Abstract
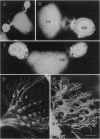
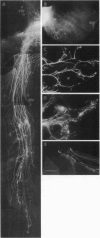
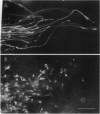
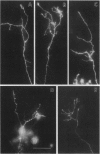
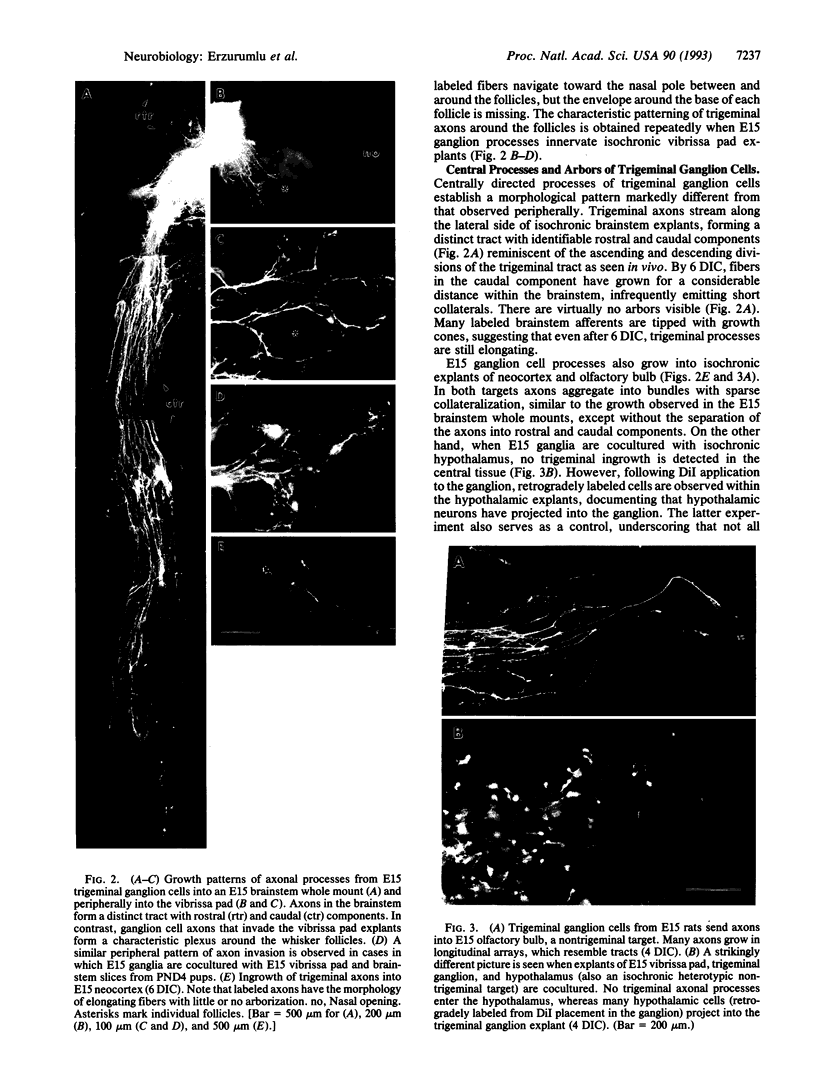
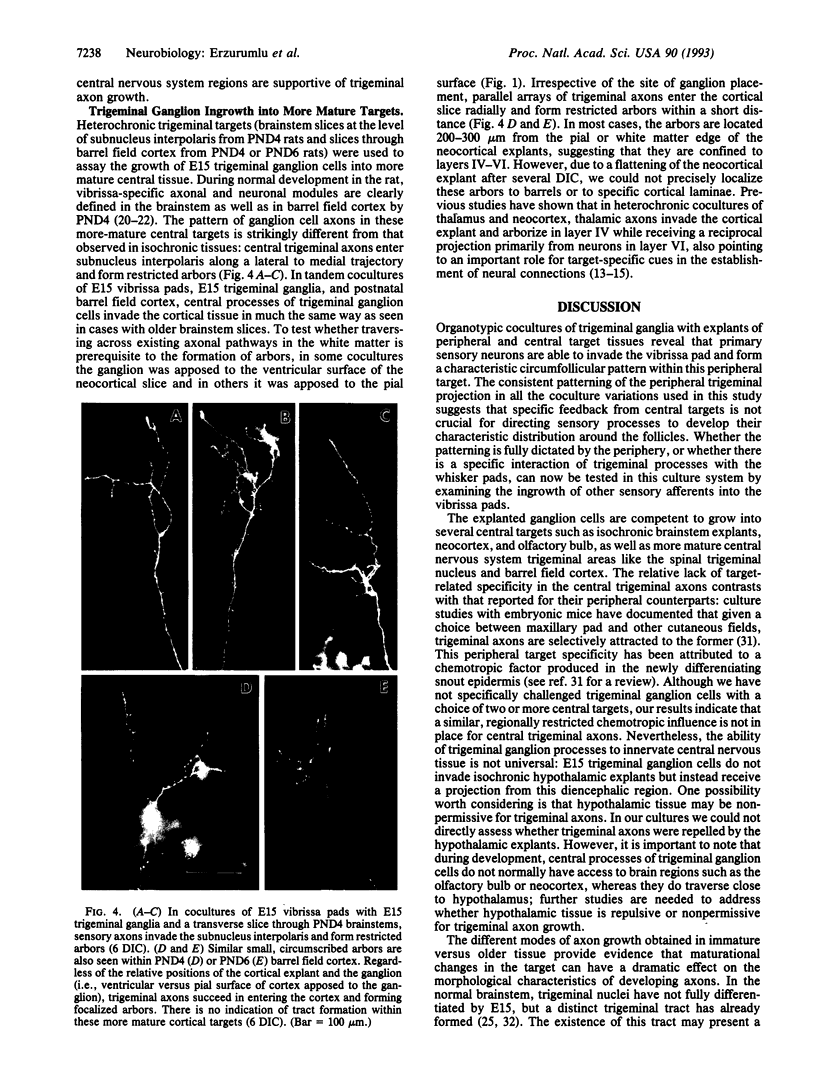
Cellular and molecular signals involved in axon elongation versus collateral and arbor formation may be intrinsic to developing neurons, or they may derive from targets. To identify signals regulating axon growth modes, we have developed a culture system in which trigeminal ganglion cells are challenged by various target tissues. Embryonic day 15 (E15) rat trigeminal ganglion explants were placed between peripheral (vibrissa pad) and central nervous system targets. Normally, bipolar trigeminal ganglion cells extend one process to the vibrissa pad and another to the brainstem trigeminal complex. Under coculture conditions, the peripheral processes invade the vibrissa pad explants and form a characteristic circumfollicular pattern. Central processes of E15 ganglion cells invade many, but not all, central nervous system tissues. In isochronic (E15) central nervous system explants such as brainstem, olfactory bulb, or neocortex, these central processes elongate and form a "tract" but have virtually no arbors. However, in more mature targets (e.g., a section from postnatal brainstem or neocortex), they form arbors instead of a tract. We conclude from these observations that whether trigeminal axons elongate to form a tract, or whether they begin to arborize, is dictated by the target tissue and not by an intrinsic developmental program of the ganglion cell body. The explant coculture system is an excellent model for analysis of the molecular basis of neuron-target interactions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Bayer S. A. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Bates C. A., Killackey H. P. The organization of the neonatal rat's brainstem trigeminal complex and its role in the formation of central trigeminal patterns. J Comp Neurol. 1985 Oct 15;240(3):265–287. doi: 10.1002/cne.902400305. [DOI] [PubMed] [Google Scholar]
- Belford G. R., Killackey H. P. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980 Sep 15;193(2):335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- Bhide P. G., Frost D. O. Stages of growth of hamster retinofugal axons: implications for developing axonal pathways with multiple targets. J Neurosci. 1991 Feb;11(2):485–504. doi: 10.1523/JNEUROSCI.11-02-00485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Molnár Z. Factors involved in the establishment of specific interconnections between thalamus and cerebral cortex. Cold Spring Harb Symp Quant Biol. 1990;55:491–504. doi: 10.1101/sqb.1990.055.01.048. [DOI] [PubMed] [Google Scholar]
- Bolz J., Novak N., Götz M., Bonhoeffer T. Formation of target-specific neuronal projections in organotypic slice cultures from rat visual cortex. Nature. 1990 Jul 26;346(6282):359–362. doi: 10.1038/346359a0. [DOI] [PubMed] [Google Scholar]
- Bolz J., Novak N., Staiger V. Formation of specific afferent connections in organotypic slice cultures from rat visual cortex cocultured with lateral geniculate nucleus. J Neurosci. 1992 Aug;12(8):3054–3070. doi: 10.1523/JNEUROSCI.12-08-03054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo D., Takahashi H., McKay R. D. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992 Oct;9(4):643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- Dörfl J. The innervation of the mystacial region of the white mouse: A topographical study. J Anat. 1985 Oct;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A. Place-dependent cell adhesion, process retraction, and spatial signaling in neural morphogenesis. Cold Spring Harb Symp Quant Biol. 1990;55:303–318. doi: 10.1101/sqb.1990.055.01.032. [DOI] [PubMed] [Google Scholar]
- English K. B., Burgess P. R., Kavka-Van Norman D. Development of rat Merkel cells. J Comp Neurol. 1980 Nov 15;194(2):475–496. doi: 10.1002/cne.901940212. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990 Nov 1;56(2):229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992 Oct;12(10):3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu R. S., Killackey H. P. Development of order in the rat trigeminal system. J Comp Neurol. 1983 Feb 1;213(4):365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- Friauf E., McConnell S. K., Shatz C. J. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990 Aug;10(8):2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Shatz C. J. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992 Jan;12(1):39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H. Organotypic cultures of neural tissue. Trends Neurosci. 1988 Nov;11(11):484–489. doi: 10.1016/0166-2236(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Heffner C. D., Lumsden A. G., O'Leary D. D. Target control of collateral extension and directional axon growth in the mammalian brain. Science. 1990 Jan 12;247(4939):217–220. doi: 10.1126/science.2294603. [DOI] [PubMed] [Google Scholar]
- Jhaveri S., Edwards M. A., Schneider G. E. Initial stages of retinofugal axon development in the hamster: evidence for two distinct modes of growth. Exp Brain Res. 1991;87(2):371–382. doi: 10.1007/BF00231854. [DOI] [PubMed] [Google Scholar]
- Molnár Z., Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991 Jun 6;351(6326):475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Beech J. N., Heizmann C. W. Two distinct phases and mechanisms of axonal growth shown by primary vestibular fibres in the brain, demonstrated by parvalbumin immunohistochemistry. Neuroscience. 1988 Nov;27(2):571–596. doi: 10.1016/0306-4522(88)90290-4. [DOI] [PubMed] [Google Scholar]
- Moya K. L., Benowitz L. I., Jhaveri S., Schneider G. E. Changes in rapidly transported proteins in developing hamster retinofugal axons. J Neurosci. 1988 Dec;8(12):4445–4454. doi: 10.1523/JNEUROSCI.08-12-04445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya K. L., Benowitz L. I., Sabel B. A., Schneider G. E. Changes in rapidly transported proteins associated with development of abnormal projections in the diencephalon. Brain Res. 1992 Jul 24;586(2):265–272. doi: 10.1016/0006-8993(92)91635-r. [DOI] [PubMed] [Google Scholar]
- Nakamura H., O'Leary D. D. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci. 1989 Nov;9(11):3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary D. D., Bicknese A. R., De Carlos J. A., Heffner C. D., Koester S. E., Kutka L. J., Terashima T. Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harb Symp Quant Biol. 1990;55:453–468. doi: 10.1101/sqb.1990.055.01.045. [DOI] [PubMed] [Google Scholar]
- O'Leary D. D., Terashima T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and "waiting periods". Neuron. 1988 Dec;1(10):901–910. doi: 10.1016/0896-6273(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Munger B. L. The ultrastructure and innervation of rat vibrissae. J Comp Neurol. 1966 Mar;126(3):423–435. doi: 10.1002/cne.901260305. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F., Bossy B., Carbonetto S., de Curtis I., Emmett C., Hall D. E., Ignatius M. J., Lefcort F., Napolitano E., Large T. Neuronal receptors that regulate axon growth. Cold Spring Harb Symp Quant Biol. 1990;55:341–350. doi: 10.1101/sqb.1990.055.01.035. [DOI] [PubMed] [Google Scholar]
- Rice F. L., Mance A., Munger B. L. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986 Oct 8;252(2):154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Luskin M. B. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat's primary visual cortex. J Neurosci. 1986 Dec;6(12):3655–3668. doi: 10.1523/JNEUROSCI.06-12-03655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Placzek M., Lumsden A. G., Dodd J., Jessell T. M. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988 Dec 22;336(6201):775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Yamakado M., Yohro T. Subdivision of mouse vibrissae on an embryological basis, with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. Am J Anat. 1979 Jun;155(2):153–173. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Kurotani T., Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989 Jul 14;245(4914):192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]