Abstract
Minimally processed fresh produce has been implicated as a major source of foodborne microbial pathogens globally. These pathogens must attach to the produce in order to be transmitted. Cut surfaces of produce that expose cell walls are particularly vulnerable. Little is known about the roles that different structural components (cellulose, pectin, and xyloglucan) of plant cell walls play in the attachment of foodborne bacterial pathogens. Using bacterial cellulose-derived plant cell wall models, we showed that the presence of pectin alone or xyloglucan alone affected the attachment of three Salmonella enterica strains (Salmonella enterica subsp. enterica serovar Enteritidis ATCC 13076, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, and Salmonella enterica subsp. indica M4) and Listeria monocytogenes ATCC 7644. In addition, we showed that this effect was modulated in the presence of both polysaccharides. Assays using pairwise combinations of S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 showed that bacterial attachment to all plant cell wall models was dependent on the characteristics of the individual bacterial strains and was not directly proportional to the initial concentration of the bacterial inoculum. This work showed that bacterial attachment was not determined directly by the plant cell wall model or bacterial physicochemical properties. We suggest that attachment of the Salmonella strains may be influenced by the effects of these polysaccharides on physical and structural properties of the plant cell wall model. Our findings improve the understanding of how Salmonella enterica and Listeria monocytogenes attach to plant cell walls, which may facilitate the development of better ways to prevent the attachment of these pathogens to such surfaces.
INTRODUCTION
According to the World Health Organization (WHO), almost 1.5 million cases of human salmonellosis are reported globally every year (1–3). Fresh produce is an important vehicle for the transmission of human pathogens and a major source of foodborne microbial outbreaks worldwide. While Salmonella enterica serovar Enteritidis and Salmonella enterica serovar Typhimurium were historically linked to the majority of the outbreaks from food of animal origin, a number of Salmonella serovars have now been strongly linked to fresh produce (4–6).
Pathogenic bacteria associated with fresh produce may establish themselves on plant surfaces and cause disease; therefore, the initial process of bacterial attachment is a crucial step in their transmission (7, 8). A study by Saggers et al. (9) suggested that plant cell wall (PCW) components at the PCW junction, particularly pectin, may provide receptor sites for bacterial attachment. In addition to the structural components of the PCW, the physicochemical properties (such as hydrophobicity and charge) of both the attachment surface and the attaching bacteria influence bacterial adhesion. For example, it has been suggested that bacteria that exhibit greater surface hydrophobicity than other strains attach preferably to hydrophobic surfaces, whereas bacteria that exhibit greater surface hydrophilicity than other strains prefer hydrophilic surfaces (10). Other studies have suggested that greater bacterial hydrophobicity favors bacterial adhesion to most surfaces (11, 12). Increased hydrophobicity also favors cell-to-cell adhesion, which leads to greater autoaggregation (13, 14). Similarly, coaggregation has been shown to be dependent on autoaggregation (14). Bujnakova and Kmet (15), for example, showed that only autoaggregating strains coaggregate with other strains.
We have used a bacterial cellulose (BC)-based PCW model to investigate the effects of PCW components on the attachment of pathogenic bacteria (16). The BC-based PCW model is produced by culturing Gluconacetobacter xylinus, a BC-producing bacterium, in Hestrin-Schramm (HS) growth medium with the addition of pectin and/or xyloglucan. Formation of the PCW model mimics the natural phenomenon of PCW deposition in native plants (17). The PCW model has been shown to possess molecular and architectural properties similar to those of native primary PCWs (17, 18). This model gives a more realistic picture of what occurs within actual PCWs because it is based on a constructive approach, in comparison with destructive chemical and physical treatments used to obtain PCW fractions (19). The PCW model can also be produced in relatively large quantities and at desired thicknesses, which makes it convenient to use (20, 21). More importantly, the chemical composition of the BC-based PCW model can be easily manipulated through the specific addition or removal of PCW components; this enables direct investigation of the effects of different levels of specific PCW components on pathogenic bacterial attachment. The PCW model used in this study has limitations and cannot fully replace the study of bacterial attachment to plant tissues in situ. It is subject to less variability, however, and can provide a substantial amount of information that can be used subsequently to design better experiments for plant tissue studies.
In this study, we investigated the roles of the major structural components of the PCW (cellulose, pectin, and xyloglucan) in the attachment of three Salmonella enterica strains to PCW models. The effect of attachment surface hydrophobicity on Salmonella attachment was also investigated, using abiotic surfaces of different hydrophobicities. A Gram-positive Listeria monocytogenes strain that was used in a previous study (16) was included in this study for comparison, as it showed different attachment characteristics than did the Gram-negative Salmonella strains. The overall aim of this study was to understand how bacteria attach to PCWs, to aid in the development of decontamination strategies that can effectively eliminate pathogens on fresh produce without damaging the organoleptic qualities of the produce (22).
MATERIALS AND METHODS
Bacterial strains.
Salmonella enterica subsp. enterica serovar Enteritidis ATCC 13076, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, Listeria monocytogenes ATCC 7644, and Gluconacetobacter xylinus ATCC 53524 were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Salmonella enterica subsp. indica M4 was isolated from lettuce in Malaysia and was used in this study as a control and fresh produce isolate. The Salmonella and Listeria strains were grown aerobically at 37°C on tryptic soy agar (Merck, Darmstadt, Germany) for maintenance or in tryptic soy broth (Merck), with shaking (150 rpm), for experiments (Lab Companion SK-600 benchtop shaker; Medline, United Kingdom). The Gluconacetobacter strain was grown as described below.
Production of BC-based PCW models.
A primary inoculum was prepared and then used for the production of all BC composites, as described by Mikkelsen and Gidley (23). The primary inoculum was prepared by culturing G. xylinus ATCC 53524 at 30°C for 72 h in Hestrin-Schramm (HS) broth containing 2% (wt/vol) glucose, 0.5% (wt/vol) peptone, 0.5% (wt/vol) yeast extract, 0.27% (wt/vol) Na2HPO4, and 0.115% (wt/vol) citric acid and adjusted to pH 5.0 (24). The different BC composites were prepared individually and grown at the same time. Triplicates of each type of BC composite were grown for each attachment assay. These composites were produced in enclosed plastic containers (1.5 cm by 1.5 cm by 1.5 cm) and incubated statically at 30°C for 72 h. Harvested BC composites (1.5 cm by 1.5 cm; thickness, ∼2 mm) were purified by rinsing in 6 mM CaCl2 at 100 rpm for 1 h, to remove medium components. The different types of PCW models produced were as follows: (i) bacterial cellulose (BC); (ii) BC-pectin (BCP) composites produced by adding 0.1%, 0.3%, or 0.5% (wt/vol) apple pectin, with a degree of methyl esterification of about 30% (Herbstreith & Fox, Neuenbürg, Germany), to the HS medium with calcium chloride (R&M Chemicals, Malaysia) (3 mM CaCl2 for 0.1% [wt/vol] pectin, 6 mM CaCl2 for 0.3% [wt/vol] pectin, or 12.5 mM CaCl2 for 0.5% [wt/vol] pectin); (iii) BC-xyloglucan (BCX) composites produced by adding 0.1%, 0.3%, or 0.5% (wt/vol) xyloglucan (Megazyme, Bray, Ireland) to the HS medium; and (iv) BC-pectin-xyloglucan (BCPX) composites produced by adding different combinations of pectin and xyloglucan (0.1%, 0.3%, or 0.5% [wt/vol]) with CaCl2 (3 mM, 6 mM, or 12.5 mM, respectively, corresponding to the amount of pectin added).
Chemical composition analysis.
The chemical compositions of the BC composites were analyzed as described by Mikkelsen et al. (19). BC composites were first cryoground using a 6850 SPEX freezer/mill (SPEX, Metuchen, NJ, USA). Saeman hydrolysis was then performed to hydrolyze the cryoground composites into monosaccharides (25). The derived monosaccharides were reduced to their corresponding alditol acetates before analysis by gas chromatography (GC-17A system; Shimadzu, Kyoto, Japan); this allowed determination of the percentage of each PCW component incorporated into the composites.
Attachment assays with individual strains of bacteria.
Early-stationary-phase cultures of S. Enteritidis ATCC 13076, S. Typhimurium ATCC 14028, Salmonella enterica M4, and L. monocytogenes ATCC 7644 (18 h for the Salmonella strains and 42 h for the L. monocytogenes strain) were centrifuged at 5,500 × g (1620A rotor; Hettich, Tuttlingen, Germany) for 10 min at 4°C. The pellet was washed twice with phosphate-buffered saline (PBS) (pH 7.4) (1st BASE, Singapore) and suspended in PBS to optical densities at 600 nm (OD600) (Shimadzu UVmini-1240 UV-visible spectrophotometer) of 0.143 for S. Enteritidis ATCC 13076, 0.500 for S. Typhimurium ATCC 14028, 0.333 for Salmonella enterica M4, and 0.100 for L. monocytogenes ATCC 7644, which corresponds to 108 CFU/ml for each isolate.
BC composites (BC, BCP, BCX, and BCPX) were incubated in 10 ml of pathogenic bacterial suspension for 20 min at 25°C, with gentle shaking (100 rpm), followed by gentle rinsing (100 rpm) with 6 mM CaCl2 solution for 1 min to remove loosely attached cells. Each composite was then placed in a stomacher bag with 50 ml of PBS and pummeled for 1 min at 8 strokes/s in a BagMixer 400 (Interscience, France). The numbers of pathogenic bacteria attached to the BC composites were enumerated by serial dilution of the stomacher bag fluid followed by plating of appropriate dilutions on xylose lysine deoxycholate agar (XLDA) (Oxoid, United Kingdom) (for Salmonella strains) or Listeria selective agar (LSA) with supplement SR0140 (Oxoid, United Kingdom) (for the L. monocytogenes strain). The numbers of attached bacterial cells were expressed as CFU per square centimeter of composite.
Attachment assays with pairwise combinations of bacteria.
In order to determine whether the bacterial attachments to BC composites were stochastic or based on bacterial characteristics, attachment assays were carried out using pairwise combinations of two bacteria with different attachment characteristics, as described by Chia et al. (26). Our previous study showed that S. Typhimurium ATCC 14028 (∼7.3 log CFU) and L. monocytogenes ATCC 7644 (∼5.7 log CFU) attached in significantly different numbers (16). Three different pairwise ratios (i.e., 0.428 [30:70], 1 [50:50], and 2.333 [70:30]) of S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 were prepared at a total density of 108 CFU/ml for the attachment assay. Studies of the attachment of different combinations of the S. Typhimurium-L. monocytogenes bacterial pair to different BC composites (BC, BCP, BCX, 0.1% BCPX, 0.3% BCPX, and 0.5% BCPX) were carried out as described above for the attachment studies with individual strains of bacteria. BC composites were incubated for 20 min at 25°C with a total of 10 ml of mixed S. Typhimurium-L. monocytogenes pathogenic bacterial suspension, prepared at a given pairwise ratio (108 CFU/ml), with gentle shaking at 100 rpm. Incubation was followed by gentle rinsing with CaCl2 solution for 1 min before the composite was pummeled in 50 ml of PBS. Enumeration of attached bacterial cells (CFU per square centimeter of composite) was carried out by spread plating appropriate dilutions of the stomacher bag liquid on XLDA and LSA.
Similarly, attachment of S. Typhimurium-L. monocytogenes at the three different ratios to abiotic surfaces of different hydrophobicities, namely, glass slides (Premier slides; Azer Scientific, Morgantown, PA, USA), stainless steel coupons (type 302, no. 4 finishing, 1-mm thickness), and Teflon coupons (Tekdon, Myakka City, FL, USA), was carried out. Each slide (75 mm by 25 mm) was sterilized before incubation for 20 min in 20 ml of the mixed S. Typhimurium-L. monocytogenes bacterial suspension (108 CFU/ml), in an ESCO Airstream horizontal laminar flow clean bench (ONBoard Solutions, Australia), and then rinsed in CaCl2 before swabbing of the bottom part of the slide. The cotton swabs were placed in 50 ml of PBS and pummeled for 1 min in the stomacher before spread plating on XLDA and LSA. Attachment ratios for the S. Typhimurium-L. monocytogenes pair were calculated by dividing the number of attached S. Typhimurium cells (log CFU per square centimeter) by the number of attached L. monocytogenes cells (log CFU per square centimeter).
Bacterial surface hydrophobicity.
Bacterial surface hydrophobicity was determined with the assay of bacterial adhesion to hydrocarbons described by Rosenberg et al. (27), with modifications. Briefly, bacteria were pelleted, washed twice with PBS, and then suspended in PBS to achieve an OD550 of 1 ± 0.1. One milliliter of xylene (Fischer, Leicestershire, United Kingdom) was added to the 3-ml cell suspension, the mixture was vortex-mixed (VTX-3000L; LMS, Japan) for 2 min, and the phases were allowed to separate for 1 h. The absorbance of the aqueous phase was measured at 550 nm before (A0) and after (A1) the addition of xylene. The hydrophobicity index was expressed as follows: % attachment to xylene = (1 – A1/A0) × 100%. As described by Ahumada et al. (28), bacterial hydrophobicity can be classified into three groups according to the attachment to xylene, as follows: strongly hydrophobic, 71 to 100%; moderately hydrophobic, 36 to 70%; weakly hydrophobic, 0 to 35%.
Aggregation assays.
The autoaggregation assay was performed as described by Collado et al. (14), with slight modifications. Briefly, bacteria were pelleted, washed, and resuspended to an OD600 of 0.25 ± 0.05. A 1.5-ml aliquot of bacterial suspension was then incubated at room temperature, and optical density was measured after 6 h. The coaggregation assay was performed as described by Grześkowiak et al. (29), with slight modifications. S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 at three different pairwise ratios (i.e., 0.428 [30:70], 1 [50:50], and 2.333 [70:30]) were prepared at a total density of 108 CFU/ml of each bacterium. Suspensions of the strain combination at the three pairwise ratios were diluted with PBS to achieve an OD600 of 0.25 ± 0.05 (Ai), and absorbance was measured after 6 h (Af). Autoaggregation and coaggregation were expressed as follows: % aggregation = 100 – [(Af/Ai) × 100]. As described by Binetti et al. (30), strains were classified into three groups according to their autoaggregation and coaggregation abilities, as follows: high, >60%; moderate, 30 to 60%; low, <30%.
Data analysis.
All experiments were performed in triplicate (3 independently grown bacterial cultures). Statistical analysis of results was performed using SPSS software (PASW Statistics 18; SPSS Inc., USA). The attachment of bacterial cells to the BC composites was expressed as CFU per square centimeter, and the data obtained were parametric. Significant differences in the overall attachments of the four strains to the BC composites were determined using one-way analysis of variance (ANOVA). One-way ANOVA was also used to establish significant differences among the types of BC composites, as well as among the different levels of PCW structural components used within each type of composite, in the attachment of each bacterial strain. Paired t tests were used to determine the significance of differences between the initial inoculum ratio and the final ratio of attached bacteria for all surfaces. Two-way ANOVA was used to establish the significance of differences in the attachment of S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 when attached individually and at different initial inoculum ratios on the BC composites and abiotic surfaces. Pearson's correlation was used to determine the correlations between bacterial physicochemical properties and attachment. Differences between the means were determined using Tukey's method at a 95% confidence level.
RESULTS
Chemical composition analysis.
The chemical composition analysis showed that the incorporation of pectin and xyloglucan increased when higher concentrations of these components were added to the HS medium (Table 1). Pectin was found to be incorporated in the composites at a relatively higher percentage than that of xyloglucan.
TABLE 1.
Chemical compositions of BC composites
| Composite | Component(s) added (% [wt/vol]) |
Incorporation (%) |
|||
|---|---|---|---|---|---|
| Pectin | Xyloglucan | Bacterial cellulose | Pectin | Xyloglucan | |
| BCP | 0.1 | 0 | 44.7 | 55.3 | 0 |
| 0.3 | 0 | 43.9 | 56.1 | 0 | |
| 0.5 | 0 | 42.8 | 57.2 | 0 | |
| BCX | 0 | 0.1 | 76.5 | 0 | 23.5 |
| 0 | 0.3 | 72.3 | 0 | 27.7 | |
| 0 | 0.5 | 67.7 | 0 | 32.3 | |
| BCPX with 0.1% xyloglucan | 0.1 | 0.1 | 34.0 | 41.5 | 24.5 |
| 0.3 | 0.1 | 33.9 | 44.7 | 21.4 | |
| 0.5 | 0.1 | 32.5 | 48.5 | 18.3 | |
| BCPX with 0.3% xyloglucan | 0.1 | 0.3 | 33.9 | 40.7 | 25.4 |
| 0.3 | 0.3 | 33.2 | 43.1 | 23.7 | |
| 0.5 | 0.3 | 32.9 | 47.8 | 19.3 | |
| BCPX with 0.5% xyloglucan | 0.1 | 0.5 | 33.5 | 39.4 | 26.7 |
| 0.3 | 0.5 | 33.1 | 42.6 | 24.3 | |
| 0.5 | 0.5 | 33.2 | 47.4 | 20.1 | |
Effects of pectin and xyloglucan on attachment of bacteria to PCW models.
An overall comparison of the attachment of S. Enteritidis ATCC 13076, S. Typhimurium ATCC 14028, Salmonella enterica M4, and L. monocytogenes ATCC 7644 showed that the bacterial strains attached to the BC composites in significantly different numbers. Specifically, L. monocytogenes attached in the lowest numbers (mean attachment to all composites, ∼5.7 log CFU/cm2 composite), followed by Salmonella enterica M4 (∼6.5 log CFU/cm2 composite) and lastly S. Enteritidis (∼7.0 log CFU/cm2 composite) and S. Typhimurium (∼7.3 log CFU/cm2 composite), which attached in the highest numbers and were not statistically different from each other.
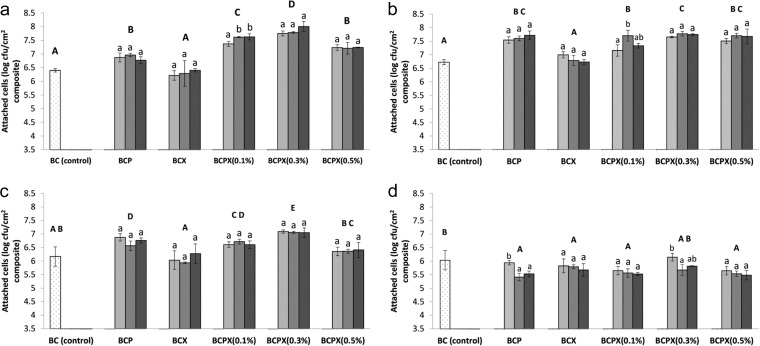
Generally, the presence of pectin and xyloglucan in the BCP and BCX composites affected the attachment of L. monocytogenes differently from that of the Salmonella strains (Fig. 1). The presence of pectin in the BCP composites was associated with increased attachment of the Salmonella strains, whereas L. monocytogenes attachment was reduced. Xyloglucan in the BCX composites significantly reduced the attachment of L. monocytogenes but had no significant effect on the attachment of the Salmonella strains.
FIG 1.
Attachment of Salmonella Enteritidis ATCC 13076 (a), Salmonella Typhimurium ATCC 14028 (b), Salmonella enterica M4 isolated from lettuce (c), and Listeria monocytogenes ATCC 7644 (d) to BC, BCP, BCX, 0.1% BCPX, 0.3% BCPX, and 0.5% BCPX. Different uppercase letters indicate significant differences between types of composites, whereas different lowercase letters indicate significant differences within each type of composite (one-way ANOVA and Tukey's pairwise comparison, P < 0.05). Stippled bars, 0% PCW components (for BC); light gray bars, 0.1% PCW components; medium gray bars, 0.3% PCW components; dark gray bars, 0.5% PCW components added into the growth medium.
There was no significant interaction between pectin and xyloglucan in the BCPX composites with respect to the attachment of the four bacterial strains. This indicates that the effect of the level of pectin on bacterial attachment is independent of the effect of the level of xyloglucan and vice versa. When the effects of pectin and xyloglucan levels in the BCPX composites on bacterial attachment were compared, xyloglucan had a greater influence on bacterial attachment than did pectin. Xyloglucan significantly affected the attachment of all strains, whereas pectin significantly affected only the attachment of the S. Typhimurium strain. Interestingly, all Salmonella strains showed the greatest attachment to the 0.3% BCPX composite, which had 0.3% (wt/vol) xyloglucan added to the medium, regardless of the amount of pectin present in the growth medium.
Attachment of pairwise combinations of Salmonella Typhimurium and Listeria monocytogenes to BC composites and abiotic surfaces.
A protocol developed by Chia et al. (26) was used to determine the stochasticity (or randomness) of the attachment process. Two bacteria with different attachment characteristics (S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644) were allowed to attach to the BC composites and abiotic surfaces in different pairwise ratios (0.428, 1, and 2.333). If the attachment process is stochastic, then the numbers of bacteria attached to the substratum are dependent on the concentration of the initial bacterial suspension. Therefore, attachment is considered stochastic when the initial ratio of the bacterial pair in a bacterial suspension does not differ significantly from the final ratio of the bacterial pair attached to the attachment surfaces. Similarly, attachment is deemed nonstochastic when the process is influenced by other factors which cause the initial and final ratios of the bacterial pair before and after attachment to be significantly different from each other.
Bacterial attachments to all BC composites and abiotic surfaces in this study were nonstochastic, and the attachment surface had a significant effect on the attached cell ratio (Tables 2 and 3). A higher initial S. Typhimurium/L. monocytogenes inoculum ratio resulted in a significantly higher S. Typhimurium/L. monocytogenes attachment ratio for most BC composites, whereas the trend was less distinct for abiotic surfaces. We also observed that the pairwise attachment of S. Typhimurium-L. monocytogenes was not significantly different on the different abiotic surfaces.
TABLE 2.
Attachment ratios for pairwise combinations of S. Typhimurium ATCC 14028-L. monocytogenes ATCC 7644 at different initial inoculum ratios on BC compositesa
| Composite | Component(s) added (% [wt/vol]) |
Final attachment ratio of S. Typhimurium to L. monocytogenes with an inoculum ratio ofb: |
|||
|---|---|---|---|---|---|
| Pectin | Xyloglucan | 0.428 | 1 | 2.333 | |
| BC | 0 | 0 | 1.230 ± 0.013 A | 1.225 ± 0.002 A | 1.342 ± 0.021 B |
| BCP | 0.1 | 0 | 1.308 ± 0.008 A | 1.379 ± 0.014 B | 1.475 ± 0.033 C |
| 0.3 | 0 | 1.516 ± 0.041 A | 1.512 ± 0.057 A | 1.560 ± 0.040 A | |
| 0.5 | 0 | 1.516 ± 0.019 A | 1.504 ± 0.045 A | 1.524 ± 0.039 A | |
| BCX | 0 | 0.1 | 1.321 ± 0.015 A | 1.284 ± 0.027 A | 1.384 ± 0.009 B |
| 0 | 0.3 | 1.323 ± 0.045 A | 1.372 ± 0.020 A | 1.360 ± 0.025 A | |
| 0 | 0.5 | 1.216 ± 0.068 A | 1.397 ± 0.050 B | 1.389 ± 0.014 B | |
| BCPX with 0.1% xyloglucan | 0.1 | 0.1 | 1.194 ± 0.100 A | 1.188 ± 0.031 A | 1.378 ± 0.055 B |
| 0.3 | 0.1 | 1.173 ± 0.026 A | 1.229 ± 0.045 A | 1.334 ± 0.017 B | |
| 0.5 | 0.1 | 1.244 ± 0.032 A | 1.225 ± 0.015 A | 1.310 ± 0.029 B | |
| BCPX with 0.3% xyloglucan | 0.1 | 0.3 | 1.180 ± 0.016 A | 1.230 ± 0.041 A | 1.337 ± 0.011 B |
| 0.3 | 0.3 | 1.296 ± 0.052 A | 1.279 ± 0.016 A | 1.364 ± 0.066 A | |
| 0.5 | 0.3 | 1.304 ± 0.030 B | 1.230 ± 0.025 A | 1.373 ± 0.023 C | |
| BCPX with 0.5% xyloglucan | 0.1 | 0.5 | 1.128 ± 0.026 A | 1.191 ± 0.028 AB | 1.227 ± 0.032 C |
| 0.3 | 0.5 | 1.116 ± 0.048 A | 1.231 ± 0.026 B | 1.286 ± 0.020 B | |
| 0.5 | 0.5 | 1.109 ± 0.023 A | 1.218 ± 0.009 B | 1.306 ± 0.019 C | |
Initial inoculum ratios were 0.428, 1, and 2.333.
Different letters indicate significant differences between final attachment ratios for pairwise combinations of S. Typhimurium and L. monocytogenes with the same BC composite (one-way ANOVA and Tukey's pairwise comparison, P < 0.05).
TABLE 3.
Attachment ratios for pairwise combinations of S. Typhimurium ATCC 14028-L. monocytogenes ATCC 7644 at different initial inoculum ratios on abiotic surfaces of different hydrophobicitiesa
| Initial inoculum ratio of S. Typhimurium to L. monocytogenes | Final attachment ratio of S. Typhimurium to L. monocytogenes onb: |
||
|---|---|---|---|
| Glass | Stainless steel | Teflon | |
| 0.428 | 1.073 ± 0.082 A | 1.247 ± 0.113 A | 1.371 ± 0.082 A |
| 1 | 1.140 ± 0.085 A | 1.211 ± 0.134 A | 1.547 ± 0.070 B |
| 2.333 | 1.145 ± 0.029 A | 1.172 ± 0.036 AB | 1.293 ± 0.091 B |
Initial inoculum ratios were 0.428, 1, and 2.333.
Different letters indicate significant differences between final attachment ratios of S. Typhimurium to L. monocytogenes on different abiotic surfaces for the same initial inoculum ratio of S. Typhimurium to L. monocytogenes (one-way ANOVA and Tukey's pairwise comparison, P < 0.05).
The numbers of S. Typhimurium and L. monocytogenes cells attached to the various surfaces were also examined. Compared to the attachment of S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 to the BC composites, both bacteria generally showed significantly smaller differences in the numbers attached to abiotic surfaces, with the exception of 0.5% BCPX; this was because S. Typhimurium ATCC 14028 attached at ∼102 CFU/cm2 less to abiotic surfaces than to the BC composites. In contrast, L. monocytogenes ATCC 7644 cells attached at similar levels to BC composites and abiotic surfaces.
Differences in the attachment of S. Typhimurium and L. monocytogenes strains were generally greater at higher initial S. Typhimurium/L. monocytogenes inoculum ratios. In addition, the individual attachments of S. Typhimurium and L. monocytogenes strains were not significantly different from the attachment of the S. Typhimurium-L. monocytogenes pair at an initial inoculum ratio of 1 (50% S. Typhimurium and 50% L. monocytogenes).
Influence of bacterial physicochemical properties on attachment of four pathogens to BC composites.
The hydrophobicity of all bacterial strains used in this study was relatively low, and most of the strains were classified as weakly hydrophobic (S. Enteritidis ATCC 13076, 34.70%; S. Typhimurium ATCC 14028, 15.00%; L. monocytogenes ATCC 7644, 9.13%). Salmonella enterica M4 (42.53%) was considered moderately hydrophobic. No significant correlation between hydrophobicity and bacterial attachment was found.
Most strains used in our study also showed low levels of autoaggregation (S. Enteritidis ATCC 13076, 25.60%; Salmonella enterica M4, 27.60%; L. monocytogenes ATCC 7644, 28.40%), with only S. Typhimurium ATCC 14028 displaying moderate autoaggregation (34.80%). We found a very weak positive correlation between hydrophobicity and autoaggregation (Pearson's correlation: r = 0.27, R2 = 7.29%, P > 0.05). Overall, there were no significant correlations of hydrophobicity, autoaggregation, and adhesion ability.
The coaggregation assays showed that the pairwise combinations of S. Typhimurium and L. monocytogenes had intermediate coaggregation abilities which were not significantly different at different initial inoculum ratios (S. Typhimurium/L. monocytogenes ratio of 0.428, 39.87% coaggregation; ratio of 1, 39.47% coaggregation; ratio of 2.333, 36.40% coaggregation). The resulting coaggregation values for the two strains were very similar to the autoaggregation values obtained for S. Typhimurium (∼35%) but not to those for L. monocytogenes (∼28%). The ability to coaggregate did not significantly affect the final S. Typhimurium/L. monocytogenes attachment ratios for all initial inoculum ratios.
DISCUSSION
Chemical composition analysis.
A BC-based PCW model was used in our study, as there are many difficulties associated with using native PCWs to study bacterial adhesion. For example, the use of physical or chemical extraction of PCWs distorts the PCW structure, while the heterogeneous nature of PCW compositions makes it difficult to study how individual PCW components affect bacterial attachment (31, 32).
We examined the effects of varying the concentrations of PCW components added to the growth medium on their incorporation within the PCW models. According to Zykwinska et al. (33), the amount of pectin absorbed onto cellulose decreases as the level of xyloglucan increases, with no pectin being bound to cellulose at xyloglucan concentrations above 500 μg/ml; however, we observed that the incorporation of both pectin and xyloglucan increased when greater amounts of the PCW components were added to the medium, even up to a concentration of 5,000 μg/ml. Zykwinska et al. (33) measured the binding of pectin and xyloglucan to cellulose by mixing the polysaccharide solutions with cellulose; however, our study used a constructive approach that resembled the formation of native PCWs, which may be more representative of the occurrence in nature.
Our BC composites were shown to have compositions similar to those of other BC-based PCW models reported in the literature (34, 35). Our BCPX composites also showed chemical compositions similar to those of average native PCWs (approximately 35% cellulose, 40% pectin, and 15% xyloglucan) (36). This is important because our BC composites are designed to be used as models for understanding the interactions of pathogenic bacteria with native PCWs.
Effects of pectin and xyloglucan on attachment of pathogenic bacteria to PCW models.
We found that all Salmonella strains attached to BC composites in significantly greater numbers than did L. monocytogenes ATCC 7644, which is in agreement with our previous findings (16). In another study, Kutter et al. (37) found that Salmonella enterica was able to colonize barley roots while Listeria strains were not. Jablasone et al. (38) showed that S. Typhimurium was internalized into plant tissues while L. monocytogenes attached only to the plant surface and was not internalized in seedlings. One possible reason that may account for the greater attachment of Salmonella strains is the availability of a larger number of adhesive appendages, such as flagella, fimbriae, thin aggregative fimbriae (tafi), lipopolysaccharides, and outer membrane proteins (39–41), produced by Salmonella for attachment. In contrast, Listeria attachment generally relies only on flagella (42). It should be noted, however, that Jablasone et al. (38) and Takeuchi et al. (43) observed greater L. monocytogenes attachment than Salmonella species attachment on cut lettuce leaves and on various vegetable seedlings.
Higher levels of pectin in the BCP composite increased the attachment of all Salmonella strains in our study. This result is consistent with the findings of Saggers et al. (9), who observed that another strain of S. Typhimurium attached preferentially to the pectin layer at the potato cell wall junction, while less attachment was observed where less pectin was present. Xyloglucan did not significantly affect the attachment of Salmonella strains in the BCX composites, but it significantly affected the attachment of all Salmonella strains when present with pectin in the BCPX composites.
Higher levels of pectin increased the attachment of only the Salmonella strains and not the Listeria strain, which suggests specific interactions between Salmonella cells and the pectin molecules. Most of the receptor-ligand interactions required for bacterial adhesion are mediated by carbohydrates and bacterial surface adhesins (44, 45). Extensive research has been undertaken regarding the role of sugar residues that serve as receptors for the binding of animal pathogens to animal cells (46, 47). However, only a few studies have focused on the role of sugar residues in the attachment of human pathogens to plants. Klerks et al. (4) found that root exudates that contain many monosaccharides, such as fructose and glucose, cause chemotaxis of Salmonella strains, which use these signals to move toward the plants. Root exudates also condition S. enterica cells for attachment and colonization of the plant roots. Another study proposed that exudates of germinating seeds and developing roots trigger Escherichia coli O157 to colonize seedlings (48).
Based on these data, we hypothesized that pectin and xyloglucan could have interacted physically and given the BCPX composites surface structures that are distinct from those of the BCP and BCX composites. This may also explain why all Salmonella strains showed the highest levels of attachment to the BCPX composite with 0.3% (wt/vol) xyloglucan, regardless of the amount of pectin added to the growth medium. The BCPX composite with 0.3% (wt/vol) xyloglucan may have distinct structural features (such as porosity and surface roughness) that are ideal for the attachment of the Salmonella cells. Cybulska et al. (35) found that the cellulose fibrils of BCPX (∼75 nm) were significantly thicker than those of BC (∼37 nm) and BCP (∼46 nm); the thicker fibrils may provide additional surface area for the attachment of bacteria. Very few studies have investigated the effects of pectin and xyloglucan on the structural properties of BC composites. Fanta et al. (49) found that the fibril diameters for BC (110 ± 33 nm) and BCPX (123 ± 29 nm) were similar, while the fibril diameter for BCP was much smaller (45 ± 9 nm). Both Cybulska et al. (35) and Fanta et al. (49) found that BC has the highest porosity, BCP has lower porosity after the addition of pectin, and BCPX has the lowest porosity and greatest compactness among the composites. The effects of various concentrations of pectin and xyloglucan on the structural properties of BC composites have not been determined, however, and mechanical studies and surface profiling of the BC composites need to be performed in future studies.
Attachment of L. monocytogenes ATCC 7644 to the BCP and BCX composites was significantly lower than that to BC, but we have yet to determine a reason for this occurrence. A possible explanation is that the BC matrix, which is more porous than the BCP and BCX composites, has increased area for bacterial attachment and its porous structure enables it to trap liquids and small particles (50, 51).
Attachment of pairwise combinations of Salmonella Typhimurium and Listeria monocytogenes to BC composites and abiotic surfaces.
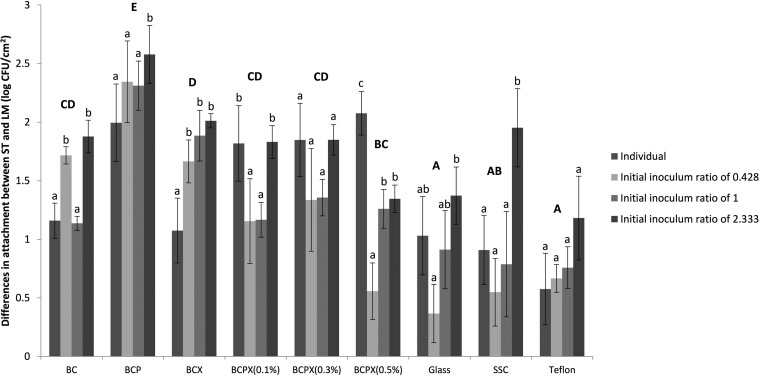
Our findings showed that bacterial attachment to all BC composites was nonstochastic, as the numbers of attached cells of the S. Typhimurium and L. monocytogenes strains did not depend on the levels of the bacterial strains present in the initial inoculum (Fig. 2). Instead, the attachment of the bacterial pair to the BC composites was shown to be strongly influenced by individual bacterial attachment characteristics, especially for S. Typhimurium ATCC 14028. An increased concentration of S. Typhimurium ATCC 14028 in the bacterial suspension (higher initial S. Typhimurium/L. monocytogenes inoculum ratio) led to significant increases in the final S. Typhimurium/L. monocytogenes attachment ratio in most cases. The finding that the two strains did not interact with each other and attached similarly when they were present together or separately supported the importance of the role of individual bacterial attachment characteristics in adhesion.
FIG 2.
Differences in attachment numbers between S. Typhimurium ATCC 14028 (ST) and L. monocytogenes ATCC 7644 (LM) when present individually and at different initial S. Typhimurium/L. monocytogenes inoculum ratios (0.428, 1, or 2.333) on BC composites and abiotic surfaces. SSC, stainless steel coupons. Different uppercase letters indicate significant differences between attachment surfaces, whereas different lowercase letters indicate significant differences between individual attachment and attachment at different initial inoculum ratios for the same attachment surface (two-way ANOVA and Tukey's pairwise comparison, P < 0.05).
Several studies have suggested that bacterial attachment can be influenced by the physicochemical properties of the attachment surface and the attaching bacteria (13, 14). To investigate whether the strong attachment of the Salmonella strain to BC composites was influenced by these factors, assays of the attachment of the bacterial pair to abiotic surfaces with different hydrophobicities and other physicochemical properties were carried out. We established that the numbers of S. Typhimurium ATCC 14028 cells that attached to different abiotic surfaces were similar to those of L. monocytogenes ATCC 7644, whereas the numbers that attached to the BC composites were far greater. This finding suggests that the hydrophobicity of the attachment surface does not greatly affect the attachment of S. Typhimurium ATCC 14028 and thus that other features of bacterial and surface interactions are responsible for the attachment of Salmonella strains to the BC composites.
Role of bacterial physicochemical properties in attachment of four pathogens to BC composites.
There have been some findings suggesting that the physicochemical properties of bacteria influence bacterial attachment to various surfaces. As mentioned earlier, there is strong evidence that autoaggregation is correlated with the ability to attach to surfaces (14, 52, 53). However, S. Typhimurium ATCC 14028 and L. monocytogenes ATCC 7644 demonstrated hydrophobicities and autoaggregation levels that were not significantly different from each other, although their abilities to attach to surfaces were significantly different. As for the pairwise combinations of S. Typhimurium and L. monocytogenes, coaggregation values obtained for the bacterial pair were similar to the autoaggregation value obtained for S. Typhimurium ATCC 14028. This again emphasizes that some specific characteristics of the S. Typhimurium strain, including its autoaggregation and attachment abilities, strongly influence the attachment of the bacterial pair.
There were no significant correlations of hydrophobicity, autoaggregation, coaggregation, and adhesion. In addition to our study, other studies have come to the conclusion that these physicochemical characteristics vary in importance for the attachment of different species of bacteria (54, 55). In contrast to Hood and Zottola (56), who found that cell surface charge, polysaccharide production, and hydrophobicity affect bacterial attachment to surfaces, Flint et al. (55) observed that the role of these factors appears to be species specific. Their study was unable to show an association between bacterial attachment and any of the three factors. Similarly, a study by Oliveira et al. (57) showed that cell surface hydrophobicity did not play a major role in the attachment of Salmonella strains to stainless steel surfaces. This indicates that other factors may be more important for Salmonella attachment than the physicochemical properties of the bacterial cells and the attachment surface.
Only a limited number of bacterial strains were investigated in this study. Ideally, a larger number of individual strains or a cocktail of strains could be used. The data provided in this study represent a resource for further studies of this type, as well as providing insights into differences in strain interactions with the composites and ultimately PCWs.
Overall, our findings demonstrated that PCW components significantly affect bacterial attachment. Pectin in the BCP composites and xyloglucan in association with pectin in the BCPX composites were shown to increase Salmonella attachment significantly. We confirmed that the attachment of the bacterial strains, particularly S. Typhimurium ATCC 14028, to the BC composites was not stochastic and was most likely controlled by specific interactions between the bacteria and the attachment surface. We also found that bacterial attachment was not significantly influenced by the hydrophobicity of the attachment surface or the physicochemical properties of the bacteria. It is still unclear, however, whether the attachment of the Salmonella strains to the BC composites was due to the influence of the PCW polysaccharides on the physical and structural characteristics of the BC composites.
Surface roughness and porosity are known to favor bacterial attachment by providing a greater surface area for attachment (50). The effects of these factors on Salmonella attachment to PCWs remain to be investigated. The findings of this study improve our understanding of how bacteria attach to PCWs. This may in turn aid in the development of more effective methods for fresh produce decontamination, as the current sanitation method using chlorine has limited effectiveness (58).
ACKNOWLEDGMENT
We acknowledge Herbstreith & Fox (Neuenbürg, Germany) for kind donation of the apple pectin.
Funding Statement
This research was funded by the School of Science, Monash University Malaysia.
REFERENCES
- 1.Brandl MT. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu Rev Phytopathol 44:367–392. doi: 10.1146/annurev.phyto.44.070505.143359. [DOI] [PubMed] [Google Scholar]
- 2.Heaton JC, Jones K. 2008. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J Appl Microbiol 104:613–626. doi: 10.1111/j.1365-2672.2007.03587.x. [DOI] [PubMed] [Google Scholar]
- 3.Krtinić G, Durić P, Ilić S. 2010. Salmonellae in food stuffs of plant origin and their implications on human health. Eur J Clin Microbiol Infect Dis 29:1321–1325. doi: 10.1007/s10096-010-1001-4. [DOI] [PubMed] [Google Scholar]
- 4.Klerks MM, Franz E, van Gent-Pelzer M, Zijlstra C, van Bruggen AHC. 2007. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J 1:620–631. doi: 10.1038/ismej.2007.82. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Namvar A, Kostryzynska M, Hora R, Warriner K. 2007. Persistence and growth of different Salmonella serovars on pre- and postharvest tomatoes. J Food Prot 70:2725–2731. [DOI] [PubMed] [Google Scholar]
- 6.Lynch MF, Tauxe RV, Hedberg CW. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect 137:307–315. doi: 10.1017/S0950268808001969. [DOI] [PubMed] [Google Scholar]
- 7.Bordas MA, Balebona MC, Zorrilla I, Borrgo JJ, Morinigo MA. 1996. Kinetics of adhesion of selected fish-pathogenic Vibrio strains to skin mucus of gilt-head sea bream (Sparus aurata L.). Appl Environ Microbiol 62:3650–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L, Walker WA. 2001. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr 73:1124S–1130S. [DOI] [PubMed] [Google Scholar]
- 9.Saggers EJ, Waspe CR, Parker ML, Waldron KW, Brocklehurst TF. 2008. Salmonella must be viable in order to attach to the surface of prepared vegetable tissues. J Appl Microbiol 105:1239–1245. doi: 10.1111/j.1365-2672.2008.03795.x. [DOI] [PubMed] [Google Scholar]
- 10.An YH, Friedman RJ. 1998. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43:338–348. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Dykes GA, Amarowicz R, Pegg RB. 2003. An antioxidant bearberry (Arctostaphylos uva-ursi) extract modulates surface hydrophobicity of a wide range of food-related bacteria: implications for functional food safety. Food Control 14:515–518. doi: 10.1016/S0956-7135(02)00110-X. [DOI] [Google Scholar]
- 12.Voravuthikunchai SP, Suwalak S. 2009. Changes in cell surface properties of Shiga toxigenic Escherichia coli by Quercus infectoria G. Olivier. J Food Prot 72:1699–1704. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Yang SF, Tay JH, Liu QS, Qin L, Li Y. 2004. Cell hydrophobicity is a triggering force of biogranulation. Enzyme Microb Technol 34:371–379. doi: 10.1016/j.enzmictec.2003.12.009. [DOI] [Google Scholar]
- 14.Collado MC, Meriluoto J, Salminen S. 2007. Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073. [Google Scholar]
- 15.Bujnakova D, Kmet V. 2002. Aggregation of animal lactobacilli with O157 enterohemorrhagic Escherichia coli. J Vet Med B Infect Dis Vet Public Health 49:152–154. doi: 10.1046/j.1439-0450.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- 16.Tan MSF, Wang Y, Dykes GA. 2013. Attachment of bacterial pathogens to a bacterial cellulose-derived plant cell wall model: a proof of concept. Foodborne Pathog Dis 10:992–994. doi: 10.1089/fpd.2013.1536. [DOI] [PubMed] [Google Scholar]
- 17.Chanliaud E, Gidley M. 1999. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J 20:25–35. doi: 10.1046/j.1365-313X.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 18.Whitney S, Gothard M, Mitchell J, Gidley M. 1999. Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol 121:657–664. doi: 10.1104/pp.121.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikkelsen D, Gidley MJ, Williams BA. 2011. In vitro fermentation of bacterial cellulose composites as model dietary fibers. J Agric Food Chem 59:4025–4032. doi: 10.1021/jf104855e. [DOI] [PubMed] [Google Scholar]
- 20.Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ. 2002. Mechanical properties of primary plant cell wall analogues. Planta 215:989–996. doi: 10.1007/s00425-002-0783-8. [DOI] [PubMed] [Google Scholar]
- 21.Padayachee A, Netzel G, Netzel M, Day L, Zabaras D, Mikkelsen D, Gidley MJ. 2012. Binding of polyphenols to plant cell wall analogues. 1. Anthocyanins. Food Chem 134:155–161. doi: 10.1016/j.foodchem.2012.02.082. [DOI] [PubMed] [Google Scholar]
- 22.López-Gálvez F, Gil MI, Truchado P, Selma MV, Allende A. 2010. Cross-contamination of fresh-cut lettuce after a short-term exposure during pre-washing cannot be controlled after subsequent washing with chlorine dioxide or sodium hypochlorite. Food Microbiol 27:199–204. doi: 10.1016/j.fm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen D, Gidley MJ. 2011. Formation of cellulose-based composites with hemicelluloses and pectins using Gluconacetobacter fermentation. Methods Mol Biol 715:197–208. doi: 10.1007/978-1-61779-008-9_14. [DOI] [PubMed] [Google Scholar]
- 24.Hestrin S, Schramm M. 1954. Synthesis of cellulose by Acetobacter xylinum: preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352. doi: 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeman JF. 1945. Kinetics of wood saccharification: hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind Eng Chem 37:43–52. doi: 10.1021/ie50421a009. [DOI] [Google Scholar]
- 26.Chia TWR, Nguyen VT, McMeekin T, Fegan N, Dykes GA. 2011. Stochasticity of bacterial attachment and its predictability by the extended Derjaguin-Landau-Verwey-Overbeek theory. Appl Environ Microbiol 77:3757–3764. doi: 10.1128/AEM.01415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg M, Gutnick D, Rosenberg E. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33. doi: 10.1111/j.1574-6968.1980.tb05599.x. [DOI] [Google Scholar]
- 28.Ahumada M, López ME, Colloca ME, Nader-Macıìas ME. 2001. Lactobacilli isolation from dental plaque and saliva of a group of patients with caries and characterization of their surface properties. Anaerobe 7:71–77. [Google Scholar]
- 29.Grześkowiak Ł, Collado MC, Salminen S. 2012. Evaluation of aggregation abilities between commensal fish bacteria and pathogens. Aquaculture 356–357:412–414. [Google Scholar]
- 30.Binetti A, Carrasco M, Reinheimer J, Suárez V. 2013. Yeasts from autochthonal cheese starters: technological and functional properties. J Appl Microbiol 115:434–444. doi: 10.1111/jam.12228. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis MC. 1992. Control of thickness of collenchyma cell walls by pectins. Planta 187:218–220. [DOI] [PubMed] [Google Scholar]
- 32.Burton RA, Gidley MJ, Fincher GB. 2010. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732. doi: 10.1038/nchembio.439. [DOI] [PubMed] [Google Scholar]
- 33.Zykwinska A, Thibault JF, Ralet MC. 2008. Competitive binding of pectin and xyloglucan with primary cell wall cellulose. Carbohydr Polym 74:957–961. doi: 10.1016/j.carbpol.2008.05.004. [DOI] [Google Scholar]
- 34.Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ. 2009. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107:576–583. doi: 10.1111/j.1365-2672.2009.04226.x. [DOI] [PubMed] [Google Scholar]
- 35.Cybulska J, Vanstreels E, Tri Q, Courtin CM, van Craeyveld V, Nicolaï B, Zdunek A, Konstankiewicz K. 2010. Mechanical characteristics of artificial cell walls. J Food Eng 96:287–294. doi: 10.1016/j.jfoodeng.2009.08.001. [DOI] [Google Scholar]
- 36.Cybulska J, Konstankiewicz K, Zdunek A, Skrzypiec K. 2010. Nanostructure of natural and model cell wall materials. Int Aerophys 24:107–114. [Google Scholar]
- 37.Kutter S, Hartmann A, Schmid M. 2006. Colonization of barley (Hordeum vulgare) with Salmonella enterica and Listeria spp. FEMS Microbiol Ecol 56:262–271. doi: 10.1111/j.1574-6941.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- 38.Jablasone J, Warriner K, Griffiths M. 2005. Interactions of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system. Int J Food Microbiol 99:7–18. doi: 10.1016/j.ijfoodmicro.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Barak JD, Gorski L, Naraghi-Arani P, Charkowski AO. 2005. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl Environ Microbiol 71:5685–5691. doi: 10.1128/AEM.71.10.5685-5691.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barak JD, Jahn CE, Gibson DL, Charkowski AO. 2007. The role of cellulose and O-antigen capsule in the colonization of plants by Salmonella enterica. Mol Plant Microbe Interact 20:1083–1091. doi: 10.1094/MPMI-20-9-1083. [DOI] [PubMed] [Google Scholar]
- 41.Wagner C, Hensel M. 2011. Adhesive mechanisms of Salmonella enterica. Adv Exp Med Biol 715:17–34. doi: 10.1007/978-94-007-0940-9_2. [DOI] [PubMed] [Google Scholar]
- 42.Dussurget O. 2008. New insights into determinants of Listeria monocytogenes virulence. Int Rev Cell Mol Biol 270:1–38. doi: 10.1016/S1937-6448(08)01401-9. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi K, Matute CM, Hassan AN, Frank JF. 2000. Comparison of the attachment of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, and Pseudomonas fluorescens to lettuce leaves. J Food Prot 63:1433–1437. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt MA, Riley LW, Benz I. 2003. Sweet new world: glycoproteins in bacterial pathogens. Trends Microbiol 11:554–561. doi: 10.1016/j.tim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Odenbreit S. 2005. Adherence properties of Helicobacter pylori: impact on pathogenesis and adaptation to the host. Int J Med Microbiol 295:317–324. doi: 10.1016/j.ijmm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Brandley BK, Schnaar L. 1986. Cell-surface carbohydrates in cell recognition and response. J Leukoc Biol 40:97–111. [DOI] [PubMed] [Google Scholar]
- 47.McHan F, Cox NA, Blankenship LC, Bailey JS. 1989. In vitro attachment of Salmonella typhimurium to chick ceca exposed to selected carbohydrates. Avian Dis 33:340–344. doi: 10.2307/1590853. [DOI] [PubMed] [Google Scholar]
- 48.Quilliam RS, Williams AP, Jones DL. 2012. Lettuce cultivar mediates both phyllosphere and rhizosphere activity of Escherichia coli O157:H7. PLoS One 7:e33842. doi: 10.1371/journal.pone.0033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fanta SW, Vanderlinden W, Abera MK, Verboven P, Karki R, Ho QT, De Feyter S, Carmeliet J, Nicolaï BM. 2012. Water transport properties of artificial cell walls. J Food Eng 108:393–402. doi: 10.1016/j.jfoodeng.2011.09.010. [DOI] [Google Scholar]
- 50.Katsikogianni M, Missirlis YF. 2004. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater 8:37–57. [DOI] [PubMed] [Google Scholar]
- 51.Shah N, Ul-Islam M, Khattak WA, Park JK. 2013. Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr Polym 98:1585–1598. doi: 10.1016/j.carbpol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Del Re B, Sgorbati B, Miglioli M, Palenzona D. 2000. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442. doi: 10.1046/j.1365-2672.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 53.Kos B, Susković J, Vuković S, Simpraga M, Frece J, Matosić S. 2003. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- 54.Sinde E, Carballo J. 2000. Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17:439–447. doi: 10.1006/fmic.2000.0339. [DOI] [Google Scholar]
- 55.Flint SH, Brooks JD, Bremer PJ. 1997. The influence of cell surface properties of thermophilic streptococci on attachment to stainless steel. J Appl Microbiol 83:508–517. doi: 10.1046/j.1365-2672.1997.00264.x. [DOI] [PubMed] [Google Scholar]
- 56.Hood SK, Zottola EA. 1995. Biofilms in food processing. Food Control 6:9–18. doi: 10.1016/0956-7135(95)91449-U. [DOI] [Google Scholar]
- 57.Oliveira K, Oliveira T, Teixeira P, Azeredo J, Oliveira R. 2007. Adhesion of Salmonella Enteritidis to stainless steel surfaces. Braz J Microbiol 38:318–323. doi: 10.1590/S1517-83822007000200026. [DOI] [Google Scholar]
- 58.Aruscavage D, Lee K, Miller S, LeJeune JT. 2006. Interactions affecting the proliferation and control of human pathogens on edible plants. J Food Sci 71:R89–R99. doi: 10.1111/j.1750-3841.2006.00157.x. [DOI] [Google Scholar]