Abstract
Microbial starter cultures have extensively been used to enhance the consistency and efficiency of industrial fermentations. Despite the advantages of such controlled fermentations, the fermentation involved in the production of chocolate is still a spontaneous process that relies on the natural microbiota at cocoa farms. However, recent studies indicate that certain thermotolerant Saccharomyces cerevisiae cultures can be used as starter cultures for cocoa pulp fermentation. In this study, we investigate the potential of specifically developed starter cultures to modulate chocolate aroma. Specifically, we developed several new S. cerevisiae hybrids that combine thermotolerance and efficient cocoa pulp fermentation with a high production of volatile flavor-active esters. In addition, we investigated the potential of two strains of two non-Saccharomyces species that produce very large amounts of fruity esters (Pichia kluyveri and Cyberlindnera fabianii) to modulate chocolate aroma. Gas chromatography-mass spectrometry (GC-MS) analysis of the cocoa liquor revealed an increased concentration of various flavor-active esters and a decrease in spoilage-related off-flavors in batches inoculated with S. cerevisiae starter cultures and, to a lesser extent, in batches inoculated with P. kluyveri and Cyb. fabianii. Additionally, GC-MS analysis of chocolate samples revealed that while most short-chain esters evaporated during conching, longer and more-fat-soluble ethyl and acetate esters, such as ethyl octanoate, phenylethyl acetate, ethyl phenylacetate, ethyl decanoate, and ethyl dodecanoate, remained almost unaffected. Sensory analysis by an expert panel confirmed significant differences in the aromas of chocolates produced with different starter cultures. Together, these results show that the selection of different yeast cultures opens novel avenues for modulating chocolate flavor.
INTRODUCTION
The flavor of chocolate is influenced by many parameters, including the genotype and growing conditions of the cocoa trees and the fermentation process of the cocoa pulp, with poor fermentations resulting in astringent and bitter chocolate (1–5). Cocoa pulp is still fermented spontaneously, with reliance on the natural microbiota at cocoa farms. As a result, cocoa pulp fermentations contain a wide variety of microorganisms, with lactic acid bacteria (LAB), acetic acid bacteria (AAB), and yeasts being the main players (6, 7). LAB occur in high numbers in the first phase of the fermentation process. The main LAB metabolites are various organic acids (mainly lactic and acetic acid), carbon dioxide, ethanol, mannitol, and glycerol (8). LAB also produce volatiles, such as diacetyl, acetoin, and acetaldehyde, but their impact on the flavor of cocoa has not yet been investigated (9). However, recent data suggest that these microbes might have only a slight influence on cocoa flavor and quality, since cocoa fermentations lacking LAB had similar shell weights and gave acceptable chocolates with no differences in sensory rankings (10). AAB, which are present mainly at later stages of fermentation, affect cocoa flavor by producing acetic acid, which diffuses into the beans. Together with the increased temperatures generated during the exothermic production of acetic acid, this diffusion of acetic acid contributes to the disruption of cocoa bean cells. As a consequence, various endogenous enzymes involved in the degradation of pigments and the production of flavor precursors, like reducing sugars, amino acids, and peptides, are released (11). These precursors will act as the substrate for Strecker degradation and Maillard reactions during roasting and conching (4). Yeasts are present throughout the fermentation process, with maximal population sizes reached in the middle of the process (12–15). Apart from the primary metabolites ethanol and CO2, yeast cells produce numerous secondary flavor-active metabolites, including aldehydes, carbonyl compounds, esters, fatty acids, higher alcohols, organic acids, phenols, and sulfur-containing compounds (16). Fruity volatiles, such as esters, are especially important, because they impart the fruity character of many fermented beverages (17–19) and are suggested to positively contribute to cocoa flavor (20, 21). Importantly, the production of fruity aroma compounds varies greatly between yeast species (22) and strains (23). Additionally, yeasts have been shown to indirectly impact the flavor of fermented beans, e.g., by reducing acidity or improving the seed protein degradation (24). This way, microbial activity leads (in)directly to the production of important aroma compounds, comprising ethyl-2-methylbutanoate, 2-phenylethanol, 2- and 3-methylbutanoic acid, certain pyrazines (such as tetramethylpyrazine), and short-chain volatile free fatty acids (4, 25).
Given the economic importance of increased efficiency and consistency of cocoa pulp fermentation, several research teams have investigated the possibility of developing starter cultures for controlled fermentation (20, 24, 26–30). An important finding of these studies is that the success of the starter culture depends largely on yeasts, since they have been shown to be indispensable in the development of the chocolate flavor, while other microbes, such as lactic acid bacteria, probably play a much less prominent role (10, 26, 29, 31). Moreover, our research group recently showed that yeast strains that show tolerance to high temperatures are especially suited as starter cultures, because they can outcompete and suppress the growth of the native cocoa microbiota, which increases the consistency and quality of the end product (29). Moreover, our trials also suggested that at least part of the yeast-related flavor(s) (or flavor precursors) produced during fermentation is retained in the final chocolate, despite the high temperatures used during roasting and conching. A recent investigation of a mixed starter culture consisting of Lactobacillus fermentum, Acetobacter pasteurianus, and a highly aromatic Pichia kluyveri strain confirmed this hypothesis, since a significantly higher phenylacetaldehyde concentration was observed in the chocolate (21). However, triangle tests performed in that study did not reveal any differences between the inoculated and spontaneously fermented chocolates. Similarly, Batista and coworkers (30) investigated the influence of a mixed inoculum of Saccharomyces cerevisiae, P. kluyveri, and Hanseniaspora uvarum, but sensory analysis again did not reveal a significant difference with spontaneous fermentation. Therefore, to our knowledge, to date, no study has effectively shown that the application of specific starter cultures enables a reproducible modulation of chocolate flavor.
We hypothesized that the limited success in modulating chocolate flavor with the use of starter cultures is due to the inability of the inoculated flavor-active microbes to dominate the cocoa pulp fermentation, so wild microbes quickly overgrow the inoculated strain(s). In this study, we therefore investigated the possibility of generating novel yeast hybrids that combine fruity flavor production with thermotolerance, which is required for good growth and survival throughout cocoa pulp fermentation. Our results show that we can indeed create hybrids that combine these two properties and that these hybrids influence the final chocolate flavor both directly (by producing aroma-active flavors) and indirectly (by limiting the production of undesired off-flavors). Moreover, we show that different starter cultures yield chocolates with very different sensorial characteristics, which opens novel routes to the production of different chocolate types without the addition of artificial flavor compounds.
MATERIALS AND METHODS
Yeast strains.
All strains used in this study are listed in Table 1.
TABLE 1.
Overview of main yeast strains used in this study
| Strain code | Strain genotype | Strain source or reference |
|---|---|---|
| Natural strains | ||
| Y115 | Wild type (S. cerevisiae) | Bioethanol industry (China) |
| Y181 | Wild type (S. cerevisiae) | Wine industry (Europe) |
| Y184 | Wild type (S. cerevisiae) | Wine industry (Europe) |
| Y274 | Wild type (P. kluyveri) | Isolated from cocoa pulp fermentation (Indonesia) |
| Y354 | Wild type (S. cerevisiae) | Beer industry (ale) (Canada) |
| Y397 | Wild type (S. cerevisiae) | Beer industry (ale) (the Netherlands) |
| Y689 | Wild type (Cyb. fabianii) | Isolated from Xylion adustus (South Africa) |
| Y927 | Wild type (S. cerevisiae) | Isolated from cocoa pulp fermentation (Malaysia) (29) |
| Haploid segregants | ||
| H28-S1 | Haploid segregant of H28 (α) | 29 |
| H28-S2 | Haploid segregant of H28 (α) | 29 |
| Y115-S1 | Haploid segregant of Y115 (a) | 29 |
| Y115-S2 | Haploid segregant of Y115 (a) | 29 |
| Y115-S3 | Haploid segregant of Y115 (α) | 29 |
| Y115-S4 | Haploid segregant of Y115 (α) | 29 |
| Y354-S6 | Haploid segregant of Y354 (α) | 23 |
| Y397-S2 | Haploid segregant of Y354 (a) | 23 |
| Hybrids selected for pilot-scale fermentations | ||
| H28 | Hybrid Y115 × Y927 | 29 |
| H40 | Hybrid H28-S1 × Y397-S2 | This study |
| H42 | Hybrid Y115-S3 × Y397-S2 | This study |
| H57 | Hybrid Y927 × Y184 | This study |
| Screening reference strain | ||
| W303 | Wild type | NCYC 3467 |
Screening for thermotolerance.
Screening for thermotolerance was performed using plate assays. After 16 h of pregrowth in 150 μl of 2% YPD (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto peptone, 2% [wt/vol] glucose) at 30°C (900 rpm), yeast strains were spotted at an initial optical density at 600 nm (OD600) of 0.1 on solid YPD agar (2% [wt/vol] agar) using a high-density array robot (Singer RoToR HDA; Singer Instruments, United Kingdom). The plates were incubated at 30, 37, and 39°C for 48 h and at 40, 41, 41.5, and 42°C for 96 h. Thermotolerance was scored (−, +, ++, or +++) as the colony size at 40°C normalized to that of S. cerevisiae strain W303, a widely used thermotolerant model organism (32). Strains with a thermotolerance comparable to that of W303 were scored as ++.
Sporulation, tetrad dissection, and mating type characterization.
Yeast strains were sporulated, and tetrads were dissected in order to determine the hetero- or homothallic nature of the strains, as described in reference 33. Mating type was determined by PCR, using MAT-a (5′-ACTCCACTTCAAGTAAGAGTT-3′), MAT-α (5′-GCACGGAATATGGGACTACTTCG-3′), and MAT-R (5′-AGTCACATCAAGATCGTTTATGG-3′) as primers, with a temperature profile consisting of 30 cycles of 45 s at 94°C, 45 s at 50°C, 40 s at 72°C, and a final extension of 5 min at 72°C (34).
Genetic fingerprinting.
To analyze the genetic diversity between yeast strains and confirm the presence of both parental genomes in newly formed outcrossed hybrids, a transposon-based PCR approach called interdelta analysis was applied, as described previously (35). Genomic DNA was extracted in a 96-well format using a standard ether extraction protocol (36). PCR was executed with the delta12 (5′-TCAACAATGGAATCCCAAC-3′) and delta21 (5′-CATCTTAACACCGTATATGA-3′) primers and the temperature profile described in reference 35.
Mass mating of cells.
Mass mating of cells was used to obtain hybrids between heterothallic yeast strains (37). First, sporulation was induced on acetate medium (1% potassium acetate [wt/vol], 2% agar [wt/vol]) and incubation was performed at 23°C. After tetrad dissection using a micromanipulator (Singer SMS manual; Singer Instruments, United Kingdom), two selected haploid segregants of opposite mating types from both parental strains were picked, mixed on YPD agar with 10 μl of distilled water (dH2O), and incubated at room temperature for 24 h. A small fraction of the spot was streaked to single colonies and incubated for 48 h. Four single colonies of each spot were subsequently checked for mating type (see above) to identify hybrids; this procedure was repeated if no hybrids were identified.
Mass mating of spore cultures.
In the case of homothallic parental strains, mass mating of spore cultures was used to quickly generate a large number of yeast hybrids (33, 37). Concisely, strains were sporulated on nutrient-poor acetate medium at 23°C (see above). After 5 to 10 days, spores were harvested, and asci (together with the remaining vegetative cells) were lysed by overnight incubation at 35°C (80 rpm) in dH2O supplemented with Zymolyase (100 mg liter−1), chloramphenicol (50 mg liter−1), and 2-marcaptoethanol (2 ml liter−1). Next, the last remainders of vegetative cells were lysed by adding sterile glass beads and vigorous vortexing. The asci were resuspended in dH2O supplemented with 0.75% Triton X-100. To break the asci down into single spores, the cultures were cooled to 4°C and sonicated for 30 s (amplitude, 50%), and this was repeated three times in total. Next, the spore suspension was washed twice with 1.5% Triton X-100, after which it was cooled and sonicated (20 s; amplitude, 50%) two more times. The spores were washed with dH2O and resuspended in 1 ml of 1× phosphate buffer solution. To start mating, spore suspensions of both parental strains were mixed in 50 ml of GNA (3% [wt/vol] peptone, 1% [wt/vol] yeast extract, and 5% [wt/vol] glucose) and incubated overnight (30°C, 80 rpm). Highly thermotolerant hybrids were consequently selected by incubation at high temperatures (42°C) for 5 days. The genotypes of the strains able to grow under these conditions were analyzed further using mating type PCR and genetic fingerprinting (see above).
Lab-scale fermentations in nutrient-rich growth medium.
Lab-scale fermentations in nutrient-rich growth medium were used to assess the aroma production of the yeast strains. Yeast precultures in 5 ml of 2% YPD (4% [wt/vol] glucose) were shaken overnight at 30°C. After 16 h, 500 μl of the preculture was used to inoculate 50 ml of 4% YPD; this second preculture was shaken at 30°C (200 rpm) for 16 h. This preculture was then used to inoculate 10% YPD at an OD600 of 0.5, roughly equivalent to 107 cells ml−1. The fermentations, performed in 250-ml Schott bottles with a water lock placed on each bottle, were incubated statically for 7 days at 20°C. Weight loss was measured daily to estimate fermentation progress. After 7 days, the fermentation medium was filtered (0.15-mm paper filter; Macherey-Nagel, Germany), and samples were taken and analyzed with headspace gas chromatography coupled with flame ionization detection (HS-GC-FID). A total of 24 important aroma compounds, including esters, higher alcohols, and acetaldehyde, were measured. For more details, see the work of Steensels et al. (23).
Lab-scale fermentations in cocoa pulp.
To test strains on a small scale in a cocoa environment, lab-scale fermentations in cocoa pulp were performed in duplicate. Cocoa pulp was (i) separated directly from cocoa beans after opening the cocoa pods by pressing them, (ii) frozen immediately, and (iii) shipped as such from the Ivory Coast to Belgium. Prior to the experiment, the pulp was pasteurized for 5 min at 105°C to eliminate contaminating microbes. Yeast propagation was performed by two propagation stages; first, an overnight pregrowth in 5 ml of 2% YPD (30°C with shaking) was performed, after which 500 μl of this culture was transferred to 50 ml of 2% YPD and incubated overnight at 30°C (200 rpm). This culture was used to inoculate 100 ml of cocoa pulp at an OD600 of 0.5, roughly equivalent to 107 cells ml−1. Next, the cocoa pulp was vigorously mixed to ensure a homogenous distribution of the inoculum. Flasks were sealed with a water lock and incubated at 41°C for 96 h. The bottles were weighed every 24 h to estimate fermentation progress.
Pilot-scale cocoa pulp fermentations.
Pilot-scale cocoa pulp fermentations were performed at the Barry Callebaut Cocoa Research Facility (Pahang, Malaysia) during the main harvest season (October and November 2013). Ten different yeast strains were tested in duplicate, together with two spontaneous controls, resulting in 22 fermentations in total. The fermentations were performed in baskets (0.70 m by 0.50 m by 0.60 m) containing 50 kg of beans and covered with banana leaves. The cocoa pods were harvested and opened after 3 days. Only healthy and mature pods were used for the fermentations, and the placenta was removed after opening the pods. All equipment was thoroughly cleaned before every use. The fermenting beans were turned once after 48 h, and fermentation was stopped after 4 days by spreading the beans on a drying platform, where they were dried in the sun for 10 to 14 days. Temperature and pH were measured in real time in the middle of the fermenting mass using a digital pH meter (pH 3310 SET 2, SenTixH 41; WTW GmbH, Weilheim, Germany). Cocoa bean samples (200 g each) from the fermentations were taken after 0 (fresh cocoa beans, right after opening the pods), 4, 24, 48, 72, and 96 h. The bean samples were cooled to 4°C and analyzed within 1 h. Therefore, a 40-g sample was mixed with 160 ml of 0.1% peptone water in an aseptic stomacher bag. The suspension was powerfully shaken and manually kneaded for 5 min, and a 10-fold serial dilution in 0.1% peptone water was made of the homogeneous cocoa pulp solution. Next, 100 ml from each dilution was spread over three different selective agar plates, de Man-Rogosa-Sharpe medium (MRS) supplemented with 0.01% cycloheximide for LAB (30°C, 2 days), acetic acid medium (AAM) (42°C, 4 days) with 0.01% cycloheximide for AAB, and 2% YPD (30°C, 2 days) supplemented with 0.01% chloramphenicol for yeast growth (14).
Microbial isolation and identification during pilot-scale field trials.
For bacteria, six LAB and three AAB isolates were randomly picked from the countable plates for all time points. Sixteen (at the onset of the fermentation, when the largest yeast diversity was expected [14]) to 10 (at other time points) random yeast isolates per time point were taken to analyze the population dynamics. In total, 645 LAB, 274 AAB, and 1,148 yeast isolates were isolated. All isolates were identified in Belgium to the species level by fingerprinting and rRNA sequencing, as described previously (14, 29). Concisely, viable bacterial isolates were identified using a polyphasic approach, starting with a phenotypic characterization (catalase and oxidase activity). Bacterial isolates suspected of being LAB or AAB according to the phenotypic characterization (185 LAB and 230 AAB) were genetically fingerprinted [using (GTG)5 repetitive-element sequence-based PCR (rep-PCR)] and clustered using the unweighted pair group method using average linkages (UPGMA) algorithm, after which the 16S rRNA of several representatives of each observed cluster was sequenced. For LAB, primers LAC1 and LAC2 were used (38), and primers 27F and 1492R were used for AAB (39, 40). All bacterial sequences were compared to type strain sequences using nucleotide-nucleotide BLAST (blastn) and the EzTaxon database (41), and the percentages of identity were reported when identity was <100%. All yeast isolates were identified by sequencing the 26S rRNA D1/D2 domain amplified using NL1 and NL4 (42), followed by a comparison to the type strain sequences, as with bacterial isolates. In the case of ambiguities concerning identity, the ACT1 and internal transcribed spacer (ITS) regions were additionally amplified using primer pairs CA21 and CA22R (43) and ITS1-F and ITS4 (44, 45), respectively. The obtained sequences were compared to available type strain sequences. In cases of <100% identity, the percentages of identity were reported. For higher resolution, all S. cerevisiae strains isolated from inoculated cocoa pulp fermentations were genetically fingerprinted using interdelta analysis.
Starter culture propagation for pilot-scale field trials.
A starter culture consisting of a single yeast strain was added to the different inoculated fermentations. Yeast propagation was performed in two propagation stages; first, cells were allowed to grow overnight in 5 ml of 2% YPD (30°C, shaking), after which the entire pregrowth was transferred to 300 ml of 10% YPD and incubated overnight (30°C, 180 rpm). The cocoa pulp fermentations were inoculated at an initial density of 106 CFU g−1 using the second preculture. The inoculum was prepared by vacuum filtration over a 0.45-μm-pore-size filter (Millipore, USA) and resuspension of the cells in 0.85% NaCl. The inoculum size was confirmed by counting. Yeast starter cultures were inoculated by pouring them onto the fermentation, and they were manually mixed to ensure a homogeneous distribution.
Chocolate production and sensory analysis.
Fermented cocoa beans were used for cocoa liquor and chocolate production by Barry Callebaut (Wieze, Belgium). According to general practices used for commercial production, cocoa beans were roasted using medium roasting at 122°C. The liquor of each fermentation batch was sampled and stored for gas chromatography-mass spectrometry (GC-MS) analysis. In order to obtain a sufficient mass for chocolate production, the cocoa liquors of the fermentation duplicates were pooled and subsequently refined, supplemented with cocoa butter, and conched for 4 h. Chocolate with 60% (wt/wt) cocoa solids was made, and the liquor and chocolate samples were stored at −18°C for a maximum of 4 weeks before further analysis. All chocolates derived from inoculated fermentation were sensorially analyzed by the Barry Callebaut chocolate expert panel, which consisted of 9 experienced panelists. All chocolates were tasted in comparison to the reference sample, for which purpose the fermentations inoculated with Y115 were picked. A standardized scorecard with 32 categories was used, and panelists were asked to score all categories from 0 to 5; half points were also allowed. In the initial session, the scores for the reference sample were established by the panel. Afterwards, all other samples were randomly divided into groups consisting of two chocolates. The expert panelists tasted and scored two samples in one session and compared them to the reference, Y115.
GC-MS analysis of cocoa liquor and chocolate.
Volatiles of the cocoa liquors and chocolates were extracted and quantified using GC-MS, according to a protocol described previously (29). In short, 5 g of each sample was liquefied at 50°C, spiked with 4 μl of a 4-heptanone internal standard stock solution (0.32296 μg μl−1), and stirred. One hundred micrograms was weighed in duplicate and transferred to a thermal desorption unit (TDU) tube. The TDU temperature was set to 70°C for 30 min for thermal desorption of the volatiles. The GC oven temperature was set at 35°C (held for 5 min) and increased to 240°C (at 5°C min−1, held for 4 min). Mass-to-charge ratios (m/z) between 40 and 300 were scanned, with the mass selective detector operating at 70 eV in electron ionization mode. To identify volatile compounds, the mass spectra of the sample compounds were compared with those in the Wiley 275L database. Compounds were semiquantified by comparing their peak area to the peak area of the 4-heptanone internal standard.
GC-MS data were visualized using principal-component analysis (PCA) performed using the BioNumerics 7.1 software (Applied Maths, Belgium). Therefore, concentrations were first converted to Z-scores for standardization by subtracting the mean value and dividing by the standard deviation of the aroma compound. To investigate whether samples differed significantly, Wilk's λ likelihood ratio test was used. Correlations were calculated using the Pearson or nonparametric Spearman correlation coefficient.
Nucleotide sequence accession numbers.
The DNA sequences of representative isolates of the different species have been deposited in GenBank (accession numbers KP190150, KP190151, KP190153 to KP190159, KP190161, KP190164 to KP190168, KP190170, KP190171, KP190174, and KR136365 to KR136368).
RESULTS
The goal of this study was to investigate whether it is possible to tune chocolate flavor by inoculating highly aromatic yeasts in cocoa pulp fermentations. We generated novel hybrids of the widely used generally recognized as safe (GRAS) organism S. cerevisiae, which carries the qualified presumption of safety (QPS) status, with the aim of combining compounds with a high production of flavor with characteristics important for an efficient cocoa pulp fermentation process, such as high thermotolerance and fermentation capacity. We generated hybrids from different parental strains to investigate whether certain combinations yielded better strains and to evaluate whether different hybrids would yield different chocolate flavors. Additionally, two non-Saccharomyces strains with a high bioflavoring potential (P. kluyveri and Cyberlindnera fabianii) were included in these trials. All strains were used as single-strain starter cultures for cocoa pulp fermentations, after which the resulting beans were further processed into chocolate.
Selection of parental strains and aromatic non-Saccharomyces yeasts.
Robust S. cerevisiae strains (i.e., strains that thrive in the highly selective cocoa pulp environment) were previously selected and developed to increase the efficiency and consistency of cocoa pulp fermentations (29). In this study, three such robust strains were selected for further breeding: Y927, an indigenous cocoa strain dominant in spontaneous fermentations; Y115, a bioethanol strain that outcompetes Y927 in a cocoa pulp environment; and their hybrid H28, which shows even better growth in cocoa pulp (Table 2).
TABLE 2.
Selected parental S. cerevisiae strains for the generation of novel hybridsa
| Strain name | Parent type | Thermotoleranceb | Isoamyl acetate production (AU)c | Spore viability (%) | Mating type segregantsd |
|---|---|---|---|---|---|
| H28 | Robust | +++ | 0.5 | 75 | a, α, or a/α |
| Y115 | Robust | +++ | 0.7 | 100 | a or α |
| Y181 | Aromatic | + | 3.6 | 50 | a/α |
| Y184 | Aromatic | ++ | 4.0 | 94 | a/α |
| Y354 | Aromatic | ++ | 3.6 | 31 | a or α |
| Y397 | Aromatic | + | 2.7 | 44 | a, α, or a/α |
| Y927 | Robust | +++ | 1.6 | 100 | a/α |
Strains Y115, Y927, and H28 were selected based on their thermotolerance and excellent performance during inoculated cocoa pulp fermentations. Y181, Y184, Y354, and Y397 were selected based on their high fruity aroma production.
+, less than that of the reference strain; ++, equal to that of the reference; +++, more than that of the reference. The reference for thermotolerance was S. cerevisiae W303 (32).
Isoamyl acetate production was measured in nutrient-rich growth medium (10% YPD) and compared to the average production of 236 S. cerevisiae strains (23). AU, arbitrary units.
Mating types were determined using mating-type PCR.
Aromatic parents were selected out of a large pool of 236 industrial S. cerevisiae strains used for the production of beer, sake, bread, bioethanol, and wine (23). Strains were selected for high isoamyl acetate production, the main determinant of fruity flavors in fermented beverages, and for the production of longer-chain acetate esters that yield an apple-like flavor and are slightly less volatile and more fat soluble. This led to the selection of four aromatic parents, two industrial ale (Y354 and Y397) and two industrial wine (Y181 and Y184) strains. Both ale yeasts have already been shown to yield highly aromatic hybrids in breeding experiments (23). The wine strains Y181 and Y184 showed high isoamyl acetate (Table 2) and phenylethyl acetate production (respectively, 210% and 170% of the average phenylethyl acetate production of the 236 industrial S. cerevisiae strains).
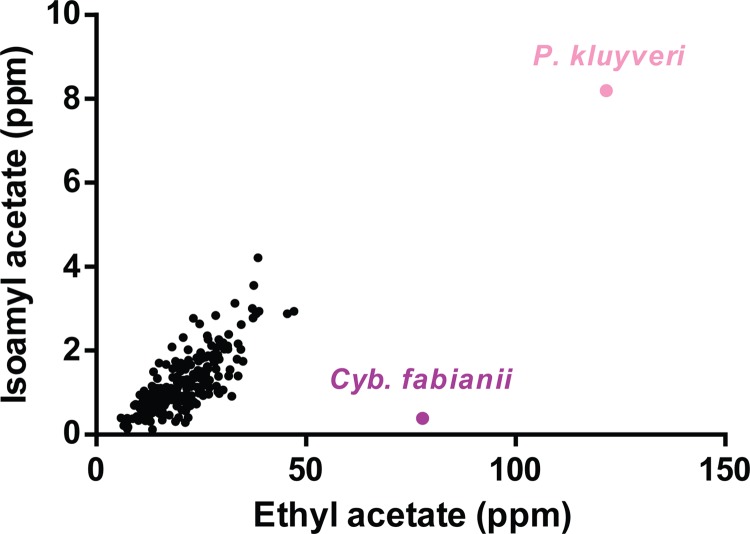
In addition to selecting the S. cerevisiae parental strains to generate novel hybrids, we selected two aromatic non-Saccharomyces yeast strains. P. kluyveri Y274 and Cyb. fabianii Y689 produced very large quantities of the volatile acetate esters isoamyl acetate and/or ethyl acetate (Fig. 1). Moreover, since a previous study hypothesized that P. kluyveri starter cultures might influence the aroma profile of cocoa liquor and chocolate (even though these differences were not perceived by a consumer panel), we considered it a good reference for our assays (21). Importantly, the P. kluyveri strain Y274 used in this study showed a lower thermotolerance than that of the selected S. cerevisiae parents, showing no growth at 39°C (see Fig. S1 in the supplemental material). The selected Cyb. fabianii strain, on the other hand, was the best-growing strain at 42°C.
FIG 1.
Aroma production of selected aromatic yeasts, P. kluyveri and Cyb. fabianii. Levels of isoamyl acetate and ethyl acetate production in nutrient-rich growth medium were compared in 236 industrial S. cerevisiae strains (the strain collection and data were derived from reference 23).
Development of hybrids that combine robust and aromatic characteristics.
To generate S. cerevisiae hybrids that combine the desired characteristics of both the efficiently fermenting dominant parents (“robust parents”) and the aromatic S. cerevisiae parents, mass mating of cells or spore cultures was performed, depending on whether the parental strains were heterothallic or homothallic. For strains that were able to produce stable haploid segregants (H28, Y115, Y354, and Y397), mass mating of cells was applied (Table 2). Previously identified thermotolerant segregants of Y115 and H28 (29) were crossed with two previously identified aromatic segregants of Y354 and Y397 (23). Next, six hybrids were selected for further analysis: two between segregants of Y115 and Y354 (H38 and H39), two with segregants of H28 and Y397 (H40 and H41), and two with segregants of Y115 and Y397 (H42 and H43) (see Table S1 in the supplemental material).
For the homothallic wine strains Y181 and Y184, the mass mating of the spore culture technique was used to generate hybrids. These two aromatic parents were mated with the robust parents Y115 and Y927 by mixing parental spore solutions in all four possible pairwise combinations (the pairs always included one robust and one aromatic strain). The resulting heterogeneous pools of hybrids were subjected to a selection step at 42°C directly after mass mating of spore cultures to select hybrids with tolerance to high temperatures (see Materials and Methods for details). This selection yielded 90 hybrids that were able to grow at 42°C. It has to be noted here that the high temperature tolerance of the hybrids might be due to either favorable mutations that occurred during the experiment or factors related to the hybridization event (e.g., dominance complementation, overdominance, or a combination of favorable, purging, or disadvantageous alleles). To distinguish between hybrids of both parents (outcrossed hybrids) and inbred hybrids, the strains were fingerprinted using interdelta analysis (see Fig. S2 in the supplemental material). This analysis identified 16 outcrossed hybrids (H44 to H59 [two Y181 × Y115 hybrids, one Y181 × Y927 hybrid, one Y184 × Y115 hybrid, and 12 Y184 × Y927 hybrids]) (see Table S1 in the supplemental material), which were further analyzed for their aroma production and ability to ferment cocoa pulp, together with the six hybrids obtained through mass mating of cells.
Hybrids combine desired characteristics of robust and aromatic parental strains.
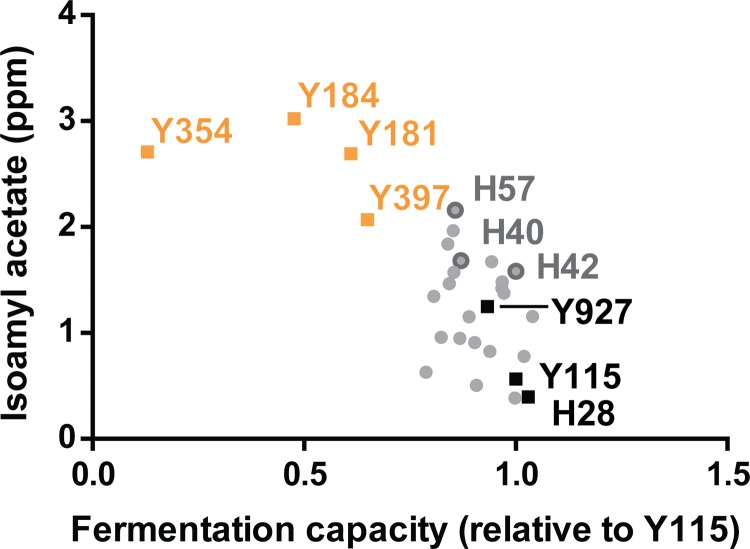
The fermentation capacity in cocoa pulp and aroma production of the 22 newly generated S. cerevisiae hybrids were investigated and compared to those of the parental strains to evaluate their potential as starter cultures for cocoa pulp fermentation. These lab-scale trials confirmed that the aromatic parents combined high isoamyl acetate production with a relatively limited fermentation capacity in cocoa pulp at 41°C, while the opposite was observed for the robust parents (Fig. 2). Interestingly, many hybrids combined the two characteristics, producing up to almost five times more isoamyl acetate than their robust parents and utilizing up to eight times more sugar in cocoa pulp at a high temperature than their aromatic parents. Additionally, thermotolerance was investigated, confirming that the hybrids showed an improved tolerance compared to that of the aromatic parents (and sometimes even compared to that of the robust parental strains) (see Fig. S1 in the supplemental material).
FIG 2.
Aroma production and cocoa pulp fermentation capacities of parental strains and 22 newly generated hybrids (all S. cerevisiae). Fermentation capacity is expressed as CO2 production (a measure for sugar utilization) in cocoa pulp at 41°C, relative to that of the robust parent Y115. Fruity aroma production is expressed as isoamyl acetate production in nutrient-rich growth medium (see Materials and Methods for details). Robust and aromatic parents are indicated with black and orange squares, respectively; hybrids are represented by filled gray circles. Hybrids selected for inoculated pilot-scale cocoa pulp fermentations are outlined with a dark-gray circle.
Three hybrids, H40, H42, and H57, which showed a desired combination of fruity aroma production and high fermentation capacity in cocoa pulp, were selected for further pilot-scale tests (Fig. 2). H40 and H42 were generated using mass mating of cells of segregants Y397-S2 with H28-S1 and Y115-S3, respectively. H57 was the result of mass mating of spore cultures Y927 and Y184.
Pilot-scale testing in the field identifies interesting yeast starter cultures.
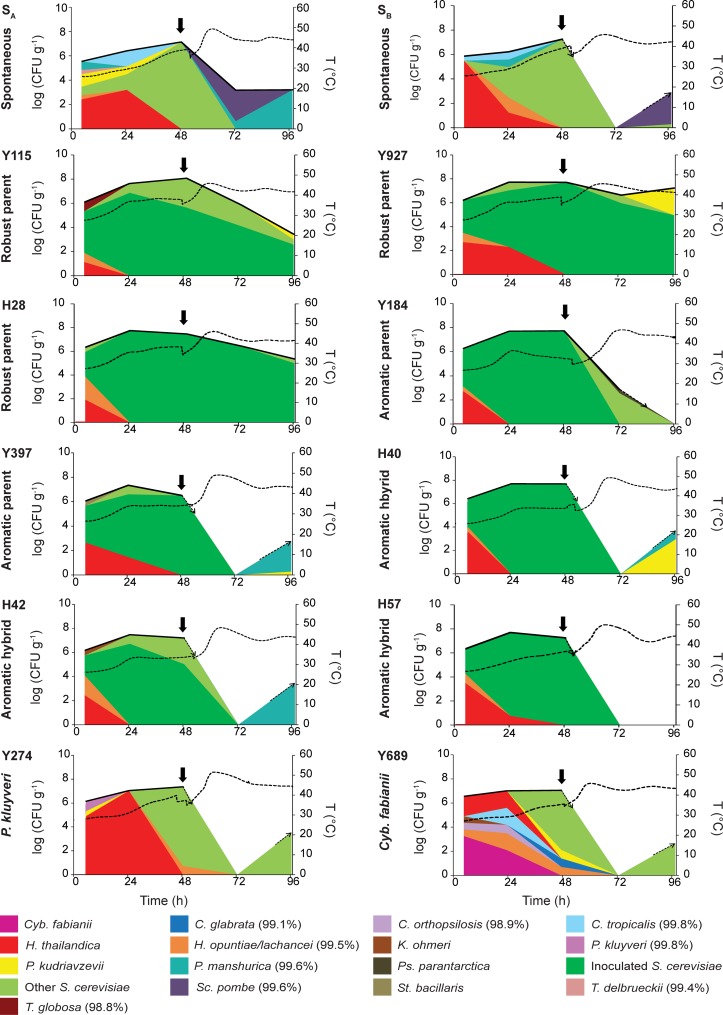
In order to assess the influence of the selected strains on cocoa flavor, a series of inoculated cocoa pulp fermentation field trials were set up in Malaysia under industrially relevant conditions. In total, 10 strains (three selected hybrids, the five parental strains of these three hybrids, and the two non-Saccharomyces strains) were tested in duplicate. All strains were inoculated at a density of 106 CFU g−1 as single-strain starter cultures. Two spontaneous fermentations (previously described in reference 29), carried out simultaneously with the inoculated fermentations, were used as a control, and temperature and pH were measured in real time in all fermentations (Fig. 3; see also Table S2 in the supplemental material).
FIG 3.
Yeast populations in spontaneous and inoculated cocoa pulp pilot-scale fermentations. Two spontaneous fermentations (SA and SB, previously described in reference 29) and 20 inoculated fermentations (10 strains in duplicate; “A” replicates are shown here, and “B” replicates are shown in Fig. S3 in the supplemental material) were monitored (see Materials and Methods for details). Temperature (T) (right y axis) is indicated by a dashed black line. The dashed arrows indicate when the yeast cell count rises to or drops below the detection limit. Note that the scale for the total yeast count (left y axis) is logarithmic, whereas individual yeast species in the population at a given time point (indicated by the colors) are presented as a fraction of the total population. The absolute concentrations for individual species are given in Table S3 in the supplemental material. Percentages of identity are reported when there was <100% identity with type strain sequences (see Materials and Methods). Cocoa beans were turned after 48 h of fermentation (arrows). Cyb., Cyberlindnera; C., Candida; H., Hanseniaspora; K., Kodamaea; P., Pichia; Ps., Pseudozyma; S., Saccharomyces; Sc., Schizosaccharomyces; St., Starmerella; T., Torulaspora.
To investigate the yeast population dynamics in the cocoa pulp fermentations, samples were taken at regular time points, and a large number of yeast isolates was identified by rRNA sequencing; all S. cerevisiae strains isolated from the inoculated fermentations were genetically fingerprinted using interdelta analysis for identification to the strain level (Fig. 3; see also Table S3 in the supplemental material). The spontaneous reference fermentations were characterized by a large yeast diversity, with six to eight different species identified. Hanseniaspora thailandica, S. cerevisiae, Schizosaccharomyces pombe, and Pichia manshurica were shown to be the most abundant. The inoculation of an S. cerevisiae strain resulted in a significant decrease in the number of yeast species (P = 0.0131 for all S. cerevisiae strains in the pilot-scale tests). The yeast diversity was the largest at the start of the fermentations, right after the starter culture was added. H. thailandica was observed in all fermentations at 4 h, except for Y397B. Hanseniaspora opuntiae/Hanseniaspora lachancei was also frequently observed at the start, occurring in 6 of the 20 inoculated fermentations. After 24 h of fermentation, all inoculated strains reached an average fraction of 85% (Y397) to 100% (H40 and Y184) of the total yeast population. Between 24 h and 48 h, the relative fraction of the starter culture decreased slightly (Y115, Y397, and H42), remained stable (Y184, H28, and H40), or increased further (Y927 and H57) but always remained between 80% (H42 and Y397) and 100% (H40, H57, and Y184). The robust S. cerevisiae parents Y115, Y927, and H28 were characterized by their continuous presence until the end of the fermentation, surviving the high temperatures observed after turning (29). However, the aromatic parents Y184 and Y397 and the three hybrids disappeared after turning at 48 h. Despite their faster disappearance, the aromatic parents and hybrids were still able to repress the growth of naturally occurring yeasts, as shown by a significant reduction in the yeast species present compared to that in the spontaneous fermentations (P = 0.0303, Mann-Whitney test).
The two inoculated non-Saccharomyces strains, P. kluyveri and Cyb. fabianii, never reached high relative population sizes and quickly disappeared below the detection limit. Moreover, the inoculation of these strains did not result in a significant reduction in the number of observed yeast species compared to that in spontaneous fermentations (P = 0.4000, Mann-Whitney test). Up to nine different yeast species were detected in the fermentation inoculated with Cyb. fabianii (Y689A). P. kluyveri was still detected at 4 h (Y274A) but disappeared thereafter. The inoculated Cyb. fabianii strain was detected up until 24 h but reached a maximal relative population size of only 40% (Y689B).
Interestingly, in some cases, the inoculation of different yeasts led to significant differences in the bacterial populations. Fermentations inoculated with the robust parental strains H28, Y115, and Y927 contained fewer LAB and AAB at the turning at 48 h (PLAB < 0.0001, PAAB = 0.0209, unpaired t test) but more at 72 h (PLAB = 0.0174, PAAB = 0.0263, unpaired t test) than the nonrobust strains. Additionally, fewer LAB were found in the former fermentations at 24 h (P < 0.0001). Maximal temperatures (typically after 64 h) were lower in the fermentations inoculated with the robust parents than in the other fermentations (P = 0.0031, unpaired t test), whereas no significant differences were found in the times needed to reach these maximal temperatures after the turning. In line with previous studies, only a limited number of LAB and AAB species were present in the inoculated fermentations (see Table S4 in the supplemental material). Lactobacillus fermentum, Leuconostoc pseudomesenteroides, and Acetobacter pasteurianus were the most abundant species present in both the spontaneous and inoculated fermentations.
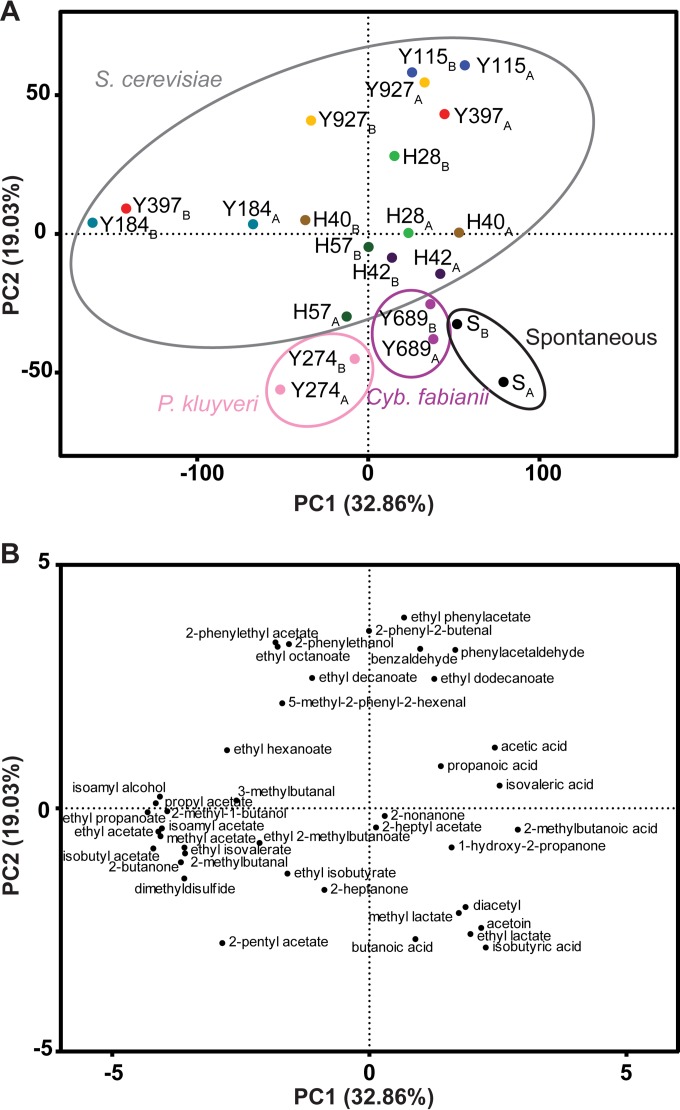
Fruity aromas produced by yeasts during fermentation are conserved in cocoa liquor.
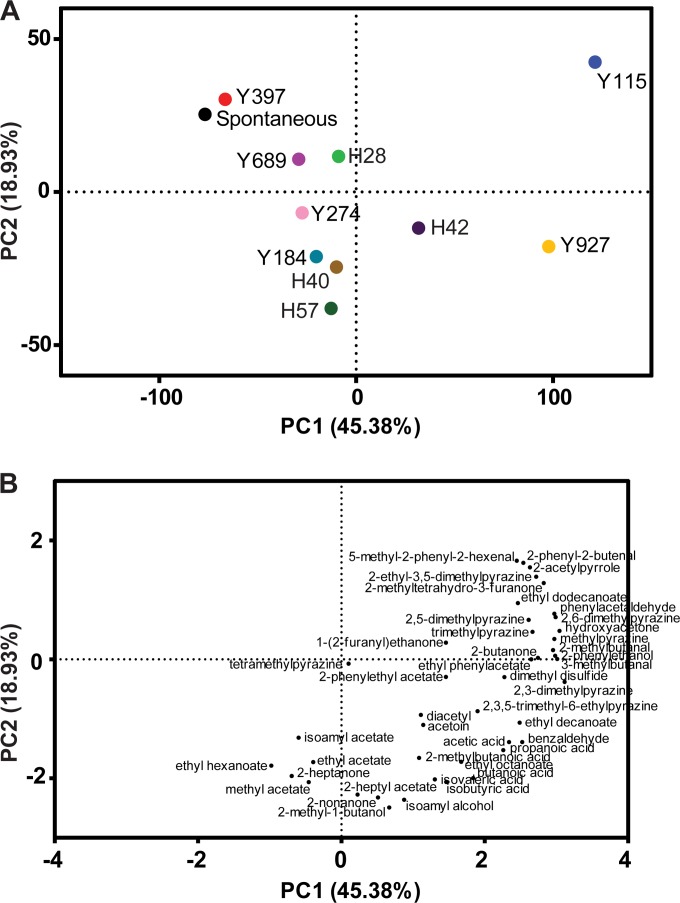
Next, we analyzed to what extent the aromas produced by the starter culture were still present after the downstream processing involved in the production of cocoa bean to liquor, e.g., drying and roasting. To this end, fermented cocoa beans from both inoculated and spontaneous fermentations were further processed into cocoa liquor, and the volatile fractions of these liquor samples were analyzed using GC-MS, enabling the identification of 41 volatile compounds related to fermentation, including esters, higher alcohols, aldehydes, volatile acids, and ketones (Table 3). Principal-component analysis (PCA) was used to determine the influence of the yeast starter culture and to compare the flavor profiles of the duplicate fermentations (Fig. 4). Liquors from spontaneous fermentations contained significantly more of the rancid and buttery flavors isobutyric acid, diacetyl, acetoin, ethyl lactate, and butanoic acid than did all inoculated fermentations (P < 0.033, Wilk's λ likelihood ratio test). The second principal component (PC2) indicated a clear separation between the fermentations inoculated with an S. cerevisiae strain on one hand and the spontaneous controls and liquor samples inoculated with the two non-Saccharomyces yeasts on the other hand (Fig. 4A). This corresponded to higher concentrations of acetate and ethyl esters in the S. cerevisiae-inoculated fermentations (Fig. 4B). The influence of the P. kluyveri starter culture on isoamyl acetate concentrations in the liquor was limited, while some S. cerevisiae strains showed a much larger effect (Table 3). The P. kluyveri liquor samples were characterized by the highest 2-pentyl acetate and 2-heptanone concentrations (P = 0.003 and 0.001, respectively, Wilk's λ likelihood ratio test) (Table 3). Cyb. fabianii liquors contained the most acetic acid (P = 0.046, Wilk's λ likelihood ratio test). All in all, the influence of the two non-Saccharomyces strains on the liquor volatiles was limited, likely because these yeasts never dominated the fermentations and quickly disappeared below the detection limit.
TABLE 3.
Volatiles identified using GC-MS in cocoa liquors made from spontaneous and inoculated cocoa pulp fermentationsa
| Volatile | Concn (μg/kg) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robust parents |
Aromatic parents |
Aromatic hybrids |
P. kluyveri Y274 | Cyb. fabianii Y689 | S | ||||||
| Y115 | Y927 | H28 | Y184 | Y397 | H40 | H42 | H57 | ||||
| Aldehydes | |||||||||||
| 2-Methylbutanal | 21.6 ± 2.7 | 13.3 ± 4.9 | 7.2 ± 0.4 | 46.5 ± 35.8 | 27.9 ± 23.2 | 30.8 ± 21.7 | 15.5 ± 8.3 | 22.7 ± 6.8 | 46.9 ± 14.4 | 17.7 ± 5.1 | 11.3 ± 3.0 |
| 2-Phenyl-2-butenal | 27.1 ± 16.0 | 17.4 ± 1.0 | 11.9 ± 3.3 | 8.6 ± 1.5 | 13.8 ± 1.3 | 12.5 ± 2.6 | 7.4 ± 2.5 | 4.9 ± 2.8 | 4.7 ± 0.7 | 4.4 ± 1.2 | 5.3 ± 1.6 |
| 3-Methylbutanal | 81.8 ± 10.5 | 43.0 ± 1.1 | 25.5 ± 2.4 | 105.8 ± 60.8 | 64.1 ± 9.6 | 93.2 ± 29.8 | 48.4 ± 21.6 | 81.0 ± 30.1 | 82.3 ± 19.7 | 42.2 ± 4 | 40.9 ± 10.6 |
| 5-Methyl-2-phenyl-2-hexenal | 4.7 ± 1.2 | 4.7 ± 1.8 | 2.1 ± 0.2 | 5.3 ± 3.2 | 3.9 ± 3.0 | 4.4 ± 3.5 | 1.6 ± 0.7 | 3.4 ± 1.2 | 2.9 ± 0.3 | 2.3 ± 0.2 | 1.8 ± 0.8 |
| Benzaldehyde | 70.2 ± 6.2 | 50.0 ± 10.1 | 34.7 ± 9.9 | 32.5 ± 8.6 | 58.6 ± 30.0 | 68.0 ± 33.9 | 37.5 ± 21.9 | 27.5 ± 14.4 | 21.1 ± 2.6 | 21.2 ± 6.2 | 19.8 ± 5.5 |
| Phenylacetaldehyde | 29.2 ± 1.9 | 14.6 ± 6.0 | 7.2 ± 0.6 | 8.1 ± 0.7 | 16.1 ± 10.2 | 13.2 ± 0.4 | 10.0 ± 3.6 | 13.2 ± 10.5 | 5.2 ± 2.6 | 9.4 ± 2.4 | 9.9 ± 1.3 |
| Alcohols | |||||||||||
| 2-Methyl-1-butanol | 4.7 ± 5.2 | 8.1 ± 2.5 | 7.2 ± 0.2 | 17.4 ± 1.8 | 10.4 ± 9.5 | 6.2 ± 2.0 | 5.5 ± 1.2 | 7.0 ± 1.2 | 7.5 ± 3.1 | 6.7 ± 2.0 | 5.5 ± 1.2 |
| 2-Phenylethanol | 115.2 ± 12.1 | 85.7 ± 14.3 | 49.6 ± 26.2 | 82.1 ± 1.7 | 103.8 ± 35.5 | 62.8 ± 11.9 | 40.2 ± 6.8 | 59.4 ± 40.2 | 41.8 ± 2.5 | 36.7 ± 13.9 | 48.1 ± 4.3 |
| Isoamyl alcohol | 22.7 ± 19.6 | 65.0 ± 22.9 | 37.3 ± 8.4 | 109.2 ± 12.6 | 83.9 ± 92.5 | 45.2 ± 20.6 | 50.9 ± 6.7 | 57.0 ± 5.8 | 38.5 ± 2.0 | 33.0 ± 5.4 | 32.2 ± 6.6 |
| Esters | |||||||||||
| 2-Heptyl acetate | 9.4 ± 2.0 | 9.0 ± 0.4 | 9.1 ± 5.1 | 4.7 ± 0.5 | 10.1 ± 3.3 | 23.0 ± 11.5 | 8.7 ± 1.8 | 20.4 ± 13.4 | 19.3 ± 3.1 | 4.4 ± 0.7 | 6.6 ± 1.9 |
| 2-Pentyl acetate | 6.1 ± 0.1 | 10.8 ± 6.5 | 8.9 ± 0.2 | 26.9 ± 6.5 | 19.7 ± 13.6 | 17.4 ± 6.5 | 22.8 ± 2.8 | 25.0 ± 12.4 | 38.4 ± 2.5 | 18.9 ± 4.6 | 12.3 ± 6.6 |
| 2-Phenylethyl acetate | 89.4 ± 9.5 | 61.7 ± 6.9 | 41.9 ± 23.5 | 58.3 ± 4.9 | 79.3 ± 48.7 | 43.7 ± 5.2 | 27.6 ± 0.8 | 30.6 ± 16.7 | 25.4 ± 0.8 | 25.5 ± 5.6 | 22.8 ± 5.8 |
| Ethyl 2-methylbutanoate | 0.4 ± 0.4 | 0.5 ± 0.5 | 0.3 ± 0.1 | 0.4 ± 0.5 | 0.7 ± 0.9 | 0.6 ± 0.6 | 0.5 ± 0.1 | 0.3 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Ethyl acetate | 4.0 ± 3.2 | 15.3 ± 18.2 | 3.1 ± 1.8 | 260.5 ± 98.3 | 76.2 ± 105.7 | 27.4 ± 36.4 | 15.7 ± 12.6 | 47.8 ± 21.5 | 81.8 ± 12.7 | 13.5 ± 7.4 | 3.1 ± 1.6 |
| Ethyl decanoate | 3.6 ± 1.9 | 7.3 ± 0.0 | 3.4 ± 2.3 | 4.5 ± 0.5 | 2.5 ± 0.1 | 3.3 ± 2.4 | 2.7 ± 0.5 | 2.4 ± 0.5 | 2.2 ± 0.1 | 1.9 ± 0.3 | 2.6 ± 0.3 |
| Ethyl dodecanoate | 5.2 ± 2.8 | 3.4 ± 0.1 | 2.4 ± 0.3 | 1.8 ± 0.5 | 5.6 ± 3.1 | 3.6 ± 0.8 | 3.7 ± 2.1 | 1.5 ± 0.8 | 2.3 ± 0.1 | 1.7 ± 0.0 | 2.1 ± 0.6 |
| Ethyl hexanoate | 3.3 ± 0.9 | 9.8 ± 2.0 | 5.4 ± 1.0 | 8.3 ± 0.5 | 6.2 ± 1.9 | 6.1 ± 1.5 | 6.2 ± 0.3 | 5.5 ± 1.8 | 5.6 ± 1.3 | 3.8 ± 0.2 | 4.1 ± 0.8 |
| Ethyl isobutyrate | 0.6 ± 0.8 | 0.4 ± 0.4 | 0.1 ± 0.0 | 0.7 ± 0.4 | 0.6 ± 0.7 | 0.4 ± 0.4 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.8 ± 0.4 | 0.9 ± 0.2 | 0.7 ± 0.9 |
| Ethyl isovalerate | 0.3 ± 0.3 | 0.7 ± 0.8 | 0.7 ± 0.0 | 1.2 ± 0.4 | 1.0 ± 0.9 | 0.9 ± 0.6 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.0 |
| Ethyl lactate | 1.0 ± 0.3 | 2.1 ± 0.2 | 1.0 ± 0.5 | 3.4 ± 1.0 | 2.1 ± 2.9 | 1.2 ± 0.3 | 13.6 ± 7.3 | 3.9 ± 0.2 | 4.3 ± 0.6 | 8.8 ± 1.3 | 19.7 ± 0.7 |
| Ethyl octanoate | 12.5 ± 1.9 | 23.9 ± 0.4 | 11.5 ± 5.4 | 17.9 ± 0.1 | 14.7 ± 4.4 | 12.2 ± 2.4 | 13.6 ± 5.0 | 7.3 ± 3.2 | 3.6 ± 0.4 | 4.2 ± 0.9 | 5.8 ± 1.5 |
| Ethyl phenylacetate | 6.6 ± 1.1 | 4.4 ± 0.0 | 2.9 ± 1.3 | 3.2 ± 0.2 | 3.3 ± 1.4 | 2.5 ± 0.4 | 2.1 ± 0.9 | 2.0 ± 1.4 | 1.1 ± 0.3 | 2.4 ± 0.2 | 2.4 ± 0.8 |
| Ethyl propanoate | 0.1 ± 0.0 | 0.2 ± 0.2 | 0.3 ± 0.3 | 1.0 ± 0.3 | 0.5 ± 0.6 | 0.3 ± 0.3 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Isoamyl acetate | 31.0 ± 20.6 | 115.3 ± 55.0 | 84.9 ± 9.7 | 209.9 ± 33.4 | 224.3 ± 241.1 | 105.9 ± 44.7 | 124.6 ± 14.5 | 125.6 ± 33.1 | 118.3 ± 18.2 | 93.5 ± 2.3 | 59.2 ± 33.6 |
| Isobutyl acetate | 1.8 ± 0.8 | 4.7 ± 3.9 | 2.6 ± 0.3 | 24.1 ± 10.0 | 21.5 ± 28.9 | 7.3 ± 7.4 | 7.0 ± 0.6 | 10.4 ± 4.8 | 13.6 ± 2.8 | 8.0 ± 3.5 | 2.6 ± 1.3 |
| Methyl acetate | 1.8 ± 0.6 | 1.4 ± 0.8 | 0.9 ± 0.1 | 50.1 ± 51.7 | 33.4 ± 45.4 | 11.0 ± 14.5 | 2.8 ± 2.4 | 10.8 ± 1.2 | 24.3 ± 2.2 | 3.3 ± 0.9 | 6.2 ± 2.9 |
| Methyl lactate | 0.5 ± 0.6 | 0.9 ± 0.4 | 0.6 ± 0.2 | 0.9 ± 0.0 | 0.3 ± 0.3 | 2.3 ± 2.3 | 2.1 ± 1.8 | 1.1 ± 0.4 | 1.0 ± 0.1 | 1.9 ± 0.0 | 2.6 ± 0.6 |
| Propyl acetate | 0.4 ± 0.5 | 0.7 ± 0.8 | 0.4 ± 0.1 | 5.6 ± 1.1 | 2.0 ± 2.6 | 0.8 ± 1.1 | 0.7 ± 0.5 | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Ketones | |||||||||||
| 1-Hydroxy-2-propanone | 36.9 ± 8.5 | 34.6 ± 0.3 | 28.3 ± 1.8 | 33.4 ± 6.7 | 27.7 ± 5.9 | 40.9 ± 0.1 | 31.5 ± 8.6 | 33.5 ± 0.1 | 36.8 ± 1.2 | 36.4 ± 1.7 | 37.1 ± 9.8 |
| 2-Butanone | 2.1 ± 0.2 | 1.1 ± 0.6 | 0.8 ± 0.0 | 5.0 ± 1.5 | 3.3 ± 2.2 | 2.8 ± 1.1 | 1.4 ± 0.5 | 2.6 ± 1.1 | 4.3 ± 0.4 | 2.1 ± 0.9 | 1.5 ± 0.4 |
| 2-Heptanone | 6.5 ± 3.4 | 13.5 ± 3.5 | 10.8 ± 1.1 | 8.9 ± 0.0 | 11.7 ± 5.4 | 23.3 ± 3.6 | 13.8 ± 4.0 | 28.7 ± 18.3 | 36.9 ± 6.0 | 7.0 ± 0.3 | 8.6 ± 0.6 |
| 2-Nonanone | 18.5 ± 6.2 | 16.4 ± 0.5 | 15.2 ± 7.2 | 7.8 ± 1.1 | 14.6 ± 3.0 | 37.0 ± 20.4 | 9.6 ± 4.3 | 45.8 ± 41.3 | 36.4 ± 7.9 | 4.6 ± 1.6 | 9.5 ± 0.5 |
| Acetoin | 598.0 ± 183.6 | 405.6 ± 89.1 | 426.6 ± 19.0 | 388.0 ± 78.8 | 366.7 ± 5.0 | 482.0 ± 163.0 | 602.6 ± 102.2 | 526.9 ± 227.1 | 681.6 ± 214.2 | 811.4 ± 191.2 | 1,208.0 ± 337.0 |
| Diacetyl | 89.4 ± 34.0 | 61.1 ± 16.8 | 56.2 ± 0.1 | 50.0 ± 7.1 | 52.7 ± 9.0 | 69.1 ± 25.1 | 70.1 ± 3.0 | 75.0 ± 39.3 | 96.9 ± 24.9 | 87.2 ± 4.7 | 165.6 ± 38.8 |
| Sulfide | |||||||||||
| Dimethyl disulfide | 1.8 ± 0.8 | 0.9 ± 0.2 | 0.6 ± 0.0 | 9.7 ± 6.2 | 4.9 ± 4.1 | 4.6 ± 1.8 | 2.8 ± 1.8 | 4.4 ± 0.7 | 8.6 ± 4.8 | 2.9 ± 1.9 | 1.1 ± 0.2 |
| Volatile acids | |||||||||||
| 2-Methylbutanoic acid | 77.6 ± 5.5 | 51.0 ± 20.4 | 47.0 ± 2.6 | 45.3 ± 5.8 | 58.6 ± 22.9 | 83.5 ± 21.7 | 61.3 ± 43.9 | 57.2 ± 17.6 | 58.6 ± 5.1 | 81.2 ± 1.0 | 78.6 ± 31.2 |
| Acetic acid | 10,163.4 ± 882.0 | 10,696.5 ± 1,780.1 | 9,383.5 ± 1,627.1 | 7,062.3 ± 2,066.6 | 6,264.2 ± 462.0 | 7,710.1 ± 190.7 | 9,374.9 ± 1,153.9 | 5,058.7 ± 567.4 | 5,181.7 ± 1,666.9 | 11,565.8 ± 2,901.1 | 9,346.5 ± 206.4 |
| Butanoic acid | 9.7 ± 0.0 | 7.7 ± 1.4 | 6.8 ± 1.5 | 8.7 ± 2.8 | 6.6 ± 0.1 | 10.5 ± 0.5 | 12.5 ± 1.8 | 6.6 ± 1.3 | 18.2 ± 7.7 | 9.7 ± 2.1 | 16.4 ± 2.7 |
| Isobutyric acid | 148.2 ± 9.5 | 119.7 ± 42.9 | 99.4 ± 5.1 | 140.5 ± 20.9 | 193.9 ± 69.7 | 295.2 ± 65.3 | 211.2 ± 105.2 | 243.9 ± 55.6 | 242.2 ± 66.9 | 390.5 ± 11.7 | 435.0 ± 117.9 |
| Isovaleric acid | 253.2 ± 102.3 | 204.1 ± 77.4 | 148.4 ± 21.0 | 201.5 ± 61.0 | 265.1 ± 113.1 | 306.8 ± 67.6 | 268.3 ± 100.2 | 186.9 ± 5.3 | 161.6 ± 49.2 | 246.5 ± 16.6 | 279.6 ± 27.5 |
| Propanoic acid | 62.4 ± 22.2 | 40.5 ± 3.7 | 38.4 ± 5.4 | 44.1 ± 17.7 | 38.9 ± 2.6 | 53.0 ± 0.1 | 61.7 ± 20.8 | 33.2 ± 0.3 | 43.5 ± 4.5 | 34.5 ± 3.6 | 48.9 ± 1.1 |
Two spontaneous (S) and 20 inoculated fermentations were performed, with 10 yeast strains tested in duplicate. Cocoa liquors were made separately from all fermentations, and the values represent the average concentrations ± SD of fermentation duplicates.
FIG 4.
Principal-component analysis of volatile compounds in cocoa liquor derived from spontaneous and yeast-inoculated fermentations. (A) Score plot of two spontaneous controls (SA and SB, previously described in reference 29) and 20 inoculated fermentations (10 strains tested in duplicate, represented as A and B). All yeasts are S. cerevisiae strains, except for Y274 (P. kluyveri) and Y689 (Cyb. fabianii). (B) Loading plot of 41 volatile compounds related to cocoa pulp fermentation, identified using GC-MS.
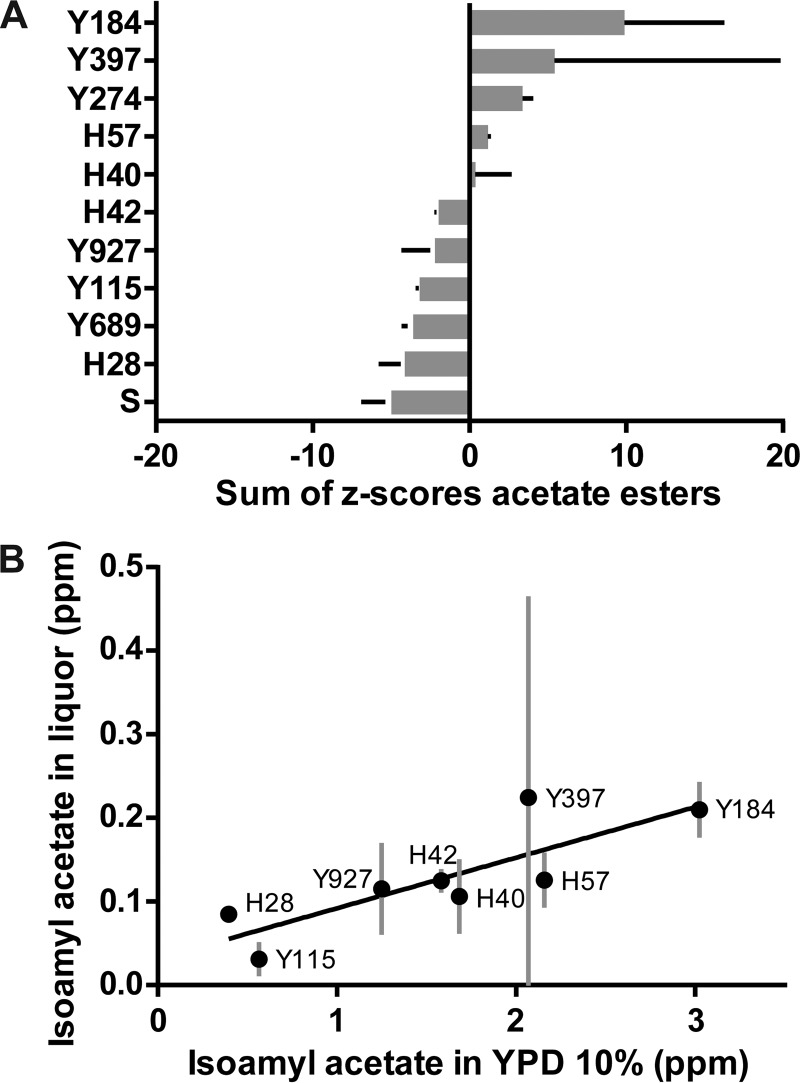
Next, we evaluated the influence of specific S. cerevisiae starter cultures on the acetate ester fraction in cocoa liquor. Isoamyl acetate, ethyl acetate, and phenylethyl acetate were quantitatively the most prominent acetate esters, and the presence of these esters varied greatly between strains (Table 3). Inoculation of the aromatic parents (Y184 and Y397) yielded the highest acetate ester concentrations, but the variation between duplicates was also large (Fig. 5A), which might limit their industrial potential. Fermentations inoculated with a newly generated hybrid (H40, H42, or H57) showed more consistent profiles, with an average increase in acetate ester concentrations of >100% compared to those in the spontaneous fermentations. Fermentations inoculated with the robust parents and the spontaneous controls generally contained the lowest acetate ester concentrations. Of all acetate esters, isoamyl acetate was present at the highest absolute concentration in the cocoa liquor (Table 3). Interestingly, the isoamyl acetate concentration retained in the liquor correlated strongly and significantly with the S. cerevisiae isoamyl acetate production in lab-scale fermentation experiments, suggesting that the results obtained in lab-scale trials can be extrapolated to industrial settings (Pearson's r = 0.8270, P = 0.0113) (Fig. 5B). Moreover, isoamyl acetate production of the inoculated strains in lab-scale fermentations also correlated with the sum of the Z-scores of the total acetate ester fraction in liquor (Spearman r = 0.9762, P = 0.0004).
FIG 5.
Concentration of fruity aromas in cocoa liquors produced from the different fermentation trials. (A) Sum of the Z-scores of all acetate esters detected in cocoa liquor for the different spontaneous and inoculated fermentations. The bar graphs indicate the averages ± SD of results from duplicated fermentations. S, spontaneous; Y274, P. kluyveri; Y689, Cyb. fabianii; all other strains are S. cerevisiae. (B) Scatter plot of isoamyl acetate detected in cocoa liquor made from cocoa beans fermented using S. cerevisiae strains and their concomitant isoamyl acetate production in 10% YPD (error bars indicate SD) (see Materials and Methods for more details).
Conversion of liquor into chocolate significantly reduces short-chain ester concentrations.
The different cocoa liquors were further processed into chocolates with 60% (wt/vol) cocoa solids, according to the general practices used for commercial chocolate production. In order to obtain a sufficient bean mass for chocolate production, liquor pools from the two biological duplicates were pooled. GC-MS analysis of the conched chocolates detected 32 volatiles related to fermentation, indicating that nine volatiles (all esters) dropped below the detection limits during the conversion of liquor into chocolate (Table 4). Moreover, the absolute concentration of all esters decreased, except for that of methyl acetate. Interestingly, the proportion of the esters that was conserved in the chocolate was positively correlated with the boiling points of the esters (Pearson's r = 0.8981, P < 0.0001); short-chain acetate esters, such as propyl acetate, isobutyl acetate, and 2-pentyl acetate, disappeared completely. Although isoamyl acetate, ethyl acetate, and phenylethyl acetate were quantitatively the most abundant esters in the liquor, this changed to phenylethyl acetate and methyl acetate in chocolate. Isoamyl acetate and ethyl acetate concentrations decreased by 93% ± 4% and 66% ± 30% (means ± standard deviations [SD]), respectively (Tables 3 and 4; see also Fig. S4 in the supplemental material). For the ethyl esters, a similar trend was observed. While short-chain ethyl esters were not detected anymore in the final chocolate (ethyl propanoate, ethyl isobutyrate, ethyl 2-methylbutanoate, and ethyl isovalerate) or disappeared to a large extent (ethyl hexanoate), the longer, more-fat-soluble ethyl esters, such as ethyl octanoate and especially ethyl decanoate and dodecanoate, remained almost unaffected.
TABLE 4.
Volatiles identified using GC-MS in conched chocolate made from spontaneous and inoculated cocoa pulp fermentationsa
| Volatile by stage | Sensorial descriptor(s) (references) | Concn (μg/kg) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robust parents |
Aromatic parents |
Hybrids |
P. kluyveri Y274 | Cyb. fabianii Y689 | S | |||||||
| Y115 | Y927 | H28 | Y184 | Y397 | H40 | H42 | H57 | |||||
| Fermentation | ||||||||||||
| Aldehydes | ||||||||||||
| 2-Methylbutanal | Chocolate (61), malty (62) | 37.8 | 38.3 | 19.2 | 14.6 | 3.9 | 19.4 | 15.0 | 11.3 | 12.3 | 13.1 | 8.4 |
| 2-Phenyl-2-butenal | Roasted (61), rum (61), cocoa (61), sweet (61) | 22.9 | 11.2 | 10.0 | 6.1 | 8.2 | 5.1 | 6.5 | 4.1 | 5.9 | 7.3 | 2.1 |
| 3-Methylbutanal | Chocolate (61), malty (57, 62) | 127.8 | 132.1 | 58.9 | 54.3 | 15.9 | 63.8 | 58.0 | 45.0 | 45.9 | 49.9 | 28.9 |
| 5-Methyl-2-phenyl-2-hexenal | Roasted cocoa (63), sweet (63) | 12.6 | 4.1 | 4.2 | 1.7 | 2.1 | 2.3 | 3.3 | 1.0 | 3.0 | 2.2 | 1.3 |
| Benzaldehyde | Almond (64) | 37.0 | 41.7 | 30.1 | 31.5 | 12.1 | 38.7 | 30.4 | 27.0 | 22.1 | 17.0 | 11.7 |
| Phenylacetaldehyde | Floral (61), honey (57, 61, 62) | 53.5 | 36.6 | 17.5 | 11.4 | 8.6 | 19.4 | 20.2 | 11.7 | 15.3 | 18.4 | 4.9 |
| Alcohols | ||||||||||||
| 2-Methyl-1-butanol | 0.1 | 3.7 | 0.8 | 3.7 | 1.2 | 2.3 | 2.0 | 2.3 | 1.2 | 0.8 | 0.2 | |
| 2-Phenylethanol | Floral (61, 62) | 142.0 | 122.9 | 67.6 | 73.2 | 62.8 | 78.1 | 70.7 | 76.4 | 60.3 | 40.2 | 30.6 |
| Isoamyl alcohol | Malty (25), bitter (25), chocolate (25) | 0.1 | 27.1 | 7.0 | 16.3 | 2.7 | 17.4 | 5.4 | 11.6 | 10.9 | 4.6 | 1.2 |
| Esters | ||||||||||||
| 2-Heptyl acetate | 1.7 | 3.2 | 1.3 | 1.4 | 1.8 | 7.6 | 5.0 | 8.7 | 2.3 | 1.9 | 1.5 | |
| Ethyl acetate | Solvent (64), fruity (17) | 2.0 | 3.7 | 3.4 | 28.9 | 1.4 | 9.1 | 5.3 | 7.7 | 11.5 | 3.3 | 1.8 |
| Ethyl decanoate | Pear (58), grape (58), brandy (58), floral (65) | 3.4 | 3.7 | 1.4 | 0.9 | 0.6 | 2.2 | 1.8 | 3.6 | 1.4 | 0.9 | 0.6 |
| Ethyl dodecanoate | Fruity (58), floral (58) | 7.1 | 3.1 | 3.1 | 1.5 | 1.4 | 2.2 | 3.2 | 2.7 | 4.0 | 1.4 | 1.6 |
| Ethyl hexanoate | Apple (64), fruity (57), banana (57) | 0.1 | 0.1 | 0.1 | 2.4 | 0.1 | 2.5 | 0.1 | 2.4 | 0.1 | 0.1 | 1.5 |
| Ethyl octanoate | Apple (64), fruity (57), pineapple (57) | 7.5 | 19.1 | 5.0 | 15.8 | 7.3 | 9.2 | 14.7 | 8.8 | 4.5 | 4.4 | 2.5 |
| Ethyl phenylacetate | Sweet (57), waxy (57) | 3.0 | 1.9 | 1.6 | 2.0 | 0.9 | 1.2 | 2.2 | 1.8 | 1.1 | 1.0 | 1.0 |
| Isoamyl acetate | Banana (64), fruity (17), pear (17) | 2.4 | 5.5 | 11.9 | 6.8 | 2.4 | 12.4 | 5.1 | 6.6 | 5.5 | 2.7 | 7.2 |
| Methyl acetate | 17.7 | 13.7 | 33.0 | 35.7 | 16.6 | 30.0 | 25.7 | 42.6 | 20.3 | 20.1 | 14.1 | |
| Phenylethyl acetate | Floral (62), fruity (57), sweet (57) | 30.3 | 29.5 | 16.7 | 37.1 | 27.2 | 15.6 | 21.1 | 22.9 | 16.8 | 13.2 | 12.3 |
| Ketones | ||||||||||||
| 1-Hydroxy-2-propanone | 26.7 | 23.0 | 8.3 | 6.3 | 5.4 | 8.1 | 13.2 | 8.4 | 6.1 | 9.6 | 2.2 | |
| 2-Butanone | 5.1 | 5.3 | 4.3 | 3.3 | 1.1 | 3.2 | 3.0 | 2.0 | 1.9 | 2.0 | 1.8 | |
| 2-Heptanone | 1.5 | 1.9 | 2.5 | 2.4 | 1.1 | 5.2 | 2.3 | 4.0 | 4.3 | 1.4 | 2.9 | |
| 2-Nonanone | Floral (66), fatty (66) | 9.5 | 15.6 | 6.3 | 6.3 | 5.1 | 22.4 | 13.1 | 33.5 | 15.6 | 7.6 | 4.2 |
| Acetoin | Buttery (67), cream (67) | 278.7 | 276.5 | 106.0 | 147.5 | 52.3 | 252.8 | 238.9 | 258.3 | 505.2 | 260.7 | 55.5 |
| Diacetyl | Buttery (57, 61) | 78.1 | 70.4 | 64.7 | 58.6 | 25.3 | 75.9 | 78.0 | 61.4 | 124.4 | 50.5 | 48.7 |
| Sulfides | ||||||||||||
| Dimethyl disulfide | Cabbage (66) | 1.0 | 0.9 | 0.9 | 0.9 | 0.4 | 0.6 | 1.0 | 0.6 | 0.6 | 0.4 | 0.6 |
| Volatile acids | ||||||||||||
| 2-Methylbutanoic acid | Sweaty (57, 62) | 0.1 | 48.1 | 35.0 | 19.7 | 0.1 | 16.7 | 22.1 | 18.7 | 11.0 | 17.4 | 5.8 |
| Acetic acid | Sour (57, 62) | 2,555.7 | 2,489.6 | 1,087.5 | 1,984.2 | 734.0 | 1,711.0 | 2,907.2 | 2,303.5 | 1,818.9 | 1,983.9 | 279.7 |
| Butanoic acid | Sweat (62), buttery (57), rancid (57) | 2,555.7 | 10.0 | 3.4 | 4.8 | 1.3 | 4.4 | 10.1 | 7.7 | 6.1 | 6.1 | 0.3 |
| Isobutyric acid | Sweaty (57, 62) | 26.9 | 48.8 | 20.1 | 36.6 | 7.7 | 25.7 | 47.4 | 44.3 | 45.4 | 41.6 | 3.8 |
| Isovaleric acid | Sweaty (57, 62) | 0.1 | 173.1 | 44.7 | 62.5 | 0.1 | 51.4 | 102.2 | 64.5 | 48.4 | 74.5 | 15.8 |
| Propanoic acid | Rancid (25) | 24.0 | 24.8 | 12.9 | 18.8 | 8.8 | 17.7 | 28.3 | 25.8 | 15.6 | 19.3 | 8.2 |
| Roasting | ||||||||||||
| 1-(2-Furanyl)ethanone | Sweet (57), balsamic (57), cocoa (57) | 1.3 | 2.1 | 1.6 | 0.8 | 1.0 | 1.2 | 1.3 | 0.9 | 1.4 | 0.9 | 1.5 |
| 2,3,5-Trimethyl-6-ethylpyrazine | Candy (67), sweet (67) | 16.1 | 13.8 | 8.2 | 4.5 | 6.7 | 10.4 | 13.8 | 20.2 | 7.9 | 10.1 | 6.0 |
| 2,3-Dimethylpyrazine | Caramel (58), cocoa (58), hazelnut (61) | 6.5 | 6.5 | 2.9 | 3.0 | 1.3 | 3.9 | 4.6 | 3.1 | 2.6 | 3.1 | 1.2 |
| 2,5-Dimethylpyrazine | Cocoa (58), roasted nuts (58) | 2.6 | 2.4 | 1.7 | 1.9 | 1.3 | 1.6 | 1.4 | 0.9 | 1.1 | 1.3 | 1.0 |
| 2,6-Dimethylpyrazine | Nutty (58), coffee (58), green (58) | 5.4 | 4.7 | 1.8 | 0.9 | 0.6 | 1.7 | 2.3 | 0.9 | 0.8 | 1.6 | 0.8 |
| 2-Acetylpyrrole | Bread (58), walnut (58), licorice (58) | 91.6 | 42.4 | 23.8 | 8.0 | 24.9 | 17.4 | 43.2 | 11.6 | 13.8 | 25.9 | 10.2 |
| 2-Ethyl-3,5-dimethylpyrazine | Earthy (62), potato-chip (57, 62) | 17.5 | 10.7 | 4.9 | 1.9 | 4.9 | 3.6 | 7.8 | 3.8 | 2.5 | 5.5 | 3.6 |
| 2-Methyltetrahydro-3-furanone | 8.6 | 4.9 | 3.3 | 1.3 | 0.8 | 1.3 | 3.1 | 1.1 | 0.9 | 1.8 | 1.5 | |
| Methylpyrazine | Green (61), hazelnut (61), cocoa (58), roasted nuts (58) | 5.4 | 3.8 | 2.2 | 1.4 | 0.8 | 2.8 | 2.8 | 1.3 | 1.3 | 0.8 | 0.4 |
| Tetramethylpyrazine | Cocoa (58, 61), mocha (61), milk coffee (58, 61) | 212.9 | 247.7 | 123.1 | 86.1 | 86.7 | 198.5 | 336.1 | 288.4 | 198.2 | 316.7 | 396.9 |
| Trimethylpyrazine | Earthy (57, 62), cocoa (58, 61), roasted (61), green (61) | 30.1 | 29.2 | 13.0 | 7.2 | 8.1 | 15.5 | 26.8 | 14.0 | 9.3 | 19.5 | 15.2 |
Two spontaneous (S) and 20 inoculated fermentations were performed, with 10 yeast strains tested in duplicate. The cocoa liquors of the fermentation duplicates were pooled prior to being processed into chocolate (see Materials and Methods for details). 2-Pentyl acetate, ethyl 2-methylbutanoate, ethyl isobutyrate, ethyl isovalerate, ethyl lactate, ethyl propanoate, isobutyl acetate, methyl lactate, and propyl acetate were detected in the cocoa liquors but not in the chocolates.
Inoculation of different yeast starter cultures introduces different chocolate flavors.
Apart from fermentation-related aroma compounds, GC-MS analysis detected 11 roasting-related volatiles in the chocolates, mostly pyrazines (Table 4). Chocolates produced using the robust parents Y115, Y927, and H28 contained significantly more 2-phenyl-2-butenal, 5-methyl-2-phenyl-2-hexenal, 2-phenylethanol, 1-hydroxy-2-propanone, 2-butanone, and the Strecker aldehydes 2-methylbutanal, 3-methylbutanal, and phenylacetaldehyde (P < 0.014, Wilk's λ likelihood ratio test). These Strecker degradation aldehydes are known precursors of pyrazines during Maillard reactions (4). Moreover, the inoculation of the robust parental strains led to significantly higher concentrations of all 11 roasting volatiles than in the other chocolates, except for those of trimethylpyrazine and tetramethylpyrazine (P < 0.029, Wilk's λ likelihood ratio test). Y115, Y927, and hybrid H42 chocolates contained the highest acetic acid concentrations (P = 0.024, Wilk's λ likelihood ratio test). Y397 and the spontaneously fermented chocolate generally contained the lowest concentrations of the detected volatiles. These results were represented in a two-dimensional PCA plot (Fig. 6). Chocolates in the upper left part of Fig. 6, the spontaneous control, Y397, and the non-Saccharomyces strains Y274 and Y689 contained fewer volatiles than the other chocolates (Table 4 and Fig. 6). The H40 chocolate contained the highest concentration of isoamyl acetate. While the clear correlation for isoamyl acetate observed in liquor was lost, a similar (but nonsignificant) correlation between isoamyl acetate production in lab-scale fermentations and the total amount of acetate esters in chocolate was still apparent (Pearson's r = 0.6805, P = 0.0632).
FIG 6.
Principal-component analysis of volatile compounds related to fermentation and roasting in chocolate. (A) Score plot derived from spontaneous fermentations and fermentations inoculated with 10 different yeasts. All inoculated yeasts are S. cerevisiae strains, except for Y274 (P. kluyveri) and Y689 (Cyb. fabianii). (B) Loading plot of 43 volatile compounds related to cocoa pulp fermentation and roasting, identified using GC-MS.
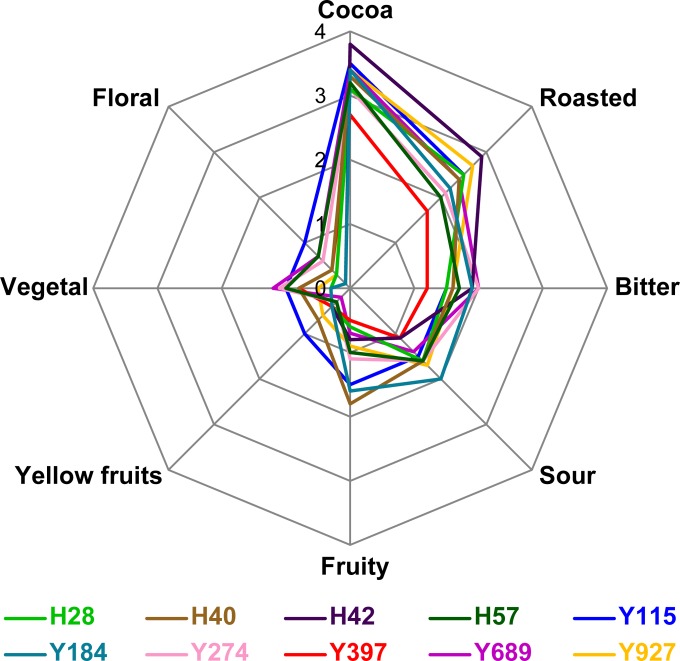
Next, the chocolates produced were sensorially analyzed by an expert panel. Significant differences between chocolates were found for eight descriptors (P < 0.05) shown in Fig. 7 (see also Table S5 in the supplemental material), indicating several clear sensorial differences between the chocolates produced with different starter cultures. Interestingly, the “fruitiness” of the chocolate, as determined by the expert panel, was nonsignificantly linked with the sum of the acetate esters in the chocolate (Spearman r = 0.5273, P = 0.1231), suggesting that acetate esters potentially influence, but are not the sole determinant of, the fruitiness of the chocolate. Chocolate produced using H40 was perceived to be the fruitiest, with a significant difference in fruitiness from that detected with all except two other starter cultures (Y184 and Y115; P < 0.05, Tukey's multiple-comparison test). The expert panel also detected other clear differences between the different starter cultures, even beyond the strain properties that we targeted in this study. For example, the use of H42 and the robust parents Y115, Y927, and H28 as starter cultures resulted in very strong cocoa and roasting notes, whereas the use of H57 led to a chocolate with distinctly sweet flavors. This indicates that it is possible to modulate the overall chocolate flavor by using different microbial starter cultures for cocoa pulp fermentation.
FIG 7.
Flavor profiles of chocolates made from inoculated cocoa pulp fermentations. The chocolates were sensorially analyzed by an expert panel, and descriptors that showed significant differences are displayed here (see Materials and Methods for details; see also Table S5 in the supplemental material for significant differences). All inoculated yeasts are S. cerevisiae strains, except for Y274 (P. kluyveri) and Y689 (Cyb. fabianii).
DISCUSSION
Gourmet premium-quality chocolate is the fastest-growing segment in the chocolate market globally, with an annual growth of 6% in 2014 (46). These chocolates stand out by their high quality, special flavors, or fragrances, and they target a consumer market that is willing to pay a premium for quality, consistency, and specific flavors. Our study shows that the development of specific yeast starter cultures could provide a cheap, natural, and easy-to-use method to expand the array of natural chocolate flavors. This study describes the development of specific cocoa starter cultures that are able to modulate the flavor of the final chocolate. While the robust parental yeast strains used in this study successfully dominate cocoa pulp fermentations, they do not yield chocolate with fruity flavors and instead result in elevated cocoa and roasted aromas. We therefore generated a set of novel hybrid strains that combine thermotolerance with high acetate ester production. Field trials with these strains and subsequent analysis of the resulting cocoa liquor and chocolate reveal that the different hybrids yield chocolates that show significant and reproducible differences in their aromas. For example, the hybrid H40 seems especially promising for the commercial production of specialty chocolate with fruity notes, whereas other strains yield more floral, roasted, and/or cocoa aromas.
Flavor development in chocolate is a complex process that is influenced by a broad array of factors. Specific chocolate flavor notes have been related to cocoa tree variety and growing conditions, postharvest treatments, and the chocolate manufacturing process (4, 5). For example, it has been shown that different cocoa tree genotypes influence the flavor of fermented beans and liquor (2, 47–50). However, modulating chocolate flavor by changing the cocoa cultivar would require elaborate plantation management by planting (a combination of) specific cocoa tree varieties, which in turn might be more disease susceptible or have a lower yield. Alternatively, different fermentation techniques are shown to alter the flavor characteristics of chocolate, with tray-fermented chocolates containing higher concentrations of volatiles (21). However, the possibility of generating a broad spectrum of chocolates by varying the fermentation method seems limited, and the implementation of tray fermentations would require additional investments in infrastructure, especially in regions where heap fermentations are used, such as the Ivory Coast and Ghana (5, 51).
Most studies describing cocoa starter cultures have focused on fermentation efficiency (27), consistency (29), or influence on cocoa sweatings (24, 28, 52). Our results confirm previous studies that suggest that the flavor of chocolate is heavily influenced by the fermentation process (1, 3, 26). Moreover, we also demonstrate that the use of different “designer” starter cultures allows producers to obtain very different natural aromas.
Our study also shows the potential of developing designer hybrid yeasts that combine efficient cocoa pulp fermentation with desirable aroma production. Specifically, we developed strains with increased acetate ester production. Acetate esters are produced by yeast alcohol acetyl transferases, encoded by ATF1 and ATF2, and are responsible for the fruity notes in many fermented beverages (18). Moreover, we show that high isoamyl acetate production in lab-scale fermentations (our main selection criterion for parental strains and hybrids) leads to high acetate ester concentrations in cocoa liquor and chocolate (r = 0.8732 and 0.6805, respectively). Field trials show that fermentations performed with the aromatic parents yield the highest acetate ester concentrations on average; yet, the aroma profiles of the cocoa liquors show the highest variability between duplicates. The robust parents, on the other hand, result in reproducible liquor aroma profiles but low acetate ester concentrations. Interestingly, fermentations performed with the newly developed hybrids show both high acetate ester concentrations and a reproducible aroma profile. Increased acetate ester concentrations in chocolates generally lead to a higher perception of fruity notes in the chocolates (r = 0.5273) during sensory analysis by an expert panel. Chocolate produced using the hybrid H40 contains the highest isoamyl acetate concentration and is perceived as the fruitiest. These results indicate that acetate esters play a prominent role in determining the fruitiness of chocolate and that the selection or development of high-acetate ester-producing strains can yield fruitier chocolates. Moreover, since the presence of different esters can have a synergistic effect on the individual flavors, the ratios of different esters might also influence the perception of fruitiness (17). A determination of the sensory thresholds for esters in cocoa liquor and chocolate and a more in-depth study on the synergetic effects of different flavor compounds will give a more comprehensive image of the influence of acetate esters on chocolate aroma.
In addition to the variation in fruitiness levels, several remarkable differences between chocolates produced using different starter cultures were detected. For example, several chocolates differed significantly in cocoa, bitter, sour, and floral notes. This indicates that apart from influencing the fruity notes derived from volatile esters that are produced by fermenting yeast cells, the fermentation process influences many other aromas, either directly or indirectly. Yeasts have been reported to influence storage protein degradation and reduce the acidity of fermented cocoa beans (24). This acidification is a prerequisite for the proteolytic formation of important flavor precursors during fermentation, such as hydrophobic amino acids and hydrophilic peptides, and therefore has a large impact on chocolate flavor (53, 54). Strong acidification accompanied by a rapid steep temperature rise leads to strong unspecific proteolysis and hence a lower flavor potential. However, moderate acidification, together with a slower and delayed temperature rise, yields a higher flavor potential (55). Therefore, any factor that influences bean acidification or temperature rise might impact chocolate flavor. As an example, yeast starter cultures with different metabolic respiration-to-fermentation ratios produce different quantities of ethanol, which can be exothermically metabolized by AAB (56); these in turn lead to different acetic acid concentrations and fermentation temperatures. However, further research on the physiology and properties of the applied starter culture and an in-depth analysis of the physiochemical changes within the cocoa bean during fermentation are required to strengthen these hypotheses.
Thermotolerance of the yeast starter culture seems to play an important role in the dominance observed toward the end of the fermentation (29). However, this study points out that other yet-to-be-determined factors contribute to robustness, as increasing the thermotolerance of the hybrids did not ensure dominance throughout the whole fermentation course. Therefore, other traits, such as acetic or lactic acid tolerance or pH tolerance, might be interesting targets for further strain improvement.
Chocolates produced using the robust thermotolerant strains show high cocoa and roasting notes, which may be explained by higher concentrations of Maillard products, such as 1-(2-furanyl)ethanone, 2-acetylpyrrole, 2-methyltetrahydro-3-furanone, various pyrazines, and the Strecker aldehydes 2-methylbutanal, 3-methylbutanal, and phenylacetaldehyde (2, 57, 58). Hydrophobic amino acids and hydrophilic peptides released during bean acidification are important precursors for Strecker aldehydes and pyrazines. Therefore, the nonester aroma differences between starter cultures might be explained by the variability in their abilities to acidify the bean. It seems plausible that the quickly fermenting robust yeasts affect the formation of these flavor precursors by producing large quantities of ethanol. This hypothesis is in keeping with the high acetic acid concentrations found in these chocolates and the large AAB population sizes at 72 h, when bean proteolysis occurs (24). The slower increase in temperature observed in fermentations inoculated with the robust yeasts most likely allowed moderate acidification and a high flavor potential (55). This observation clears the path for in-depth investigation of these traits, which in turn might allow further exploitation of the potential of yeasts to modulate chocolate flavor.
Several recent studies target the use of starter cultures consisting of bacteria and non-Saccharomyces yeasts, such as P. kluyveri and Kluyveromyces marxianus, to improve cocoa flavor or fermentation efficiency (20, 21, 30, 59). Although conceptually interesting, these studies conclude that sensorial differences between chocolates produced from inoculated and spontaneous fermentations are small or even undetectable, thus limiting industrial applicability. In line with these results, the fruity P. kluyveri and Cyb. fabianii strains investigated in this study do not yield significantly higher ester concentrations in the liquor. This can be explained by the poor competitiveness of these non-Saccharomyces strains in a cocoa environment, in which they disappear quickly after inoculation and do not dominate the yeast population at any given time point. GC-MS analysis and sensorial tests performed by an expert panel describe that the outcomes with P. kluyveri- and Cyb. fabianii-inoculated chocolates closely resemble the profile of spontaneous fermentations and are perceived as more bitter and vegetal than the S. cerevisiae-inoculated chocolates. Taken together, our results show that these non-Saccharomyces yeasts might not be the best choice for cocoa starter cultures.
Our results also highlight the influence of conching on flavor. Conching is a ripening step at high temperatures for a long time, which is required for texture and flavor improvement (5). It reduces the fractions of many volatiles, including those producing fruity flavors (60). In line with these findings, we established a clear correlation between the boiling point of a compound and its retention in the final chocolate (r = 0.8981). Short-chain esters largely disappear during conching, while longer-chain esters, such as phenylethyl acetate, ethyl phenylacetate, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate, are largely retained. Optimization of the conching parameters, e.g., duration, temperature profile, and stirring speed, might reduce this large loss of flavor and potentially increase even further the effect of the developed starter cultures on chocolate flavor.
Supplementary Material
ACKNOWLEDGMENTS
We thank Niels Vanhoudt and the members of the Verstrepen lab for their help and suggestions. We especially thank the Barry Callebaut innovations team for their support in the execution and successful conclusion of this project, and in particular Renata Januszewska for the sensory analysis, Isabelle Van Leuven for the GC-MS analysis, and Herwig Bernaert.
Research in the lab of Kevin J. Verstrepen is supported by Barry Callebaut, ERC starting grant 241426, VIB, the EMBO YIP program, FWO, and IWT.
Funding Statement
Barry Callebaut provided funding to Jan Steensels and Kevin J. Verstrepen. Agentschap voor Innovatie door Wetenschap en Technologie (IWT) provided funding to Jan Steensels and Kevin J. Verstrepen (Baekeland project 100697). Vlaams Instituut voor Biotechnologie (VIB) provided funding to Kevin J. Verstrepen.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02556-15.
REFERENCES
- 1.Thompson SS, Miller KB, Lopez AS. 2001. Cocoa and coffee, p 721–733. In Doyle MJ, Beuchat LR, Montville TJ (ed), Food microbiology: fundamentals and frontiers, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 2.Counet C, Ouwerx C, Rosoux D, Collin S. 2004. Relationship between procyanidin and flavor contents of cocoa liquors from different origins. J Agric Food Chem 52:6243–6249. doi: 10.1021/jf040105b. [DOI] [PubMed] [Google Scholar]
- 3.Reineccius G. 2005. Flavour chemistry and technology, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 4.Afoakwa EO, Paterson A, Fowler M, Ryan A. 2008. Flavor formation and character in cocoa and chocolate: a critical review. Crit Rev Food Sci Nutr 48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- 5.Beckett ST. 2008. The science of chocolate, 2nd ed Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 6.Schwan RF, Wheals AE. 2004. The microbiology of cocoa fermentation and its role in chocolate quality. Crit Rev Food Sci Nutr 44:205–221. doi: 10.1080/10408690490464104. [DOI] [PubMed] [Google Scholar]
- 7.Lopez SA, Dimick PS. 1995. Cocoa fermentation, p 562–577. In Reed G, Nagodawithana TW (ed), Biotechnology: enzymes, biomass, food and feed, 2nd ed Wiley-VCH, New York, NY. [Google Scholar]
- 8.Lima LJ, Almeida MH, Nout MJ, Zwietering MH. 2011. Theobroma cacao L., “The food of the Gods”: quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit Rev Food Sci Nutr 51:731–761. doi: 10.1080/10408391003799913. [DOI] [PubMed] [Google Scholar]
- 9.De Vuyst L, Lefeber T, Papalexandratou Z, Camu N. 2010. The functional role of lactic acid bacteria in cocoa bean fermentation, p 301–326. In Mozzi F, Raya RR, Vignolo GM (ed), Biotechnology of lactic acid bacteria: novel applications. Wiley-Blackwell, Ames, IA. [Google Scholar]
- 10.Ho VT, Zhao J, Fleet G. 2015. The effect of lactic acid bacteria on cocoa bean fermentation. Int J Food Microbiol 205:54–67. doi: 10.1016/j.ijfoodmicro.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CE, del Olmo M, Burri C. 1998. Enzyme activities in cocoa beans during fermentation. J Sci Food Agric 77:273–281. doi:. [DOI] [Google Scholar]
- 12.Camu N, De Winter T, Verbrugghe K, Cleenwerck I, Vandamme P, Takrama JS, Vancanneyt M, De Vuyst L. 2007. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl Environ Microbiol 73:1809–1824. doi: 10.1128/AEM.02189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jespersen L, Nielsen DS, Hønholt S, Jakobsen M. 2005. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res 5:441–453. doi: 10.1016/j.femsyr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Meersman E, Steensels J, Mathawan M, Wittocx PJ, Saels V, Struyf N, Bernaert H, Vrancken G, Verstrepen KJ. 2013. Detailed analysis of the microbial population in Malaysian spontaneous cocoa pulp fermentations reveals a core and variable microbiota. PLoS One 8:e81559. doi: 10.1371/journal.pone.0081559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arana-Sánchez A, Segura-Garcia LE, Kirchmayr M, Orozco-Ávila I, Lugo-Cervantes E, Gschaedler-Mathis A. 2014. Identification of predominant yeasts associated with artisan Mexican cocoa fermentations using culture-dependent and culture-independent approaches. World J Microbiol Biotechnol 31:359–369. [DOI] [PubMed] [Google Scholar]
- 16.Lambrechts MG, Pretorius IS. 2000. Yeast and its importance to wine aroma: a review. S Afr J Enol Vitic 21:97–129. [Google Scholar]
- 17.Verstrepen KJ, Derdelinckx G, Dufour J, Winderickx J, Thevelein J, Pretorius I, Delvaux F. 2003. Flavor-active esters: adding fruitiness to beer. J Biosci Bioeng 96:110–118. doi: 10.1016/S1389-1723(03)90112-5. [DOI] [PubMed] [Google Scholar]
- 18.Verstrepen KJ, Van Laere SDM, Vanderhaegen BMP, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR. 2003. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microbiol 69:5228–5237. doi: 10.1128/AEM.69.9.5228-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saerens SMG, Delvaux F, Verstrepen KJ, Van Dijck P, Thevelein JM, Delvaux FR. 2008. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 74:454–461. doi: 10.1128/AEM.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crafack M, Mikkelsen MB, Saerens S, Knudsen M, Blennow A, Lowor S, Takrama J, Swiegers JH, Petersen GB, Heimdal H, Nielsen DS. 2013. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int J Food Microbiol 167:103–116. doi: 10.1016/j.ijfoodmicro.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Crafack M, Keul H, Eskildsen CE, Petersen MA, Saerens S, Blennow A, Skovmand-Larsen M, Swiegers JH, Petersen GB, Heimdal H, Nielsen DS. 2014. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res Int 63:306–316. doi: 10.1016/j.foodres.2014.04.032. [DOI] [Google Scholar]
- 22.Steensels J, Verstrepen KJ. 2014. Taming wild yeast: potential of conventional and nonconventional yeasts in industrial fermentations. Annu Rev Microbiol 68:61–80. doi: 10.1146/annurev-micro-091213-113025. [DOI] [PubMed] [Google Scholar]
- 23.Steensels J, Meersman E, Snoek T, Saels V, Verstrepen KJ. 2014. Large-scale selection and breeding to generate industrial yeasts with superior aroma production. Appl Environ Microbiol 80:6965–6975. doi: 10.1128/AEM.02235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leal GA Jr, Gomes LH, Efraim P, de Almeida Tavares FC, Figueira A. 2008. Fermentation of cacao (Theobroma cacao L.) seeds with a hybrid Kluyveromyces marxianus strain improved product quality attributes. FEMS Yeast Res 8:788–798. doi: 10.1111/j.1567-1364.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 25.Frauendorfer F, Schieberle P. 2008. Changes in key aroma compounds of Criollo cocoa beans during roasting. J Agric Food Chem 56:10244–10251. doi: 10.1021/jf802098f. [DOI] [PubMed] [Google Scholar]
- 26.Lefeber T, Papalexandratou Z, Gobert W, Camu N, De Vuyst L. 2012. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol 30:379–392. doi: 10.1016/j.fm.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Schwan RF. 1998. Cocoa fermentations conducted with a defined microbial cocktail inoculum. Appl Environ Microbiol 64:1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzogbefia VP, Buamah R, Oldham JH. 1999. The controlled fermentation of cocoa (Theobroma cacao L) using yeasts: enzymatic process and associated physicochemical changes in cocoa sweatings. Food Biotechnol 13:1–12. doi: 10.1080/08905439609549958. [DOI] [Google Scholar]
- 29.Meersman E, Steensels J, Paulus T, Struyf N, Saels V, Mathawan M, Koffi J, Vrancken G, Verstrepen KJ. 2015. Breeding strategy to generate robust yeast starter cultures for cocoa pulp fermentations. Appl Environ Microbiol 81:6166–6176. doi: 10.1128/AEM.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batista NN, Ramos CL, Dias DR, Pinheiro ACM, Schwan RF. 2015. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT Food Sci Technol 63:221–227. doi: 10.1016/j.lwt.2015.03.051. [DOI] [Google Scholar]
- 31.Ho VTT, Zhao J, Fleet G. 2014. Yeasts are essential for cocoa bean fermentation. Int J Food Microbiol 174:72–87. doi: 10.1016/j.ijfoodmicro.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Warringer J, Zörgö E, Cubillos FA, Zia A, Gjuvsland A, Simpson JT, Forsmark A, Durbin R, Omholt SW, Louis EJ, Liti G, Moses A, Blomberg A. 2011. Trait variation in yeast is defined by population history. PLoS Genet 7:e1002111. doi: 10.1371/journal.pgen.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snoek T, Picca Nicolino M, Van den Bremt S, Mertens S, Saels V, Verplaetse A, Steensels J, Verstrepen KJ. 2015. Large-scale robot-assisted genome shuffling yields industrial Saccharomyces cerevisiae yeasts with increased ethanol tolerance. Biotechnol Biofuels 8:32. doi: 10.1186/s13068-015-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huxley C, Green ED, Dunham I. 1990. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet 6:236. doi: 10.1016/0168-9525(90)90190-H. [DOI] [PubMed] [Google Scholar]
- 35.Legras J-L, Karst F. 2003. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol Lett 221:249–255. doi: 10.1016/S0378-1097(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 36.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1994. Current protocols in molecular biology. John Wiley and Sons, New York, NY. [Google Scholar]
- 37.Steensels J, Snoek T, Meersman E, Nicolino MP, Voordeckers K, Verstrepen KJ. 2014. Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev 38:947–995. doi: 10.1111/1574-6976.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67:2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, NY. [Google Scholar]
- 40.Turner S, Pryer KM, Miao VPW, Palmer JD. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 42.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel HM, Vrancken G, Takrama JF, Camu N, De Vos P, De Vuyst L. 2009. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res 9:774–783. doi: 10.1111/j.1567-1364.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 44.Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes–application for the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 45.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc, New York, NY. [Google Scholar]
- 46.Barry Callebaut 2015. Roadshow presentation -HY 2014/15. Barry Callebaut AG, Zurich, Switzerland: https://www.barry-callebaut.com/sites/default/files/publications/roadshow_hy_2014-15.pdf. [Google Scholar]
- 47.Ramos CL, Dias DR, Miguel MGDCP, Schwan RF. 2014. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res Int 64:908–918. doi: 10.1016/j.foodres.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 48.Trognitz B, Cros E, Assemat S, Davrieux F, Forestier-Chiron N, Ayestas E, Kuant A, Scheldeman X, Hermann M. 2013. Diversity of cacao trees in Waslala, Nicaragua: associations between genotype spectra, product quality and yield potential. PLoS One 8:e54079. doi: 10.1371/journal.pone.0054079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luna F, Crouzillat D, Cirou L, Bucheli P. 2002. Chemical composition and flavor of Ecuadorian cocoa liquor. J Agric Food Chem 50:3527–3532. doi: 10.1021/jf0116597. [DOI] [PubMed] [Google Scholar]
- 50.Clapperton J, Yow S, Chan J, Lim D, Lockwood R, Romanczyk L, Hammerstone J. 1994. The contribution of genotype to cocoa (Theobroma cacao L.) flavor. Trop Agr 71:303–308. [Google Scholar]
- 51.ICCO. 2014. Quarterly bulletin of cocoa statistics (production of cocoa beans), vol XL, no. 1: cocoa year 2013/14. International Cocoa Organization, London, United Kingdom. [Google Scholar]
- 52.Buamah R, Dzogbefia VP, Oldham JH. 1997. Pure yeast culture fermentation of cocoa (Theobroma cacao L.): effect on yield of sweatings and cocoa bean quality. World J Microbiol Biotechnol 13:457–462. doi: 10.1023/A:1018536519325. [DOI] [Google Scholar]
- 53.Voigt J, Heinrichs H, Voigt G, Biehl B. 1994. Cocoa-specific aroma precursors are generated by proteolytic digestion of the vicilin-like globulin of cocoa seeds. Food Chem 50:177–184. doi: 10.1016/0308-8146(94)90117-1. [DOI] [Google Scholar]
- 54.Dimick PS, Hoskin JC. 1999. The chemistry of flavour development in chocolate, p 137–152. In Beckett ST. (ed), Industrial chocolate manufacture and use, 3rd ed Blackwell Science, Oxford, United Kingdom. [Google Scholar]
- 55.Biehl B, Brunner E, Passern D, Quesnel VC, Adomako D. 1985. Acidification, proteolysis and flavour potential in fermenting cocoa beans. J Sci Food Agr 36:583–598. doi: 10.1002/jsfa.2740360710. [DOI] [Google Scholar]
- 56.Adler P, Frey LJ, Berger A, Bolten CJ, Hansen CE, Wittmann C. 2014. The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl Environ Microbiol 80:4702–4716. doi: 10.1128/AEM.01048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnermann P, Schieberle P. 1997. Evaluation of key odorants in milk chocolate and cocoa mass by aroma extract dilution analyses. J Agric Food Chem 45:867–872. doi: 10.1021/jf960670h. [DOI] [Google Scholar]
- 58.Bonvehí J. 2005. Investigation of aromatic compounds in roasted cocoa powder. Eur Food Res Technol 221:19–29. doi: 10.1007/s00217-005-1147-y. [DOI] [Google Scholar]
- 59.Saerens SMG, Swiegers JH. 2013. Enhancement of cocoa quality and flavor by using Pichia kluyveri yeast starter culture for cocoa fermentation. WO patent WO2013064678.
- 60.Owusu M. 2010. Influence of raw material and processing on aroma in chocolate. Ph.D. thesis. University of Copenhagen, Copenhagen, Denmark. [Google Scholar]
- 61.Counet C, Callemien D, Ouwerx C, Collin S. 2002. Use of gas chromatography-olfactometry to identify key odorant compounds in dark chocolate. Comparison of samples before and after conching. J Agric Food Chem 50:2385–2391. [DOI] [PubMed] [Google Scholar]
- 62.Frauendorfer F, Schieberle P. 2006. Identification of the key aroma compounds in cocoa powder based on molecular sensory correlations. J Agric Food Chem 54:5521–5529. doi: 10.1021/jf060728k. [DOI] [PubMed] [Google Scholar]
- 63.Owusu M, Petersen MA, Heimdal H. 2012. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J Food Process Preserv 36:446–456. doi: 10.1111/j.1745-4549.2011.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meilgaard MC. 1982. Prediction of flavor differences between beers from their chemical composition. J Agric Food Chem 30:1009–1017. doi: 10.1021/jf00114a002. [DOI] [Google Scholar]
- 65.Saerens SMG, Delvaux FR, Verstrepen KJ, Thevelein JM. 2010. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol 3:165–177. doi: 10.1111/j.1751-7915.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belitz H-D, Grosch W, Schieberle P. 2009. Food chemistry, 4th ed Springer-Verlag, Berlin, Germany. [Google Scholar]
- 67.Afoakwa EO, Paterson A, Fowler M, Ryan A. 2009. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC-mass spectrometry and GC-olfactometry. Food Chem 113:208–215. doi: 10.1016/j.foodchem.2008.07.088. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.