Abstract
Understanding the activity of bacteria in coniferous forests is highly important, due to the role of these environments as a global carbon sink. In a study of the microbial biodiversity of montane coniferous forest soil in the Bohemian Forest National Park (Czech Republic), we succeeded in isolating bacterial strain S55T, which belongs to one of the most abundant operational taxonomic units (OTUs) in active bacterial populations, according to the analysis of RNA-derived 16S rRNA amplicons. The 16S rRNA gene sequence analysis showed that the species most closely related to strain S55T include Bryocella elongata SN10T (95.4% identity), Acidicapsa ligni WH120T (95.2% identity), and Telmatobacter bradus TPB6017T (95.0% identity), revealing that strain S55T should be classified within the phylum Acidobacteria, subdivision 1. Strain S55T is a rod-like bacterium that grows at acidic pH (3 to 6). Its phylogenetic, genotypic, phenotypic, and chemotaxonomic characteristics indicate that strain S55T corresponds to a new genus within the phylum Acidobacteria; thus, we propose the name Terracidiphilus gabretensis gen. nov., sp. nov. (strain S55T = NBRC 111238T = CECT 8791T). This strain produces extracellular enzymes implicated in the degradation of plant-derived biopolymers. Moreover, analysis of the genome sequence of strain S55T also reveals the presence of enzymatic machinery required for organic matter decomposition. Soil metatranscriptomic analyses found 132 genes from strain S55T being expressed in the forest soil, especially during winter. Our results suggest an important contribution of T. gabretensis S55T in the carbon cycle in the Picea abies coniferous forest.
INTRODUCTION
The decomposition of organic matter from plant residues in forest soils depends on microbial enzymes and environmental factors, such as temperature, moisture, pH, and soil texture. In fact, the carbon cycle in forest soils is completed principally through lignocellulose-degrading microorganisms present in the soil and guts of animals (1). The main plant cell wall polymers are polysaccharides (cellulose and hemicelluloses) and lignin. The degradation of these polymers requires the activity of extracellular enzymes (cellulases, xylanases, laccases, and peroxidases) produced from fungal and bacterial inhabitants of soil (2–6).
Bacteria belonging to the phylum Acidobacteria are common in the environment. Acidobacteria are difficult to isolate and maintain in culture, but estimations based on culture-independent studies suggest that approximately 20% (7) of the bacteria in soils worldwide belong to this phylum. Particularly, in some acidic soils, members of Acidobacteria might constitute >50% of the total bacterial community (8, 9), although they can also be abundant in more-neutral soils (10–13). Thus, this phylum is as abundant in soils as is Proteobacteria. Subdivision 1 of Acidobacteria contains most of the officially described taxa within this phylum; currently, there are only 21 validated species classified in 7 genera, plus the not-yet-validated proposed new genus and species “Acidipila rosea” (14). Most of these bacteria have been isolated from acidic soils, wetlands, tundra, and peat (15–20). The Acidobacteria phylum was originally described as including four or five subgroups or subdivisions (10, 21). As the number of available 16S rRNA gene sequences increased, the work of Barns et al. (11) expanded the known diversity encompassed within the phylum to the current 26 subdivisions. The abundance of these bacteria in soils suggests that acidobacteria play important roles in soil ecological processes. pH is the most important environmental variable affecting acidobacterial abundance (8). Besides pH, a negative correlation with organic carbon availability and total nitrogen concentration in soils was observed, suggesting an oligotrophic lifestyle of Acidobacteria (22, 23). Oligotrophy and preferential colonization of bulk soil instead of rhizosphere (22) suggest an adaptation to metabolize non-simple carbon compounds. In fact, previous studies using stable isotope probing (SIP) described members of subdivision 1 of the Acidobacteria as cellulose decomposers in soil environments (24, 25). However, difficulties in the isolation of these bacteria in pure culture hinder the study and comparison of their physiologies. On the other hand, the first genomic studies inferred that the metabolic potential encoded in acidobacterial genomes allows the breakdown and utilization of diverse polysaccharides of plant origin and resilience to fluctuating temperatures and nutrient-deficient conditions (26). However, the importance of these strains in terms of abundance and activity in the original environments remains unclear. As part of an increasing effort to isolate new strains of Acidobacteria, the combination of genome sequencing, soil diversity, and functional analysis looks promising in order to obtain deep insight into the ecophysiology of this important and ubiquitous bacterial phylum.
Acidobacteria are highly abundant in the acidic coniferous soils of the mountainous forests of the Bohemian Forest National Park (Czech Republic). We expect that the high abundance of some members of this phylum in these soils (27) indicates their active contribution to soil processes. Here, we characterize the isolated strain S55T, which corresponds to one of the most abundant soil operational taxonomic units (OTUs), according to the analysis of the 16S rRNA gene sequences (27, 28). Based on phenotypic, genotypic, and chemotaxonomic tests, we conclude that the bacterium S55T belongs to a new genus within the phylum Acidobacteria. In the present study, we describe strain S55T, belonging to a species referred to as Terracidiphilus gabretensis gen. nov., sp. nov., and analyze the potential functions of this bacterium in forest soils and nutrient cycles through a multiomics approach.
MATERIALS AND METHODS
Study site, sample collection, and DNA/RNA analyses.
The study area was located in the highest altitudes of the Bohemian Forest National Park, Czech Republic (central Europe; 49°2′38″N, 13°37′2″E), covered by an unmanaged spruce (Picea abies) forest. Sampling for DNA- and RNA-based bacterial community analyses was performed in July 2012 and is the subject of another study (27). The physicochemical parameters properties of the litter (L) and soil (S) material from the study sites were measured and published previously (29). The collection of the L and S material for the isolation of bacteria was performed at the same study sites during the summer period in 2014 (28).
RNA and DNA isolation, metatranscriptome analysis, 16S rRNA amplification, sequencing, and data analysis were carried out as previously described (27). Metatranscriptomic data are available in MG-RAST (MG-RAST project identification number [ID], 4544233.3 [30]). The amplicon sequencing data were processed with SEED 1.2.1 (31), as described in reference 27. DNA sequence abundances were expressed as the relative abundances of the bacterial genomes. To achieve this, the abundance of the sequence belonging to each OTU was divided by the count of 16S rRNA gene sequences in the genome of the most closely related bacterium with a sequenced genome prior to diversity analyses, as described previously (32). The Shannon-Wiener index was calculated using EstimateS 9.1.0 (http://viceroy.eeb.uconn.edu/estimates). The data are available in MG-RAST (MG-RAST project ID, 4603354.3 [30]).
Strain isolation.
The isolation of bacteria from forest topsoil was described by Lladó et al. (28). Briefly, 5 g of soil or litter was diluted with 45 ml of sterile 0.25% Ringer's solution (2.25 g liter−1 NaCl, 0.105 g liter−1 KCl, 0.045 g liter−1 CaCl2, and 0.05 g liter−1 NaHCO3) and agitated for 30 min. The supernatant was serially diluted with sterile 0.25% Ringer's solution to 10−4 and plated on GY-VL55 medium at pH 4.5 (1 g liter−1 glucose, 0.5 g liter−1 yeast extract, and VL55 mineral medium [pH 4.5] [33]) at least in triplicate.
Cultivation proceeded for up to 15 weeks, because long-term culturing was previously demonstrated to yield slow-growing bacteria from nutrient-limiting environments (34). The strains were stored in a sterile 20% glycerol solution at −80°C and subcultured on GY-VL55 agar plates at 24°C.
Genotypic analysis.
Total DNA extraction and 16S rRNA gene amplification and sequencing were performed according to García-Fraile et al. (35) and Rivas et al. (36). The GenBank accession number of the S55T 16S rRNA sequence is KP120762. The nearly complete 16S rRNA gene sequence (1,400 bp) was compared with those available in GenBank using the BLASTn program (37) and EzTaxon tool (38). Phylogenetic analysis of the S55T 16S rRNA gene sequence and the sequences of the type species included in subdivision 1 of the phylum Acidobacteria was performed using the MEGA5 software (39), based on the Clustal W alignment of these sequences (40, 41). The distances were calculated using Kimura's two-parameter model (42), and phylogenetic trees were inferred using maximum likelihood (ML) (43) and neighbor-joining (NJ) (44) analyses.
Chemotaxonomic analysis.
The biomass for the analysis of fatty acid methyl esters (FAME) and respiratory quinones was harvested after cultivation of the strain for 10 days in GY-VL55 liquid mineral medium at 24°C, pH 4.5, and 300 rpm. The culture was centrifuged at 10,000 rpm for 10 min, and the pellet containing the cells was freeze-dried. Analysis of FAME was performed as previously described (45). The extraction and identification of quinones were performed at the identification service of the DSMZ.
Bacterial characterization.
Gram staining was performed using a previously described protocol (46). Motility was studied through phase-contrast and transmission electron microscopy (TEM) after growth in GY-VL55 liquid medium at 24°C for 7 days. Bacterial culture was processed using alcian blue, according to Fassel et al. (47), to preserve the outer envelope structure and extracellular polymeric substances (EPS). In parallel, a classical method based on 3% buffered glutaraldehyde fixation (48) followed by OsO4 postfixation was also performed. The fixed and extensively washed samples were dehydrated through an alcohol series and embedded into Vestopal resin (Sigma-Aldrich). Ultrathin sections were contrasted according to a previously described method (49), and the samples were examined using a Philips CM100 electron microscope (FEI, the Netherlands) at 80 kV. The images were processed using the Analysis 3.2 software (Olympus Soft Imaging Solutions GmbH, Germany). Cell morphology and sporulation were assessed through scanning electron microscopy (SEM). One milliliter of culture was prefixed with 1.5% glutaraldehyde for 1 h. The samples were subsequently washed with cacodylate buffer and fixed with 3% glutaraldehyde in cacodylate buffer overnight at 4°C. After fixation and extensive washing, the cells were sedimented overnight onto poly-l-lysine-treated SPI pore filters (0.2-μm-diameter pore size) or circular coverslips at 4°C. The samples were dehydrated using an alcohol series, followed by absolute acetone and critical point drying from liquid CO2 using a K 850 device (Quorum Technologies Ltd., United Kingdom). The dried samples were sputter coated with 3 nm of platinum in a Q150T ES sputter coater (Quorum Technologies Ltd.) and examined on a FEI Nova NanoSem scanning electron microscope (FEI, USA) at 5 kV using a circular back-scatter detector (CBS).
For the catalase test, bacterial cells from a GY-VL55 agar plate were imbibed into 30% catalase drops and were observed for 5 min to detect the formation of bubbles, indicating a positive result. The oxidase test was performed according to Kovács (50).
GY-VL55 solid medium at pH 4.5 supplemented with 0, 0.1, 0.5, 1, 2.5, 5, and 10% (wt/vol) NaCl was used to assay salt tolerance at 24°C. Plates containing GY-VL55 medium at pH 4.5 were incubated at 4 to 37°C to determine the temperature range for optimal growth. To assay growth at different pH, the same medium with no agar was adjusted to a final pH in the range of 3 to 8; citric acid-sodium citrate and potassium dihydrogen orthophosphate-sodium hydroxide buffers were used to adjust the final pH to 3 to 6 and to 6.5 to 8, respectively. In all cases, the cultures were examined at 5 and 10 days after inoculation.
For the anaerobic growth test, strain S55T was inoculated in GY-VL55 medium at pH 4.5, and the plates were placed into anaerobic jars with the AnaeroGen system (Oxoid) and incubated at 24°C.
The capability of strain S55T to metabolize different carbon sources was evaluated using GY-VL55 at pH 4.5 as the basal medium, in which glucose was replaced by different substrates. Among them, we included important substrates from plant and fungal origins, such as microcrystalline cellulose (Serva), carboxymethyl cellulose (Sigma-Aldrich), xylan from beechwood (Sigma-Aldrich), starch (Sigma-Aldrich), and colloidal chitin prepared according to Nagpure and Gupta (51). The flasks were incubated at 24°C with shaking at 300 rpm for 7 days. Nitrogen sources were tested as described by Pankratov et al. (19). Additionally, the ability of strain S55T to oxidize a set of carbon substrates was assessed with the Biolog system (GN2 plates), according to the instructions provided by the manufacturer.
Enzymatic assays.
Screening for the production of enzymes involved in the degradation of cellulose, hemicellulose, and other polysaccharides and in the acquisition of nitrogen, phosphorus, and sulfur was described by Lladó et al. (28).
Draft genome sequencing and annotation.
The draft genome was sequenced on an Illumina MiSeq platform via a paired-end run (2 × 251 bp). The sequencing yielded 597,340 reads, representing approximately 27-fold coverage. The sequence data were assembled using Velvet 1.2.10 (52), generating 21 contigs of >500 bp (N50, 911,777 bp) representing the T. gabretensis strain S55T genome. Gene calling and annotation were performed using the Integrated Microbial Genome (IMG) system supported through the Department of Energy-Joint Genome Institute Microbial Annotation Pipeline (DOE-JGI MAP). Genome completeness was assessed using the PhyloSift software version 1.0.1 (53). dbCAN (54) was used to identify the coding sequences (CDSs) encoding carbohydrate-active enzymes (CAZYmes).
Subsequently, the draft genome of strain S55T was used to look for gene transcription in the real soil environment. Genome sequences were compared with the soil metatranscriptome using SEED 1.2.1 (31). Transcripts with at least 100 bp and >97% similarity with the genome sequence were considered to belong to strain S55T.
Nucleotide sequence accession numbers.
This whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession no. LAIJ00000000. The version described in this paper is version LAIJ01000000.
RESULTS
Bacterial community composition.
The sequences of the amplicons from total environmental DNA and RNA obtained in the study by Žifčáková et al. (27) clustered into 6,041 OTUs at a 97% similarity threshold, after the exclusion of singletons. The bacterial communities, as expressed by the Shannon-Wiener index, were significantly more diverse (P < 0.001) in the litter than in the soil for both DNA (L, 5.07; S, 4.54) and RNA (L, 5.21; S, 4.99) samples.
Acidobacteria and Proteobacteria were dominant at the DNA level, comprising 70 to 80% of all the bacteria (Fig. 1).
FIG 1.
Phylogenetic composition of bacterial sequences from P. abies forest litter (L) and soil (S). The data represent the mean values for the six study sites and express the estimated relative abundances of bacterial genomes (DNA) and the relative abundance of rRNA (RNA).
Members of 643 genera were found in the soil (S) and litter (L), with most DNA sequences showing closest similarity to the Acidobacteria genera “Candidatus Koribacter” (S, 20.9%; L, 7.2%), Acidipila (S, 5.8%; L, 6.4%), “Candidatus Solibacter” (S, 3.0%; L, 3.5%), Granulicella (S, 1.3%; L, 3.5%), and Telmatobacter (S, 3.1%; L, 2.3%). Several taxa showed a preference for either litter or soil (see Table S1 in the supplemental material).
Bacterial isolation and identification.
A total of 299 isolates were isolated using GY-VL55 agar medium, and 13% belonged to Acidobacteria subdivision 1 (28). Among them, we isolated strain S55T. A comparison of the nearly complete 16S rRNA gene sequence of S55T (1,400 bp) with those obtained from the amplicon libraries derived from DNA and cDNA indicated that this isolate corresponds to one of the most abundant OTUs, B19 (see Table S1 in the supplemental material), suggesting that S55T is an active and abundant bacterium in the P. abies coniferous forest soil. From 6,043 identified OTUs, the OTU B19 was 10th in terms of abundance in soil DNA, 12th in litter DNA, 27th in soil RNA, and 58th in litter RNA (see Table S1).
Analysis of the 16S rRNA gene sequence suggested that this isolate is a member of the phylum Acidobacteria and belongs to the family Acidobacteriaceae, with the most closely related strains being Bryocella elongata SN10T (95.4% identity) and Acidicapsa ligni WH120T (95.4% identity).
Both ML and NJ trees that included all the described species within the family Acidobacteriaceae showed the same phylogenetic clustering, in which strain S55T clusters with Telmatobacter bradus TPB6017T (Fig. 2). Nevertheless, the phylogenetic distances between strain S55T and its closest relative are equivalent to distances between species belonging to different genera within the family, therefore suggesting its classification into a different genus. Thus, the phylogenetic analysis of the 16 rRNA gene sequence supports the classification of S55T within a new genus of subdivision 1 in the phylum Acidobacteria.
FIG 2.
Neighbor-joining phylogenetic tree based on nearly complete (1,400 bp) 16S rRNA gene sequences of all species classified within the family Acidobacteriaceae and the species Blastocatella fastidiosa, which was included as an outgroup. The numbers at the nodes are bootstrap values that indicate the significance of the branches, calculated as a percentage for 1,000 subsets. The circles indicate that the corresponding nodes were also obtained with the maximum-likelihood algorithm. Scale bar = 2 nucleotide (nt) substitutions per 100 nt. Accession numbers are indicated in parentheses.
Colony and cellular morphology.
Strain S55T forms round and convex transparent colonies with entire borders on GY-VL55 medium, and these bacteria are visible after 5 days of incubation at 24°C. Growth was observed at a temperature range of 12 to 30°C, with optimum growth at 20 to 28°C, and at a pH range of 3 to 6, with optimum growth at pH 4 to 5. The growth rate under optimum conditions was 0.46 day−1. The cells are rod shaped, with an external capsule and an extracellular matrix (Fig. 3). Under a phase-contrast microscope, the cells are motile; however, under the culture conditions and with the TEM protocol used in the present study, no flagella were observed.
FIG 3.
Electron TEM and SEM micrographs showing the morphology of T. gabretensis S55T. (A to C) Images of ultrathin sections of strain S55T. The capsule was well preserved using an alcian blue fixation protocol. The arrow in panel B points to the border of the capsule. OM, outer membrane; IM, inner membrane; PS, periplasmic space; Inc, inclusion. (D) Ammonium molybdate negatively stained S55T cells. (E and F) Critical point-dried and platinum-coated S55T cells imaged using back-scattered electron in high-resolution SEM. Scale bars, 0.2 µm (A, B, and C) and 0.5 µm (D, E, and F).
Phenotypic and chemotaxonomic characterizations.
The major fatty acids of strain S55T included iso-C15:0 (61.2%), which is also the main fatty acid in most of the other members of the family Acidobacteriaceae, along with C15:0 (11.9%), C18:0 (8.6%), and C16:1ω7 (8.2%). The analysis of isoprenoid quinones revealed menaquinone-8 (MK-8) to be the sole respiratory quinone in S55T, which is also the respiratory quinone of other members of subdivision 1 of the phylum Acidobacteria.
The main phenotypic features analyzed in strain S55T are detailed in the species description. The main differences with the closest related genera and all other genera of subdivision 1 of the phylum Acidobacteria are listed in Table 1 and in Table S2 in the supplemental material, respectively.
TABLE 1.
Differential characteristics of strain S55T and the most closely related speciesa
| Characteristic | S55T | T. bradus TPB6017T | B. elongata SN10T | A. ligni WH120T |
|---|---|---|---|---|
| Growth in anaerobiosis | − | + | − | − |
| Catalase | − | w | + | + |
| Motility | + | + | − | − |
| External EPS capsule | + | − | + | + |
| Pigment | None | None | Light pink | None |
| Growth at: | ||||
| pH ≥7 | − | + | − | + |
| pH 3 | + | + | − | − |
| 4°C | − | + | − | − |
| 35°C | − | + | − | − |
| Inhibition by 0.1% NaCl | + | + | − | − |
| Anaerobic growth | − | + | − | − |
| Cellulose degradation | + | + | − | − |
| C15:0 as a major fatty acid | + | − | − | − |
| C18:0 as a major fatty acid | + | − | − | − |
| Assimilated carbon sources | ||||
| Ethanol | + | − | + | − |
| Glycerol | + | ND | − | − |
| Mannitol | + | − | − | |
| Methanol | + | − | − | − |
| Sorbitol | + | − | − | |
| Starch | + | + | − | + |
| Xylan | + | + | − | − |
| Cellulose | + | + | − | − |
| Carboxymethyl cellulose | + | − | − | − |
| Chitin | + | − | − | − |
| Assimilated nitrogen sources | ||||
| Arginine | + | − | ND | ND |
| Ammonium | − | + | ND | ND |
In vitro enzymatic activity detection.
Strain S55T growing in GY-VL55 (pH 4.5) medium produced enzymes involved in the degradation of polysaccharides of plant and fungal origins (see Table S3 in the supplemental material). The assays were positive for enzymes involved in the degradation of cellulose (β-glucosidase and cellobiohydrolase), hemicellulose (β-glucuronidase, β-xylosidase, α-arabinosidase, and β-galactosidase), starch (α-glucosidase), and chitin (N-acetylglucosaminidase).
Genome properties.
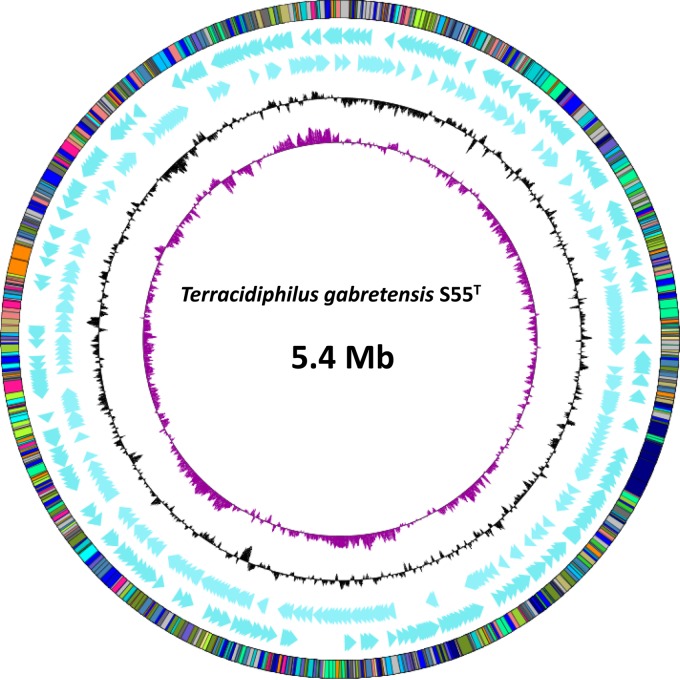
The total combined contig size of the T. gabretensis S55T genome was 5,351,935 bp. Genome completeness was assessed by searching for a list of 40 highly conserved single-copy-marker genes. All of them were found after the assembly. The annotation process predicted 4,610 coding sequences (CDSs) within the genome, of which 73.5% were assigned to known functional genes (Table 2). The distribution of the genes into functional categories of clusters of orthologous groups (COGs) is shown in Fig. 4 and Table 3.
TABLE 2.
Genome properties of T. gabretensis S55T
| Attribute | Value | % of total |
|---|---|---|
| Genome size (bp) | 5,351,935 | 100 |
| DNA coding (bp) | 4,670,294 | 87.3 |
| DNA G+C content (bp) | 3,066,659 | 57.3 |
| No. of replicons | 1 | 100 |
| Total no. of genes | 4,610 | 100 |
| No. of RNA genes | 48 | 1.04 |
| No. of tRNA genes | 46 | 1.00 |
| No. of protein-coding genes | 4,562 | 98.9 |
| No. of genes with function prediction | 3,390 | 73.5 |
| No. of genes assigned to COGs | 2,481 | 53.8 |
FIG 4.
Graphical representation of the circular map of the chromosome of T. gabretensis S55T. From outside to center: COG categories of the annotated genes, genes on forward strand (blue), genes on reverse strand (blue), G+C content, and GC skew.
TABLE 3.
Number of genes associated with general COG functional categories in the T. gabretensis S55T genome
| COG category | Code | No. of genes | % of total genes |
|---|---|---|---|
| Amino acid transport and metabolism | E | 224 | 6.6 |
| Carbohydrate transport and metabolism | G | 227 | 6.7 |
| Cell cycle control, cell division, and chromosome partitioning | D | 29 | 0.9 |
| Cell motility | N | 54 | 1.6 |
| Cell wall/membrane/envelope biogenesis | M | 193 | 5.7 |
| Coenzyme transport and metabolism | H | 141 | 4.2 |
| Cytoskeleton | Z | 1 | 0.03 |
| Defense mechanisms | V | 144 | 4.2 |
| Energy production and conversion | C | 143 | 4.2 |
| Extracellular structures | W | 14 | 0.4 |
| Function unknown | S | 135 | 4.0 |
| General function prediction only | R | 317 | 9.4 |
| Inorganic ion transport and metabolism | P | 91 | 2.7 |
| Intracellular trafficking, secretion, and vesicular transport | U | 58 | 1.7 |
| Lipid transport and metabolism | I | 116 | 3.4 |
| Mobilome: prophages and transposons | X | 19 | 0.6 |
| Nucleotide transport and metabolism | F | 74 | 2.2 |
| Posttranslational modification, protein turnover, and chaperones | O | 107 | 3.2 |
| Replication, recombination, and repair | L | 80 | 2.4 |
| Secondary metabolite biosynthesis, transport, and catabolism | Q | 80 | 2.4 |
| Signal transduction mechanisms | T | 125 | 3.7 |
| Transcription | K | 211 | 6.2 |
| Translation, ribosomal structure, and biogenesis | J | 175 | 5.2 |
| Not in COGs | 632 | 18.6 |
Analysis of the genome using dbCAN showed 346 genes encoding different carbohydrate-active enzymes (CAZYmes) involved in the decomposition of cellulose, hemicellulose, starch, pectin, and chitin. Among them, we identified gene modules belonging to five different enzyme classes of glycoside hydrolases (GHs) (n = 168), glycosyltransferases (GTs) (n = 61), polysaccharide lyases (PLs) (n = 10), carbohydrate esterases (CEs) (n = 64), auxiliary activities (AAs) (n = 10), and noncatalytic carbohydrate-binding modules (CBMs) (n = 33). These genes belonged to 56 families of GHs, 23 GTs, 3 PLs, 12 CEs, 3 AAs, and 13 CBMs (see Table S4 in the supplemental material).
We identified a large number of CDSs belonging to eight different GH families representing endocellulases (GH5, GH8, GH9, GH10, GH26, GH44, GH51, and GH74) and six GH families representing β-glucosidases (GH1, GH3, GH5, GH9, GH30, and GH116). CDSs encoding different CBMs were also identified (see Table S3 in the supplemental material).
We identified CDSs encoding 10 different families of endoxylanases (GH5, GH8, GH9, GH10, GH16, GH26, GH30, GH43, GH44, and GH51), nine different families of exoxylanases (GH1, GH3, GH5, GH30, GH39, GH43, GH51, GH54, and GH116), and seven families of xylan esterases (CE1, CE2, CE3, CE4, CE6, CE7, and CE15).
We also detected six GH families with α-l-arabinosidase activity (GH2, GH3, GH10, GH43, GH51, and GH54) and CDSs encoding α-xylosidase (GH31), α-fucosidase (GH29 and GH95), α-1-4-galactosidase (GH27, GH36, GH57, and GH97), and β-1-4-galactosidase (GH1, GH2, GH3, GH35, and GH42). Moreover, CDSs for GH families with β-mannosidase activity were also identified (GH1, GH2, and GH5).
Additionally, in the genome of T. gabretensis S55T, we detected CDSs for different GH families with enzymatic activities, such as polygalacturonases (GH28), α-rhamnosidase (GH13, GH78, and GH106), endoarabinanase (GH43), unsaturated rhamnogalacturonyl hydrolase (GH105), pectate lyase (PL9), pectin methylesterase (CE8), feruloyl esterase (CE1), α-amylase (GH13 and GH57), β-amylase (GH13), α-1-4-glucosidase (GH13, GH31, GH63, and GH97), glucoamylase (GH15 and GH97), and chitinase (GH18 and GH23).
Gene expression in soil.
One hundred thirty-two different putative transcripts belonging to S55T were detected in the metatranscriptome of litter and soil sampled during summer and winter (27). The annotated sequences encode proteins involved in a wide range of cellular functions (see Table S5 in the supplemental material). T. gabretensis transcripts related to cellular processes and signaling, information storage, processing, and metabolism were found in the soil and litter. However, 45 sequences were annotated as encoding hypothetical proteins. S55T gene transcription was significantly higher in both soil horizons in winter samples (P < 0.05).
TAXONOMY
Description of Terracidiphilus gen. nov.
Terracidiphilus (Ter.ra.ci.di.phi'lus L. n. terra, earth; L. adj. acidus, sour, acid; N.L. adj. philus -a -um [from Gr. adj. philos -ê -on], friend, loving; N.L. masc. n. Terracidiphilus, bacteria from soil-loving acid conditions).
Gram-negative, motile, non-spore-forming, with short and ovoid rod-shaped cells. Strictly aerobic. Catalase negative and oxidase negative. Acidophilic and mesophilic. The respiratory quinone is MK-8. Main fatty acids are iso-C15:0, C15:0, C18:0, and C16:1ω7. The G+C content of genomic DNA of the type strain of the type species is 57.3 mol%. The type species is T. gabretensis.
Description of T. gabretensis sp. nov.
T. gabretensis (ga.bret.en'sis N.L. masc. adj. gabretensis, pertaining to Gabreta, the Celtic name of the Bohemian Forest, the mountain range in central Europe, where the type strain was isolated). The description is as for the genus given above, but with the following further characteristics: cells are 0.6 to 1.2 μm in length and 0.5 to 0.8 μm in diameter. Colonies are transparent, circular, and convex, reaching 0.5 to 1 mm in diameter within 7 days of growth at 24°C in GY-VL55. Bacterium with temperature growth ranges of 12 to 30°C and pH 3 to 6 and unable to grow in 0.5% NaCl in GY-VL55 medium. Optimal growth occurs at 20 to 24°C and pH 4 to 5. Uses d-glucose, l-arabinose, xylose, d-mannose, d-galactose, d-cellobiose, d-sorbitol, d-mannitol, glycerol, ethanol, methanol, tartrate, histidine, Casamino Acids, microcrystalline cellulose, carboxymethyl cellulose, xylan, starch, and colloidal chitin as single carbon and energy sources and does not use glutamate. In Biolog, the assimilation of N-acetyl-d-glucosamine, amygdalin, arbutin, d-cellobiose, α-cyclodextrin, dextrin, d-fructose, l-fucose, d-galactose, gentiobiose, d-gluconic acid, d-glucosamine, α-d-glucose, glucose-1-phosphate, glycerol, glycogen, maltitol, maltose, maltotriose, d-mannitol, d-mannose, d-melezitose, d-melibiose, α-methyl-d-galactoside, α-methyl-d-glucoside, β-methyl-d-glucoside, palatinose, d-psicose, d-raffinose, salicin, d-sorbitol, l-sorbose, stachyose, sucrose, d-trehalose, turanose, fumaric acid, β-hydroxyl-butyric acid, p-hydroxyphenyl-acetic acid, l-malic acid, succinamic acid monomethyl ester, alaninamide, l-alanine, l-alanyl-glycine, l-threonine, 2-amino ethanol, adenosine, uridine, and adenosine-5′-monophosphate was positive. Nitrogen sources utilized are peptone, yeast extract, Casamino Acids, and arginine. The enzymatic in vitro assays were positive for β-glucosidase, cellobiohydrolase, β-glucuronidase, β-xylosidase, α-arabinosidase, β-galactosidase, α-glucosidase, and N-acetylglucosaminidase activities.
The G+C base composition was 57.3 mol%. The type strain S55T (NBRC 111238T, CECT 8791T) was isolated from coniferous forest soil in the Bohemian Forest National Park (Czech Republic).
DISCUSSION
The recalcitrance to culture of Acidobacteria hampers the understanding of the role of these bacteria in global biogeochemical cycles (55, 56). In addition, it is difficult to conclude the importance of acidobacterial strains in actual ecological processes without assessing the abundance and activity of these bacteria in the environment. This fact highlights the importance of determining the role of these bacteria in ecological processes occurring in the soil, such as the decomposition of organic matter derived from plant residues and fungal mycelia.
In the present study, we isolated T. gabretensis S55T from coniferous forest soil in the Czech Republic; this bacterium belongs to one of the most abundant and active OTUs in the P. abies forest soil bacterial community. To understand how T. gabretensis S55T participates in the recycling of soil organic matter, we assayed the hydrolysis of organic compounds likely present in the soil and identified the genes encoding the corresponding enzymes in the annotated draft genome. The T. gabretensis S55T genome is 5.4 Mb, a regular size for a bacterium belonging to subdivision 1 of the phylum Acidobacteria (55). The members of subdivision 1 possess genomes ranging from 2.0 to 6.2 Mb (56). Our results showed that T. gabretensis strain S55T exhibited genetic potential and produced the required enzymatic machinery in vitro for the active decomposition of biopolymers. Although the use of in vitro enzyme activities as indicators of soil functionality is still a problem when extrapolating the results to real soil environments (57), the production of β-glucosidase, cellobiohydrolase, and N-acetylglucosaminidase at high rates, the growth with microcrystalline cellulose, carboxymethyl cellulose, chitin, xylan, and starch as sole sources of carbon, and the concomitant presence of genes related to cellulose, hemicellulose, and chitin degradation, indicate the metabolic capability to degrade plant-derived oligosaccharides and chitin, the major component of fungal cell walls. The ability to degrade these polymeric compounds might allow the Acidobacteria to displace other bacterial species that are unable to use these substrates, particularly at low concentrations (55).
Organic matter decomposition in the globally widespread coniferous forests plays an important role in biogeochemical cycles (24). Decomposition of cellulose and hemicellulose is particularly important in this respect, because these compounds are the most abundant structural components of plants. The genome analysis of T. gabretensis S55T showed the high abundance of genes assigned to the transport and metabolism of carbohydrates and amino acids, gene transcription, and envelope biogenesis. Similar to other Acidobacteria subdivision 1 genomes (56), the genome of T. gabretensis S55T harbors the genes involved in nutrient utilization and biogenesis to maintain cell functionality in cold environments. Further analysis revealed the high abundance of catalytic and noncatalytic carbohydrate-binding modules (CBMs) within the genome of T. gabretensis S55T. The genes encoding carbohydrate-active enzymes (CAZYmes) represent 7.6% of the total CDSs identified in the genome, representing the highest proportion of CAZYmes described for a member of the phylum Acidobacteria (26). Among them, we identified genes related to the degradation, modification, and synthesis of glycosidic bonds.
The complete degradation of cellulose to glucose involves three types of cellulases: endoglucanases, exoglucanases/cellobiohydrolases, and β-glucosidases. Cellulases have been identified in 18 GH families (58). The large number of CDSs belonging to GH families representing endocellulases, β-glucosidases, and CBMs found in the genome of T. gabretensis S55T, along with the ability to grow with carboxymethyl cellulose and microcrystalline cellulose as sole C sources and the in vitro production of β-glucosidase and cellobiohydrolase, confirmed its metabolic potential to degrade cellulose. These results are coincident with the identification of members of subdivision 1 of the Acidobacteria as cellulose decomposers in soil environments through SIP experiments (24, 25). In another study, the cellulolytic potential of a bacterial isolate belonging to subdivision 1 of the Acidobacteria was recognized by fluorescence in situ hybridization (FISH), using an acidobacterium-specific probe. Microcolonies of these bacteria were observed developing next to cellulose microfibrils (59).
The complete degradation of hemicellulose requires at least nine different enzyme activities encompassed within many different GH and CE families (60). GH families containing endoxylanases and exoxylanases, enzymes that catalyze the cleavage of xylan molecules into oligosaccharides and eventually into xylose monomers, were highly represented in the genome of T. gabretensis S55T. In addition, xylan esterases catalyze the hydrolysis of acetyl groups from polymeric xylan, acetylated xylose, and other compounds present in hemicellulose. α-l-Arabinosidase is required for the hydrolysis of the α-l-arabinose in xylan-rich hemicelluloses. Moreover, α-xylosidase, α-fucosidase, α-1-4-galactosidase, and β-1-4-galactosidase are necessary for the degradation of xyloglucan-based hemicelluloses. β-Mannosidase activity is required for the cleavage of the β-mannan-based hemicelluloses. In contrast to other acidobacterial strains with a large number of CDSs encoding hemicellulolytic enzymes (26), T. gabretensis S55T was able to grow with xylan as a carbon source in the in vitro assays; we observed β-glucuronidase, β-xylosidase, α-arabinosidase, and β-galactosidase activities to be associated with hemicellulose degradation and also CDSs encoding different CBMs involved in xylan degradation.
Pectin is a structural heteropolysaccharide contained in the cell walls of plants in addition to cellulose and hemicellulose. The degradation of pectin involves enzymes corresponding to a diverse set of GH, PL, and CE families. In the genome of T. gabretensis S55T, we detected CDSs for different GH families containing enzymatic activities associated with pectin degradation, such as polygalacturonases, α-rhamnosidase, endoarabinanase, and unsaturated rhamnogalacturonyl hydrolase. Furthermore, we also identified CDSs for pectate lyase (PL9), pectin methylesterase (CE8), and feruloyl esterase (CE1). The presence of genes encoding pectin-degrading enzymes is a common trait in members of subdivision 1 of the Acidobacteria (26, 55).
Starch is a polysaccharide produced in plants as energy stores. In the genome of T. gabretensis S55T, we identified CDSs encoding GH families encompassing enzymatic activities necessary for the hydrolysis of starch: α-amylase, β-amylase, α-1-4-glucosidase, and glucoamylase.
Chitin is the characteristic component of the cell walls of fungi and exoskeletons of arthropods and insects. Chitin in fungi is highly abundant and active in the litter and soil of coniferous forests (27). Chitinases hydrolyze the β-1-4-linkages between N-acetyl-d-glucosamine molecules. We identified CDSs for GH18 and GH23 chitinases in the genome of T. gabretensis S55T. Similar to other Acidobacteria, we did not identify any chitosanases (GH46) that hydrolyze chitosan (26). We also identified CDSs for CBM13, CBM35, and CBM50 with chitin-binding functions. T. gabretensis S55T was able to grow with chitin and starch as carbon sources, and we identified α-glucosidase and N-acetylglucosaminidase activity associated with the degradation of starch and chitin, respectively. In conclusion, the results of carbon source utilization, in vitro enzymatic assays, and the identification of genes encoding the enzymes for the degradation of cellulose, hemicellulose, pectin, starch, and chitin revealed the potential role of T. gabretensis S55T in the decomposition of plant and fungal biomass present in the P. abies forest soil and, therefore, in the soil recycling process.
Although the metabolic potential can be demonstrated through the techniques used in the present study, limitations in DNA-based studies and in vitro analyses make it impossible to be certain of the involvement of T. gabretensis in the ecological processes carried out in the real soil environment. In order to obtain deep insight into the in situ activity of T. gabretensis, we used metatranscriptomic data from summer and winter samples. We found transcripts belonging to 132 different genes present in the T. gabretensis draft genome in the soil metatranscriptome. This fact establishes that the T. gabretensis metabolism is active in summer and winter in both soil horizons. Despite the limitations in the gene annotation, we found T. gabretensis transcripts related to energy production, carbohydrate and amino acid metabolism, and inorganic ion transport. These findings indicate again a real activity of T. gabretensis S55T in organic matter decomposition in the P. abies coniferous forest soil. Finally, is important to highlight that the gene transcription of T. gabretensis is 10-fold higher in the winter than in the summer. This observation may be related to the ecological strategy of this acidobacterium. During winter, easily available nutrients from root exudates or litter decomposition are scarce (27). It has been hypothesized that K-strategists, such as Acidobacteria, arise when a higher proportion of more recalcitrant compounds remains in the soil (61). The presence of 1 rRNA gene copy in the genome of T. gabretensis S55T is consistent with other members of Acidobacteria subdivision 1 and has been postulated to be a marker of slow growth and a K-selective lifestyle (55). This fact, together with the presence of transcripts related to cold shock proteins, might be an explanation for the increase in T. gabretensis metabolism during the winter. In conclusion, this study highlights the necessity of sequencing the genomes of important environmental strains to try to understand their ecology. The combination of in vitro assays and especially the use of genomic and metatranscriptomic data is a powerful method for determining the microbial ecology in soil environments.
Regarding the taxonomic study of this strain, the presence of MK-8 as a major quinone and iso-C15:0 as the main fatty acid supports the classification of S55T in subdivision 1 of the phylum Acidobacteria. However, the results of the phylogenetic analysis clearly confirm the classification of strain S55T in a new genus within the phylum Acidobacteria. Moreover, the isolate presents several phenotypic differences with the closest related genera. Unlike Telmatobacter, Bryocella, and Acidicapsa, strain S55T is capable of using methanol, carboxymethyl cellulose, and chitin as sole carbon sources and is catalase negative. In contrast to Acidicapsa and Telmatobacter, Terracidiphilus is able to grow using alcohols, like ethanol, mannitol, and sorbitol, as sole carbon sources; also, Bryocella has been reported to be unable to hydrolyze glycerol. Unlike Telmatobacter, strain S55T uses arginine as an N source but does not use ammonium; Terracidiphilus is unable to grow at pH 7 and produces large amounts of external exopolysaccharide (EPS). Other phenotypic differences between our isolate and the genera Bryocella and Acidicapsa are the ability of S55T to grow at pH 3 and degrade cellulose.
Therefore, taking into account all the phylogenetic, chemotaxonomic, and phenotypic data, strain S55T should be assigned to a novel species of the class Acidobacteria, order Acidobacteriales, and family Acidobacteriaceae, for which the name T. gabretensis gen. nov., sp. nov., is proposed.
Supplementary Material
Funding Statement
Additionally, the Ministry of Education, Youth and Sports of the Czech Republic provided funding to Paula García-Fraile under project CZ.1.07/2.3.00/30.0003. The Ministry of Education, Youth and Sports of the Czech Republic (project number LO1509) and the Operational Program Prague—Competitiveness (project CZ.2.16/3.1.00/24023) supported Oldrich Benada.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03353-15.
REFERENCES
- 1.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns RG. 1978. Soil enzymes. Academic Press, London, United Kingdom. [Google Scholar]
- 3.Baldrian P. 2006. Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 4.Osono T. 2007. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22:955–974. doi: 10.1007/s11284-007-0390-z. [DOI] [Google Scholar]
- 5.Wilson DB. 2008. Three microbial strategies for plant cell wall degradation, p 289–297. In Wiegel J, Maier RJ, Adams MWW (ed), Incredible anaerobes: from physiology to genomics to fuels. Blackwell, Oxford, United Kingdom. [DOI] [PubMed] [Google Scholar]
- 6.Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A. 2013. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234. doi: 10.1016/j.soilbio.2012.11.009. [DOI] [Google Scholar]
- 7.Janssen PH. 2006. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N. 2009. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453. doi: 10.1038/ismej.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Männistö MK, Tiirola M, Häggblom MM. 2007. Bacterial communities in Arctic fields of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol Ecol 59:452–465. doi: 10.1111/j.1574-6941.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuske CR, Barns SM, Busch JD. 1997. Diverse uncultivated bacterial groups from soils of the arid southwestern United States that are present in many geographic regions. Appl Environ Microbiol 63:3614–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barns SM, Takala SL, Kuske CR. 1999. Wide distribution and diversity of members of the bacterial kingdom Acidobacterium in the environment. Appl Environ Microbiol 65:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar J, Takala S, Barns SM, Davis JA, Kuske CR. 1999. Levels of bacterial community diversity in four arid soils compared by cultivation and 16S rRNA gene cloning. Appl Environ Microbiol 65:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunbar J, Barns SM, Ticknor LO, Kuske CR. 2002. Empirical and theoretical bacterial diversity in four Arizona soils. Appl Environ Microbiol 68:3035–3045. doi: 10.1128/AEM.68.6.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura K, Kawai A, Yamada T, Hiraishi A. 2011. Acidipila rosea gen. nov., sp. nov., an acidophilic chemoorganotrophic bacterium belonging to the phylum Acidobacteria. FEMS Ecol Lett 317:138–142. [DOI] [PubMed] [Google Scholar]
- 15.Koch IH, Gich F, Dunfield PF, Overmann J. 2008. Edaphobacter modestus gen. nov., sp. nov., and Edaphobacter aggregans sp. nov., acidobacteria isolated from alpine and forests soils. Int J Syst Evol Microbiol 58:1114–1122. [DOI] [PubMed] [Google Scholar]
- 16.Pankratov TA, Dedysh SV. 2010. Granulicella paludicola gen. nov., sp. nov., Granulicella pectinivorans sp. nov., Granulicella aggregans sp. nov., and Granulicella rosea sp. nov., acidophilic, polymer-degrading acidobacteria from Sphagnum peat bogs. Int J Syst Evol Microbiol 60:2951–2959. [DOI] [PubMed] [Google Scholar]
- 17.Dedysh SN, Kulichevskaya IS, Serkebaeva YM, Mityaeva MA, Sorokin VV, Suzina NE, Rijpstra WIC, Sinninghe Damsté JS. 2008. 2011. Bryocella elongata gen. nov., sp. nov., a member of subdivision 1 of the Acidobacteria isolated from a methanotrophic enrichment culture, and emended description of Edaphobacter aggregans Koch et al. Int J Syst Evol Microbiol 62:654–664. doi: 10.1099/ijs.0.031898-0. [DOI] [PubMed] [Google Scholar]
- 18.Männistö MK, Rawat S, Starovoytov V, Häggblom MM. 2012. Granulicella arctica sp. nov., Granulicella mallensis sp. nov., Granulicella tundricola sp. nov., and Granulicella sapmiensis sp. nov., novel acidobacteria from tundra soil. Int J Syst Evol Microbiol 62:2097–2106. doi: 10.1099/ijs.0.031864-0. [DOI] [PubMed] [Google Scholar]
- 19.Pankratov TA, Kirsanova LA, Kaparullina EN, Kevbrin VV, Dedysh SN. 2012. Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. Int J Syst Evol Microbiol 62:430–437. doi: 10.1099/ijs.0.029629-0. [DOI] [PubMed] [Google Scholar]
- 20.Kulichevskaya IS, Kostina LA, Valášková V, Rijpstra WIC, Sinninghe Damsté JS, de Boer W, Dedysh SN. 2012. Acidicapsa borealis gen. nov., sp. nov. and Acidicapsa ligni sp. nov., subdivision 1 Acidobacteria from Sphagnum peat and decaying wood. Int J Syst Evol Microbiol 62:1512–1520. doi: 10.1099/ijs.0.034819-0. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig W, Bauer SH, Bauer M, Held I, Kirchhof G, Schulze R, Huber I, Spring S, Hartmann A, Schleifer KH. 1997. Detection and in situ identification of representatives of a widely distributed new bacterial phylum. FEMS Microbiol Lett 153:181–190. doi: 10.1111/j.1574-6968.1997.tb10480.x. [DOI] [PubMed] [Google Scholar]
- 22.Fierer N, Morse JL, Berthrong ST, Bernhardt ES, Jackson RB. 2007. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 88:2162–2173. doi: 10.1890/06-1746.1. [DOI] [PubMed] [Google Scholar]
- 23.Foesel BU, Nägele V, Naether A, Wüst PK, Weiner J, Bonkowski M, Lohaus G, Polle A, Alt F, Oelmann Y, Fischer M, Friedrich MW, Overmann J. 2014. Determinants of Acidobacteria activity inferred from the relative abundances of 16S rRNA transcripts in German grasslands and forest soils. Environ Microbiol 16:658–675. doi: 10.1111/1462-2920.12162. [DOI] [PubMed] [Google Scholar]
- 24.Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P. 2012. Cellulose utilization in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microbiol Ecol 80:735–746. doi: 10.1111/j.1574-6941.2012.01343.x. [DOI] [PubMed] [Google Scholar]
- 25.Eichorst SA, Kuske CR. 2012. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl Environ Microbiol 78:2316–2327. doi: 10.1128/AEM.07313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawat SR, Männistö MK, Bromberg Y, Häggblom MM. 2012. Comparative genomic and physiological analysis provide insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol Ecol 82:341–355. doi: 10.1111/j.1574-6941.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- 27.Žifčáková L, Větrovský T, Howe A, Baldrian P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol, in press. doi: 10.1111/1462-2920.13026. [DOI] [PubMed] [Google Scholar]
- 28.Lladó S, Žifčáková L, Větrovský T, Eichlerová I, Baldrian P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol Fertil Soils, in press. doi: 10.1007/s00374-015-1072-6. [DOI] [Google Scholar]
- 29.Baldrian P, Kolařík M, Štursová M, Kopecký J, Valášková V, Větrovský T, Žifčáková L, Šnajdr J, Rídl J, Vlček C, Voříšková J. 2012. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J 6:248–258. doi: 10.1038/ismej.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA. 2008. The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Větrovský T, Baldrian P. 2013. Analysis of soil fungal communities by amplicon pyrosequencing: current approaches to data analysis and the introduction of the pipeline SEED. Biol Fertil Soils 49:1027–1037. [Google Scholar]
- 32.Větrovský T, Baldrian P. 2013. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS One 8:e57923. doi: 10.1371/journal.pone.0057923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sait M, Hugenholtz P, Janssen PH. 2002. Cultivation of globally distributed soil bacteria from phylogenetic lineages previously only detected in cultivation-independent surveys. Environ Microbiol 4:654–666. doi: 10.1046/j.1462-2920.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 34.George IF, Hartmann M, Liles MR, Agathos SN. 2011. Recovery of as-yet-uncultured soil Acidobacteria on dilute soil media. Appl Environ Microbiol 77:8184–8188. doi: 10.1128/AEM.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Fraile P, Chudíčková M, Benada O, Pikula J, Kolařík M. 2015. Serratia myotis sp. nov. and Serratia vespertilionis sp. nov., isolated from bats hibernating in caves in the Czech Republic. Int J Syst Evol Microbiol 65:90–94. doi: 10.1099/ijs.0.066407-0. [DOI] [PubMed] [Google Scholar]
- 36.Rivas R, García-Fraile P, Mateos PF, Martínez-Molina E, Velázquez E. 2007. Characterization of xylanolytic bacteria present in the bract phyllosphere of the date palm Phoenix dactylifera. Lett Appl Microbiol 44:181–187. doi: 10.1111/j.1472-765X.2006.02050.x. [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 42.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 43.Rogers JS, Swofford DL. 1998. A fast method for approximating maximum likelihoods of phylogenetic trees from nucleotide sequences. Syst Biol 47:77–89. doi: 10.1080/106351598261049. [DOI] [PubMed] [Google Scholar]
- 44.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetics trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 45.Šnajdr J, Valášková V, Merhautová V, Herinková J, Cajthaml T, Baldrian P. 2008. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40:2068–2075. doi: 10.1016/j.soilbio.2008.01.015. [DOI] [Google Scholar]
- 46.Doetsch RN. 1981. Determinative methods of light microscopy, p 21–33. In Gerdhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Phillips GB (ed), Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC. [Google Scholar]
- 47.Fassel TA, Schaller MJ, Remsen CC. 1992. Comparison of alcian blue and ruthenium red effects on preservation of outer envelope ultrastructure in methanotrophic bacteria. Microsc Res Tech 20:87–94. doi: 10.1002/jemt.1070200109. [DOI] [PubMed] [Google Scholar]
- 48.Kellenberger E, Sechaud J, Ryter A. 1959. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology 8:478–498. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds ES. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovács N. 1956. Eine vereinfachte Methode zum Nachweis der indolbildung durch Bakterien. Z Immunitätsforschung 55:311–315. [Google Scholar]
- 51.Nagpure A, Gupta RK. 2013. Purification and characterization of an extracellular chitinase from antagonistic Streptomyces violaceusniger. J Basic Microbiol 53:429–439. doi: 10.1002/jobm.201100648. [DOI] [PubMed] [Google Scholar]
- 52.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darling AE, Jospin G, Lowe E, Matsen FA IV, Bik HM, Eisen JA. 2014. PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 2:e243. doi: 10.7717/peerj.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin YB, Mao XZ, Yang JC, Chen X, Mao FL, Xu Y. 2012. dbCAN: a Web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 40:W445–W451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, Barabote RD, Bradley B, Brettin TS, Brinkac LM, Bruce D, Creasy T, Daugherty SC, Davidsen TM, DeBoy RT, Detter JC, Dodson RJ, Durkin AS, Ganapathy A, Gwinn-Giglio M, Han CS, Khouri H, Kiss H, Kothari SP, Madupu R, Nelson KE, Nelson WC, Paulsen I, Penn K, Ren Q, Rosovitz MJ, Selengut JD, Shrivastava S, Sullivan SA, Tapia R, Thompson LS, Watkins KL, Yang Q, Yu C, Zafar N, Zhou, Kuske CR. 2009. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microbiol 75:2046–2056. doi: 10.1128/AEM.02294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Challacombe JF, Eichorst SA, Hauser L, Land M, Xie G, Kuske CR. 2011. Biological consequences of ancient gene acquisition and duplication in the large genome of “Candidatus Solibacter usitatus” Ellin6076. PLoS One 6:e24882. doi: 10.1371/journal.pone.0024882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S. 2012. Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762. doi: 10.1007/s00374-012-0723-0. [DOI] [Google Scholar]
- 58.Wang M, Liu K, Dai L, Zhang J, Fang X. 2013. The structural and biochemical basis for cellulose biodegradation. J Chem Technol Biotechnol 88:491–500. doi: 10.1002/jctb.3987. [DOI] [Google Scholar]
- 59.Pankratov TA, Ivanova AO, Dedysh SN, Llesack W. 2011. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ Microbiol 13:1800–1814. doi: 10.1111/j.1462-2920.2011.02491.x. [DOI] [PubMed] [Google Scholar]
- 60.van den Brink J, de Vries RP. 2011. Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1149. doi: 10.1007/s00253-011-3473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontaine S, Mariotti A, Abbadie L. 2003. The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. doi: 10.1016/S0038-0717(03)00123-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.