (Table 4). The anti-LPS GMT at baseline in the rechallenge group (just prior to rechallenge) was 21-fold higher (P = 0.0480) than in the naive group. The anti-LPS antibody titers peaked on day 10 in both groups (highest titers of 1,768.36 [P = 0.0295] in naive subjects and 1,880.85 in rechallenged subjects), with an 87-fold increase compared to the baseline in naive volunteers but only a much more modest 4-fold increase in the rechallenged group.
TABLE 4.
Rates of IgA response to ETEC strain H10407 antigens as determined from fecal specimens in cohort 3a
| Antigen | No. (%) of subjects showing an IgA response |
|
|---|---|---|
| Naive (n = 10) | Rechallenge (n = 10) | |
| LPS | 5/5 (100) | 5/7 (71) |
| LTB | 4/5 (80) | 4/7 (57) |
| CFA/I | 0/5 (0) | 3/7 (43) |
A 4-fold or greater rise in titer from baseline was considered a response.
Eighty percent (4 of 5) of naive subjects responded to LTB, while 57% (4 of 7) of rechallenged subjects responded (Table 4). The GMT of the baseline level was 3.52-fold higher in the rechallenged subjects than in the naive volunteers. Of note, the anti-LTB response (titer of 76.33) peaked late on day 21 in naive volunteers, but the highest titer (218.16) was seen on day 10 for the rechallenged group. However, the fold increases from the baseline to the peak titer were similar, with a 3.63-fold increase in the naive subjects and a 2.95-fold increase in the rechallenged group (Table 1).
Fecal IgA responses to CFA/I were more meager than those to the other two ETEC antigens and appeared to have a different response pattern. Although 43% (3 of 7) of the rechallenged subjects responded, none of the naive volunteers responded (Table 4). There was a 1.6-fold increase of the GMT titer (13.48) from baseline to day 10 in rechallenged volunteers, compared to no increase at all in naive subjects (Table 1).
Lavage fluid.
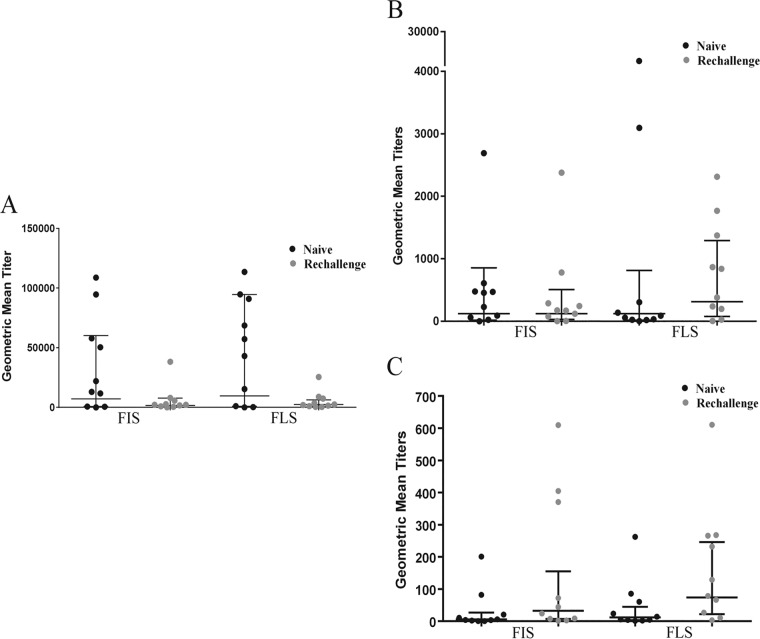
On day 10 after challenge or rechallenge, we collected the FIS and FLS from the volunteers after giving them the lavage fluid solution. We compared the specific IgA antibody responses for LPS, LTB, and CFA/I in these two specimens (Fig. 2A, B, and C, respectively). Statistically significant correlations (r = 0.75 to 0.78, P < 0.001) were observed between IgA titers of FIS and FLS for all the three antigens, though the titers were slightly higher with FLSs than FISs. Rechallenge subjects had lower antibody titers for LPS antigen than the naive subjects, while titers for LTB were either similar (FIS) or higher in rechallenge subjects. On the other hand, rechallenged volunteers had higher titers for CFA/I antigen than naive subjects. Since we collected the lavage fluid only on day 10 after challenge or rechallenge, titer increases from baseline were not available. Comparing the specific IgA antibody responses for LPS, LTB, and CFA/I on day 10 after challenge in induced fecal sample FIS and normal fecal samples, there were lower (than FIS and FLS) but significant correlations (r = 0.53 to 0.75, P < 0.0003 to < 0.031).
FIG 2.
Frequencies of antigen-specific IgA responses to LPS (A), LTB (B), and CFA/I (C) expressed in geometric mean titers (95% confidence intervals) in FIS (first induced stool) and FLS (first liquid intestinal lavage fluid) samples on day 10 following challenge or rechallenge with ETEC strain H10407. FIS and FLS are expressed as units per milligram of whole IgA.
Saliva.
The study subjects were also evaluated for IgA antibody responses to LPS, LTB, and CFA/I in parotid and sublingual saliva samples. The anti-CFA/I responses were very low or negligible for both parotid and sublingual saliva samples. The volumes of saliva from some subjects were insufficient; thus, samples from 7 to 10 of the 10 subjects in each group could be evaluated.
A 50% (4 of 8) proportion of the parotid saliva samples of naive subjects showed 4-fold or higher IgA responses to LPS compared to only 10% (1 of 10) in the rechallenged group (Table 5). The trend was similar for LTB, with 25% (2 of 8) responding in the naive group compared to 11% (1 of 9) in the rechallenged group. The anti-LPS baseline GMTs were similar in the two groups and increased 3-fold to reach the highest titer (2,103.78) on day 10 in naive volunteers but increased only 1.5-fold (highest titer, 757.62) on day 28 in rechallenged volunteers. The baseline GMT titer of anti-LTB was 1.4-fold higher in rechallenged volunteers than in naive subjects. The highest titers were found on day 10 in the naive subjects (7,130.01) and on day 28 in the rechallenged group (7,821.78), but the increase in titer from baseline to peak was minimal, at less than 2-fold (Table 1).
TABLE 5.
| Antigen | No. (%) of subjects showing an IgA response |
|||
|---|---|---|---|---|
| Parotid |
Sublingual |
|||
| Naive (n = 10) | Rechallenge (n = 10) | Naive (n = 10) | Rechallenge (n = 10) | |
| LPS | 4/8 (50) | 1/10 (10) | 6/10 (60) | 5/9 (56) |
| LTB | 2/8 (25) | 1/9 (11) | 3/9 (33) | 0/7 (0) |
A 4-fold or greater rise in titer from baseline was considered a response.
A similar result was found in sublingual saliva samples. Sixty percent (6 of 10) and 56% (5 of 9) of the subjects in the naive and rechallenged groups, respectively, responded to anti-LPS IgA. In contrast, 33% (3 of 9) of naive volunteers responded to anti-LTB but none in the rechallenged group responded (Table 5). The groups had similar GMTs at baseline for anti-LPS. In the naive group, the highest titer (3,596.752) was seen on day 10 and was 4.5-fold higher than the baseline titer, whereas the highest titer in rechallenged volunteers was seen on day 28 (1,429.53) and had increased by 2.1-fold. The anti-LTB response in sublingual saliva was quite similar to the responses in parotid saliva for both groups. However, the highest titer was reached in sublingual saliva samples earlier (on day 10) in the rechallenged group (10,247.02) compared to day 28 in naive subjects (7,931.85). The titer in parotid saliva samples (7,130.01) peaked on day 10 in naive subjects, while the highest titer (7,821.78) was seen on day 28 in the rechallenged group. Notably, the correlations between the sublingual and parotid saliva sample results for LPS were significant (r = 0.795, P = 0.00005), but the correlations between these two sample categories were low for LTB.
Immune responses in unprotected subjects and among the nonshedders.
All evaluable naive subjects except one shed ETEC strain H10407 in their postchallenge stools. Although 90% of the subjects were clinically protected in the rechallenged group, it was not sterile immunity—all of the protected subjects shed the challenge strain.
The one naive subject who did not have any diarrheal illness and did not shed the challenge strain also did not respond to any antigens in ALS, serum, or fecal samples. Surprisingly, this subject mounted significant anti-LPS and anti-LTB IgA responses (>4-fold increase) in both the parotid and sublingual saliva samples.
The subject who was not protected (had mild diarrhea) after rechallenge responded to all the antigens in the ALS samples, to only LPS and LTB in the fecal samples, and to only LPS in the parotid saliva samples. This subject did not mount a serum IgA or IgG response to any of the test antigens.
The subject in the rechallenge group who did not shed the challenge strain postrechallenge responded to LPS IgA only in the fecal sample. However, when this subject was infected with ETEC the first time, the subject had severe diarrhea with shedding of the challenge strain with 6.4 × 107 CFU/g of stool and responded to all of the antigens in most of the assays.
Comparison of various methods for assessment of mucosal immune responses in the challenge model.
Comparing the immune responses to LPS, LTB, and CFA/I in cohort 3 in the different samples using ELISA, the responses to these antigens were most often seen using the ALS samples. The only exception was a low response rate for anti-CFA/I in the naive group which was improved upon rechallenge with strain H10407.
When the titers measured with other samples were compared to IgA titers in samples of ALS, the highest sensitivity (100%) and highest PPV (100%) were noted for serum IgA responses to both LPS and LTB in naive volunteers. Combining the data from the naive and rechallenged volunteers showed that the sensitivities decreased to 75% and 69% for LPS and LTB IgA, respectively, while the PPV remained unchanged (Table 6).
TABLE 6.
Comparison of ALS assay responses to responses in other assays used in this studya
| Antigen | Serum IgA assay |
Serum IgG assay |
Fecal IgA assay |
Parotid saliva assay |
Sublingual saliva assay |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % sensitivity | PPV (%) | % sensitivity | PPV (%) | % sensitivity | PPV (%) | % sensitivity | PPV (%) | % sensitivity | PPV (%) | |
| LPS | 75 | 100 | 44 | 100 | 56 | 90 | 25 | 80 | 50 | 72 |
| LTB | 69 | 100 | 25 | 100 | 44 | 88 | 13 | 67 | 13 | 67 |
| CFA/I | 67 | 60 | 44 | 67 | 11 | 33 | ||||
Data represent combined results from naive and rechallenged subjects.
For serum IgG, the sensitivity was 100% for both LPS and LTB but the specificity and PPV were 33% and 67% for LPS and 17% and 44% for LTB. Combining the results from the two groups showed that the sensitivity decreased to 44% with a PPV of 100% for LPS and to 25% with a PPV of 100% for LTB. However, CFA/I serum responses showed sensitivity of 67% for IgA with a PPV of 60 and sensitivity of 44% for IgG with a PPV of 67% for the combined data and the sensitivities were similar only in comparisons of naive subjects (Table 6).
Comparing IgA antibody responses in the ALS and fecal samples in the two groups combined, sensitivities of 56%, 44%, and 11% and PPVs of 90%, 88%, and 33% were noted for LPS, LTB, and CFA/I, respectively. The sensitivity was 100% for LPS and LTB only in comparisons of naive subjects (Table 6).
Comparison of ALS assay responses with saliva responses in the combined groups showed that both the parotid and sublingual saliva samples had much lower sensitivity to LPS and LTB (Table 6) but that the sensitivity was increased to 83% and 50% for LPS and LTB in sublingual saliva samples and 75% and 50% in parotid saliva samples, respectively, in comparisons of the naive subjects only (Table 6).
DISCUSSION
In this study, a dose of 107 CFU of ETEC strain H10407 induced immune responses to LPS, LTB, and CFA/I in most volunteers. Although this strain has been used in many volunteer studies (21), this is the first study to evaluate antibody responses induced by this strain in a comprehensive way using different methods that enabled mucosal as well as serum antibody responses to be assessed in naive and rechallenged volunteers. Previous challenge and rechallenge studies with strain H10407 and other ETEC strains (12, 22) were done at a time when methods for measuring both mucosal and serum responses were not as readily available; thus, data on how mucosal responses, in particular, may compare in naive and immune subjects are lacking from the human experimental infection models. A better understanding of the interplay between local and serum responses to ETEC strains, such as strain H10407, in nonimmune and immune subjects is needed since these data will provide important immunological benchmarks for the evaluation of ETEC vaccines and immunization regimens in the future.
In the present study, each volunteer responded with antibodies to one or more antigens in at least one of the five different immunological assays. There were significant responses to LPS and LTB which were similar in magnitude and frequencies in serum and ALS. However, IgA antibody responses to CFA/I antigens were lower than to either LPS or LTB. For all antigens, ALS responses were short-lived, peaking on day 7 and declining by day 10. Fecal antibodies showed a trend similar to that found with the serum and ALS assays. We found that the antibody responses in parotid and sublingual saliva samples were lower than those found in the other assays. There was a high correlation between the responses for LPS in sublingual and parotid saliva samples; however, the correlation was low for LTB.
Our comparisons of the immune responses of the naive and rechallenged subjects showed that protection against disease appeared to be reflected in changes in antibody titers produced in response to these virulence antigens. Most naive subjects (64% to 96%) had significant ALS and serum IgA antibody responses to LPS and LTB (Fig. 1A and B and Tables 1, 2, and 3). However, the frequencies of IgG responses to LPS and LTB in serum from the naive challenge group were lower (41% to 73%). In contrast to the naive group, responses to LPS and LTB in ALS and serum were low in the rechallenge group. Lower frequencies and magnitudes of the anti-LPS response in rechallenge subjects have been observed previously in a limited number of subjects (22), but the impact is much clearer with the larger number of subjects evaluated in this trial. A likely explanation for the relatively modest frequency of responders among the rechallenged volunteers is the immune protection induced by the first challenge, which limited colonization of the challenge strain and thus potentially blocked or limited the production of the antigens in the gut by the referred ETEC strain. In addition, the local intestinal antibodies that are likely present in the gut of subjects previously infected with ETEC may have bound to ETEC antigens being produced by the infecting H10407 strain and blocked them from reaching the gut-associated lymphoid tissue. Thus, this lack of or reduced level of responsiveness in the rechallenged group might be considered an indicator of protection.
We noted a similar kinetics of LPS and LTB with all other samples in this study in naive and rechallenged volunteers. We also found a similar trend when subjects vaccinated with the ACE527 live attenuated ETEC vaccine were challenged with ETEC strain H10407 (23). Our findings are similar to those of previously published studies showing that an oral booster dose given at a time of active mucosal immunity results in lower or similar levels of antibody-secreting cells (ASC) and serum IgA responses to CTB (cholera toxin B) (15, 24–26). Similar blunting of responses has also been seen with Campylobacter (27) and Shigella (28) volunteer challenge models.
In this study, interestingly, antibody responses to CFA/I were quite different from those to LPS and LTB, since there appeared to be a boosting of the response to this fimbrial antigen following rechallenge. The finding of a booster response after the rechallenge is not consistent with previous findings. In prior ETEC vaccine studies (20, 29), there were higher CF-specific responses after the first dose of a killed whole-cell ETEC vaccine than after the second dose of the vaccine. With regard to anti-CFA/I immunity in our study, it seems that the level of anti-CFA/I antibody at the mucosal surface induced by the first challenge was not sufficient to neutralize the CFA/I and thus there was an increase in the anti-CFA/I titer seen after rechallenge. Alternatively, this may have represented a true immunological booster response to this protein antigen following the second dose. This trend toward increasing titers of antibody to CFA/I was also found in serum, fecal, and saliva samples. The higher titers seen following the second dose suggest that immunity based on CFA/I requires at least two doses.
Notably, a second IgA response to CFA/I peak was observed in the ALS samples on day 21 after ETEC feeding among half of the naive subjects. This suggests that B cells left the intestinal mucosa to enter the circulation a second time. It is not clear why this second peak occurred, since this has been observed only for CFA and not LPS and LTB.
A key issue, which has not yet been resolved, is that of which immunoassay is the most appropriate biomarker of the local immune responses in the intestine. Among the assays used in this study, the ALS yielded the highest number of responses to the different antigens used in both the naive and rechallenge subjects. In the present study, we evaluated the immune responses to the specific antigens of virulent strain H10407 using an ALS assay and compared the responses to serum, fecal antibody, lavage fluid, and saliva samples. Similarly to this study, antibody-secreting cell responses of the IgA isotype have been used as the gold standard in previous studies (20, 24, 29). In this study, we found a significant correlation between the magnitudes of anti-LPS and -LTB IgA antibody responses in serum with 100% sensitivity compared to ALS responses after challenge in naive volunteers. However, the sensitivity and PPV were comparatively lower for CFA/I IgA in serum. On the basis of these high correlations, both of these assays (ALS and serum IgA) may be useful to screen for intestinal immune responses after oral vaccination, which could be of considerable practical value.
Similar results were found in a report published by Qadri et al. in 2000 (20) of a study in children who were immunized with two doses of oral inactivated ETEC plus CTB subunit vaccine, where the highest sensitivity (90%) and highest PPV (100%) were noted for the IgA plasma antibody response to CTB compared with CTB-specific ASCs after the first vaccine dose. However, in the adults, the plasma IgA antibody response correlated poorly with the ASC response to the different vaccine antigens. The lack of correlation in adults was possibly because of prior exposure to ETEC antigens and is similar to our results showing lower correlations in the rechallenge subjects. A comparison with ASC responses in both children and adults in this study showed that the sensitivity and PPV of fecal IgA responses were lower and were comparable to what we observed in our trial.
Among the rechallenged volunteers in our study, the ALS responses were a more sensitive immunological indicator than the serum responses for anti-LPS and anti-LTB antibodies, as fewer volunteers mounted serum IgA responses to these antigens than ALS IgA responses, and none were positive for serum IgG. The correlations were also higher among samples from naive subjects than among those from rechallenged individuals for all the antigens in correlations with ALS. The ALS test is based on the concept that antigen-specific plasmablasts are short-lived and are present in the circulation only at times of acute infection and not during latency or previously acquired immunity (30–33). Thus, we propose that, in the endemic population, where there are possibilities of high baseline titers of ETEC antigen-specific antibody, the ALS assay is a superior method for evaluating immune responses.
One disadvantage of using the ALS assay may be the requirement of a high volume of blood, which may not be suitable for the pediatric population. In recent studies, however, ALS assays have been successfully carried out in the pediatric population in the field using a small volume of blood samples (34, 35).
The fecal IgA assay showed a trend similar to that seen with the ALS and serum IgA assays, but the sensitivity was low. Immunoglobulins in fecal specimens are often extensively degraded by intestinal and bacterial enzymes. The assay results depend on the quality of a fecal specimen, which depends on how long it stayed in the intestine, transportation time, and handling, processing, and storage of the specimens.
An alternative method of obtaining intestinal specimens for antibody determinations is the collection of fecal specimens induced in fasting volunteers who drink a laxative (GoLYTELY). Whole-gut lavage fluid acts almost like a perfusion system and can be used to assess the antibodies produced in the entire gastrointestinal tract (36, 37). In the past, lavage fluid specimens were used for determination of intestinal antibodies. In this study, the lavage was performed only on day 10 and not before challenge because of a concern that a lavage procedure performed prior to challenge might alter the intestinal microflora and would affect the response to the challenge ETEC bacteria.
Although the lavage fluid procedure is noninvasive and safe, the main disadvantage is that it is time-consuming and requires motivated and cooperative volunteers and good laboratory support conditions that are not always available for large-scale population studies, especially in developing countries. As a pilot attempt to develop an improved method for obtaining intestinal fluid that would optimally and directly reflect the antibody responses of the gut mucosa, we compared two types of fecal/lavage fluid samples (Fig. 2). We found that titers from the FIS correlated significantly with the FLS and that responses to all the ETEC antigens correlated well between lavage fluid and day 10 fecal IgA. These results suggest that serial collection of a FIS might be considered a way to obtain intestinal specimens for antibody measures and would be unlikely to change the microbiome significantly. This type of specimen might be useful in the future for monitoring intestinal antibody titers. The advantage of the FIS relates to its being freshly collected and processed in comparison to fecal samples, especially those obtained during outpatient visits, which are less well controlled and may be subject to enzymatic decay.
From our analyses, we found that both the parotid and sublingual saliva fluid samples showed lower immune responses after challenge or rechallenge, and the sensitivity of both of these determinations was low. Although there was significant correlation between parotid and sublingual saliva sample results for LPS, the correlation was low for LTB. Antibodies to CFA/I were rarely found in detectable quantities in salivary secretions, which might have been due to high dilutions of antibodies in saliva samples. However, the saliva samples showed the same trend as the serum and ALS samples. A number of studies have reported the measurement of antibodies to Vibrio cholerae in saliva from patients convalescing from cholera and after immunization with cholera vaccines (13, 16). However, the usefulness of measuring levels of salivary antibodies after enteric infection remains unclear.
To conclude, correlations between methods to assess intestinal, saliva, and systemic antibody responses to key ETEC vaccine antigens suggest that the ALS assay more consistently predicts the intestinal immune response following oral challenge in both naive and rechallenge subjects. Assays of serum IgA and IgG are also useful measures of an immune response following challenge, even though they may not reflect the response in the intestine.
Identification of immune correlates of protection is a crucial need to accelerate the development of effective ETEC vaccines. Since there was only one subject who was not protected from diarrhea when rechallenged, our data were not able to identify such a marker for protection in the present study. Given the high level of interest in ETEC vaccine development, the field could benefit from a longer follow-up after initial infection similar to the challenge-rechallenge studies performed with Campylobacter (27) which may give more insights into correlates of long-term immunity.
Although there were only two subjects who did not shed the challenge strain, it is apparent that shedding level is related to the severity of diarrhea and the magnitude of the immune responses.
There were some limitations in this study. The number of volunteers in the rechallenged group was low. Since the induced stool procedure was done only at day 10 after challenge and not prior to challenge, we were unable to measure the titer increase after infection. We also had to exclude some subjects from the fecal and salivary analysis due to unavailability of some samples.
In conclusion, the results of this study, which extensively evaluated the immune responses of strain H10407 in adult volunteers, will help to improve understanding of and establish the H10407 strain as an effective challenge model in ETEC vaccine studies.
ACKNOWLEDGMENTS
We thank all the volunteers who participated in this study. The devoted efforts of George Gomes and Fatuma Mawanda were invaluable. We are thankful to Ann Mari Svennerholm for providing the LPS and CFA/I antigens and her guidance.
The study was supported by PATH.
Funding Statement
The study was funded by PATH Vaccine Solutions. Contributions from all the authors were supported by this grant.
REFERENCES
- 1.Steffen R, Castelli F, Dieter NH, Rombo L, Zuckerman NJ. 2005. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. J Travel Med 12:102–107. doi: 10.2310/7060.2005.12207. [DOI] [PubMed] [Google Scholar]
- 2.Wennerås C, Erling V. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Popul Nutr 22:370–382. [PubMed] [Google Scholar]
- 3.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2009. Diarrhoeal diseases. Initiative for vaccine research (IVR).World Health Organization, Geneva, Switzerland: (Updated February 2009.) http://www.who.int/vaccine_research/diseases/diarrhoeal/en/index6.html. [Google Scholar]
- 5.Das JK, Tripathi A, Ali A, Hassan A, Dojosoeandy C, Bhutta ZA. 2013. Vaccines for the prevention of diarrhea due to cholera, shigella, ETEC and rotavirus. BMC Public Health 13(Suppl 3):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadri F, Svennerholm AM, Faruque AS, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill DR, Beeching NJ. 2010. Travelers' diarrhea. Curr Opin Infect Dis 23:481–487. doi: 10.1097/QCO.0b013e32833dfca5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines 11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 9.Fleckenstein J, Sheikh A, Qadri F. 2014. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev Vaccines 13:631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black RE. 1993. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine 11:100–106. doi: 10.1016/0264-410X(93)90002-F. [DOI] [PubMed] [Google Scholar]
- 11.Cravioto A, Reyes RE, Trujillo F, Uribe F, Navarro A, De La Roca JM, Hernandez JM, Perez G, Vazquez V. 1990. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol 131:886–904. [DOI] [PubMed] [Google Scholar]
- 12.Levine MM, Nalin DR, Hoover DL, Bergquist E, Hornick RB, Young CR. 1979. Immunity to enterotoxigenic Escherichia coli. Infect Immun 23:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine MM, Black RE, Brinton CC Jr, Clements ML, Fusco P, Hughes TP, O'Donnell S, Robins-Browne R, Wood S, Young CR. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl 33:83–95. [PubMed] [Google Scholar]
- 14.Sack RB, Kline RL, Spira WM. 1988. Oral immunization of rabbits with enterotoxigenic Escherichia coli protects against intraintestinal challenge. Infect Immun 56:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrén CM, Svennerholm AM. 1982. Synergistic protective effect of antibodies against Escherichia coli enterotoxin and colonization factor antigens. Infect Immun 38:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svennerholm AM, Steele D. 2004. Microbial-gut interactions in health and disease. Progress in enteric vaccine development. Best Pract Res Clin Gastroenterol 18:421–445. doi: 10.1016/j.bpg.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Walker RI, Steele D, Aguado T. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545–2566. doi: 10.1016/j.vaccine.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Stoll BJ, Svennerholm AM, Gothefors L, Barua D, Huda S, Holmgren J. 1986. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J Infect Dis 153:527–534. doi: 10.1093/infdis/153.3.527. [DOI] [PubMed] [Google Scholar]
- 19.Harro C, Chakraborty S, Feller A, DeNearing B, Cage A, Ram M, Lundgren A, Svennerholm AM, Bourgeois AL, Walker RI, Sack DA. 2011. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin Vaccine Immunol 18:1719–1727. doi: 10.1128/CVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri F, Wenneras C, Ahmed F, Asaduzzaman M, Saha D, Albert MJ, Sack RB, Svennerholm A. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704–2712. doi: 10.1016/S0264-410X(00)00056-6. [DOI] [PubMed] [Google Scholar]
- 21.Porter CK, Riddle MS, Tribble DR, Louis Bougeois A, McKenzie R, Isidean SD, Sebeny P, Savarino SJ. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885. doi: 10.1016/j.vaccine.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Levine MM, Rennels MBL, Cisneros Hughes TP, Nalin DR, Young CR. 1980. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am J Epidemiol 111:347–355. [DOI] [PubMed] [Google Scholar]
- 23.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahrén C, Jertborn M, Svennerholm AM. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect Immun 66:3311–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantele A, Kantele JM, Arvilommi H, Makela PH. 1991. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine 9:428–431. doi: 10.1016/0264-410X(91)90130-X. [DOI] [PubMed] [Google Scholar]
- 26.Qadri F, Ahmed T, Ahmed F, Bradley SR, Sack DA, Svennerholm AM. 2003. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18–36 months of age. Vaccine 21:2394–2403. doi: 10.1016/S0264-410X(03)00077-X. [DOI] [PubMed] [Google Scholar]
- 27.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, Burg EF III, Moran AP, Applebee L, Bourgeois AL. 2010. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun 78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488–1494. doi: 10.1016/0264-410X(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 29.Savarino SJ, Hall ER, Bassily S, Wierzba TF, Youssef FG, Peruski LF Jr, Abu-Elyazeed R, Rao M, Francis WM, El Mohamady H, Safwat M, Naficy AB, Svennerholm AM, Jertborn M, Lee YJ, Clemens JD, Pride Study Group . 2002. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatr Infect Dis J 21:322–330. doi: 10.1097/00006454-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Jertborn M, Svennerholm AM, Holmgren J. 1984. Gut mucosal, salivary and serum antitoxic and antibacterial antibody responses in Swedes after oral immunization with B subunit-whole cell cholera vaccine. Int Arch Allergy Appl Immunol 75:38–43. doi: 10.1159/000233587. [DOI] [PubMed] [Google Scholar]
- 31.Raqib R, Kamal SM, Rahman MJ, Rahim Z, Banu S, Bardhan PK, Chowdhury F, Ara G, Zaman K, Breiman RF, Andersson J, Sack DA. 2004. Use of antibodies in lymphocyte secretions for detection of subclinical tuberculosis infection in asymptomatic contacts. Clin Diagn Lab Immunol 11:1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manz RA, Hauser AE, Hiepe F, Radbruch A. 2005. Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 33.Tarlinton D, Radbruch A, Hiepe F, Dorner T. 2008. Plasma cell differentiation and survival. Curr Opin Immunol 20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Raqib R, Mondal D, Karim MA, Chowdhury F, Ahmed S, Luby S, Cravioto A, Andersson J, Sack D. 2009. Detection of antibodies secreted from circulating Mycobacterium tuberculosis-specific plasma cells in the diagnosis of pediatric tuberculosis. Clin Vaccine Immunol 16:521–527. doi: 10.1128/CVI.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chisti MJ, Salam MA, Raqib R, Banu S, Shahid AS, Shahunja KM, Sharmin L, Ashraf H, Faruque AS, Bardhan PK, Ahmed T. 2015. Validity of antibodies in lymphocyte supernatant in diagnosing tuberculosis in severely malnourished children presenting with pneumonia. PLoS One 10:e0126863. doi: 10.1371/journal.pone.0126863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaspari MM, Brennan PT, Solomon SM, Elson CO. 1988. A method of obtaining, processing, and analyzing human intestinal secretions for antibody content. J Immunol Methods 110:85–91. doi: 10.1016/0022-1759(88)90086-5. [DOI] [PubMed] [Google Scholar]
- 37.Svennerholm AM, Sack DA, Holmgren J, Bardhan PK. 1982. Intestinal antibody responses after immunisation with cholera B subunit. Lancet i:305–308. [DOI] [PubMed] [Google Scholar]