Abstract
The Deepwater Horizon blowout in April 2010 represented the largest accidental marine oil spill and the largest release of chemical dispersants into the environment to date. While dispersant application may provide numerous benefits to oil spill response efforts, the impacts of dispersants and potential synergistic effects with crude oil on individual hydrocarbon-degrading bacteria are poorly understood. In this study, two environmentally relevant species of hydrocarbon-degrading bacteria were utilized to quantify the response to Macondo crude oil and Corexit 9500A-dispersed oil in terms of bacterial growth and oil degradation potential. In addition, specific hydrocarbon compounds were quantified in the dissolved phase of the medium and linked to ecotoxicity using a U.S. Environmental Protection Agency (EPA)-approved rotifer assay. Bacterial treatment significantly and drastically reduced the toxicity associated with dispersed oil (increasing the 50% lethal concentration [LC50] by 215%). The growth and crude oil degradation potential of Acinetobacter were inhibited by Corexit by 34% and 40%, respectively; conversely, Corexit significantly enhanced the growth of Alcanivorax by 10% relative to that in undispersed oil. Furthermore, both bacterial strains were shown to grow with Corexit as the sole carbon and energy source. Hydrocarbon-degrading bacterial species demonstrate a unique response to dispersed oil compared to their response to crude oil, with potentially opposing effects on toxicity. While some species have the potential to enhance the toxicity of crude oil by producing biosurfactants, the same bacteria may reduce the toxicity associated with dispersed oil through degradation or sequestration.
INTRODUCTION
The Deepwater Horizon (DWH) oil spill discharged ∼4.9 million barrels of light crude oil into the ocean at a depth of 1,500 meters below the sea surface (1). In an attempt to enhance biodegradation and to prevent oil from reaching sensitive shorelines, ∼1.84 million gallons of the chemical dispersants Corexit 9500A and Corexit 9527A were applied both at the surface (1.06 million gallons) and directly to the wellhead in the deep sea (0.78 million gallons) (1). Although dispersant was used in response strategies prior to the DWH oil spill, the DWH oil spill marks the first large-scale subsea application of dispersants. Therefore, understanding the impacts of dispersants on Gulf of Mexico ecosystems is crucial.
Biodegradation is the ultimate fate of the majority of hydrocarbons that enter the marine environment (2, 3). Based on calculations of the remaining and dispersed oil in the Gulf of Mexico, it was estimated that hydrocarbon-degrading bacteria removed up to 50% of the hydrocarbons released during the DWH oil spill (4). Analyses of in situ microbial community composition, gene expression, and hydrocarbon degradation rates in oil-contaminated seawater samples support these claims (5). Amplicon sequencing of small-subunit (SSU) rRNA genes revealed that taxa with high sequence identity to known oil-degrading bacteria were enriched in oil-contaminated seawater and sediment samples compared to uncontaminated samples (6–9, 27). Additionally, genes involved in hydrocarbon degradation were significantly enriched, and enhanced rates of biodegradation were reported in deep-sea plumes and sediments exposed to Macondo oil from the DWH blowout (10–12, 63).
Dispersants, which are composed of a surfactant dissolved in a hydrocarbon-based solvent, function by reducing the interfacial surface tension between water and oil, which results in the formation of tiny oil droplets that rapidly disperse (13). Although dispersant formulations have been continuously improved since the 1960s to reduce toxicity, the possible synergistic effects of oil and dispersant mixtures on toxicity to organisms require further research (14). Based on criteria set forth by the U.S. Environmental Protection Agency (EPA), the majority of dispersants range from slightly toxic to practically nontoxic (15). Dispersant-oil mixtures, however, have been shown in numerous studies to be significantly more toxic than dispersants alone. Rico-Martínez et al. (14) demonstrated that the synergistic effect of Corexit 9500A with Macondo crude oil increased toxicity to the marine rotifer Brachionus manjavacas in the water-accommodated fraction (WAF) by 47- to 52-fold relative to that generated with Macondo crude oil and by 66-fold relative to that generated with Corexit 9500A alone. B. manjavacas is a member of the Brachionus plicatilis species complex that has routinely been used in assessments of marine ecotoxicity because the organism grows rapidly, is easy to cultivate, is genetically homozygous, and plays a central role in coastal food webs (16, 17). Furthermore, the EPA required British Petroleum (BP) to assess the toxicity of dispersed oils using the Brachionus rotifer test following the DWH oil spill (18). In another study, Hemmer et al. (19) showed that dispersant-crude oil mixtures were more toxic than dispersants alone to mysid shrimp (Americamysis bahia) and inland silverside fish (Menidia beryllina). Other studies observed that dispersed oil and dispersant constituents showed higher toxicity than crude oil alone in coral species (20, 21). Components of the dispersant also potentially inhibit or delay microbial oil degradation. For example, in one study comparing different dispersant-oil mixtures, lags in biodegradation were attributed to preferential degradation of the dispersant (22).
In addition to synthetic dispersants, biosurfactants and bioemulsifiers are produced by a diversity of hydrocarbon-degrading microorganisms from all domains of life (23). These compounds impact the physicochemical properties of oil in a manner similar to, and often more effective than, that of synthetic chemical dispersants (23–25). Microbially synthesized surfactants are classified primarily on the basis of their molecular weights. Low-molecular-weight biosurfactants are generally lipopeptides and glycolipids that serve to lower surface and interfacial tensions, increasing the solubility of hydrocarbons (24). High-molecular-weight compounds include polysaccharides, proteins, lipopolysaccharides, lipoproteins, and complex mixtures of these biopolymers that are effective at stabilizing oil-in-water emulsions (25). Biosurfactants likely play a role in hydrocarbon resource partitioning between microbial populations responding to crude oil released into the environment.
Since biodegradation represents an important fate for hydrocarbons in the environment, it is important to understand how components of Corexit 9500A ultimately impact bacterial degradation and biosurfactant production and how degradation of the synthetic dispersant and oil affects toxicity to marine life. To our knowledge, no study has investigated the effects of bacterium-mediated biodegradation of oil and dispersant constituents on overall toxicity. The two primary objectives of this study were to (i) quantify the hydrocarbon degradation potential of two bacterial strains, Alcanivorax sp. strain P2S70 and Acinetobacter sp. strain COS-3, isolated from oil-contaminated sands with crude oil alone, with Corexit 9500A-dispersed oil, and with Corexit 9500A alone and (ii) link bacterial growth and activity to the observed changes in overall toxicity, assessed using an EPA-approved rotifer assay, and the solubility of specific hydrocarbon compounds. We demonstrate that the effects of Corexit 9500A on the biodegradation of crude oil are species specific, with opposite responses in biodegradation observed. Similarly, results indicate that some populations of hydrocarbon-degrading bacteria may enhance the toxicity of light crude oil, likely through the production of biosurfactants that increase the solubility of several classes of hydrocarbon compounds. Conversely, the activities of both strains significantly reduced the overall toxicity of the chemically dispersed oil to B. manjavacas in comparison to that in uninoculated controls. In order to achieve a predictive understanding of biodegradation, the potential synergistic effects that crude oil and dispersants have on microbial processes require further research.
MATERIALS AND METHODS
Bacterial strains.
In previous work, 24 bacterial strains were isolated using Macondo oil as the sole carbon source from beach sands exposed to oil deposited from the DWH discharge at Pensacola Beach, FL (26, 27). Of these, two strains were chosen to represent contrasting functional roles in hydrocarbon degradation. Alcanivorax spp. are obligate hydrocarbon-degrading bacteria, specializing in aliphatic hydrocarbon degradation (28, 29). Alcanivorax strain P2S70 is representative of a dominant population detected in situ in contaminated beach sands (26). This environmentally abundant strain had an ∼25%-reduced genome size compared to that of another Alcanivorax strain (PN-3) isolated from the same beach sands. It also encodes ∼40% fewer genes known to be associated with hydrocarbon degradation, indicating a higher degree of specialization for growth on hydrocarbons (26). In contrast, Acinetobacter spp. are considered generalists, with a broad carbon substrate range, including polycyclic aromatic hydrocarbons (PAHs) and aliphatic hydrocarbons (30–32). Along with Alcanivorax strains, Acinetobacter spp. showed the highest potential for oil degradation in pure culture (27).
Culture conditions.
All bacterial cultures were grown in an artificial seawater medium at 25°C in the dark to prevent hydrocarbon photooxidation (33). The cultures were shaken in Erlenmeyer flasks with at least 70% headspace at 150 rpm to promote sufficient aeration. Three carbon source treatments were tested throughout this study: (i) 0.5% (vol/vol) crude oil, (ii) 0.01% Corexit 9500A, or (iii) a 1:50 Corexit 9500A-oil mixture (hereinafter referred to as crude oil, Corexit, and dispersed-oil treatments, respectively). The concentration of oil (5 g/liter) used in this study was based on concentrations in previous studies investigating the toxicity of the WAF of crude oil and the microbial degradation of crude oils (Shafir et al. at 5 g/liter [21], Gardiner et al. at 10 g/liter [34], Hemmer et al. at 25 g/liter [19], Anderson et al. at 25 g/liter [35], Campo et al. at 5 g/liter [36], Swannell and Daniel at 0.3 g/liter [37]). The crude oil used in this study was surrogate MC252 oil collected from the Marlin platform in the Dorado field and was provided by BP (38). The crude oil and dispersant mixture was adjusted to a 1:50 ratio, which is at the lower end of the range of ratios recommended by the EPA for dispersing oil (39). All experiments were performed with triplicate cultures, with the exception of the specific hydrocarbon class analysis, in which only one sample was used per treatment. For hydrocarbon analysis, cultures were sacrificed after 7 and 14 days of incubation. Only the cultures from 7 days of incubation were used for compound-specific analysis (see below).
Hydrocarbon analyses.
Extraction and analysis of hydrocarbon compounds were performed according to a modified version of EPA Method 3510C, with accompanying quality assurance/quality control (QA/QC) protocols. Briefly, bacterial and control treatments were extracted for quantification of total petroleum hydrocarbons (TPHs) and the specific hydrocarbon compound classes aliphatics (n-alkanes C12 to C40 and isoprenoids pristine and phytane) and PAHs. Extracts were concentrated under a gentle stream of nitrogen using a TurboVap and reconstituted in hexane (100%) for chromatographic analysis (see the supplemental material for more detail).
TPHs in the samples were quantified using gas chromatography-flame ionization detection (GC-FID). One milliliter of EPH surrogate spiking solution (ISM-581X, lot CL-1009; Ultra, Kingstown, RI) containing o-terphenyl and 1-chlorooctadecane was added directly to the separatory funnel before extraction. TPH concentrations were corrected for extraction efficiency based on recovery of the EPH spiking solution and the mass of oil added.
Aliphatics and PAHs entrained in the culture medium (WAF and the chemically enhanced WAF [CEWAF]) were quantified in a gas chromatograph–mass spectrometric detector (GC-MS) in full scan mode (m/z 50 to 550). Splitless injections of 1 μl of the sample were conducted, and an RXi-5Sil column (30 m by 0.25 mm by 0.25 μm) was used. Quantitative analyses of aliphatics and PAHs were conducted using the IS (internal standard) method (see the supplemental material for more details). Concentrations are expressed as the sample volume (liters), and all recoveries were generally within QA/QC criteria of 90% to 120% for aliphatics and 70% to 120% for aromatics.
Quantification of bacterial growth.
Bacterial growth was quantified as total cellular protein. Cultures were grown in 15 ml of artificial seawater medium (33) and were supplemented with crude oil, dispersed oil, or Corexit alone, as detailed above. Additional treatments included an uninoculated control (nonbacterial control) for each substrate and an inoculated control with no added carbon source but containing bacterial inoculum in the same volume as in the carbon treatments (noncarbon control). All treatments were performed in triplicate.
Treatment cultures (3 replicates each) were sacrificed at each time point. The entire volume of culture medium was added to a 15-ml Falcon tube, which was centrifuged at 3,200 × g for 20 min. The supernatant was removed without disturbing the cell pellet, and the samples were stored at −20°C until further analysis. Total cellular protein was extracted using 1 ml of 2% SDS lysis buffer (50 mM Tris-HCl buffer with 2% [wt/vol] SDS) followed by room temperature incubation for 20 min. Samples were sonicated (Fisher Scientific Sonic Dismembrator model 550, amplitude of 4) for 15 s total (half a second on, half a second off). The samples were then centrifuged at 3,200 × g for another 20 min. Total cellular protein was quantified by following the Pierce bicinchoninic acid protein assay protocol according to the manufacturer's instructions (Life Technologies, Grand Island, NY).
Ecotoxicity assays.
Static acute toxicity tests were conducted using the marine rotifer B. manjavacas. The WAF (from the crude oil treatment) and CEWAF (from the dispersed-oil treatment) were prepared by following methods outlined in the study by Singer et al. (40) and modified to permit bacterial growth. Specifically, cultures were shaken at 150 rpm instead of mixed with a stir bar, and air exchange was permitted to prevent the headspace from going anaerobic. Furthermore, the mixing time suggested by Singer et al. (40) was insufficient for bacterial growth. Thus, for our purposes, the WAF and CEWAF were prepared by following the same conditions throughout the experiments. A preliminary experiment was conducted using uninoculated controls to compare the Singer et al. method and our modified method. Our modified method did not significantly alter the toxicity to B. manjavacas (see Fig. S1 in the supplemental material).
After 7 days of incubation, based on bacterial growth curves and TPH analysis, the samples were poured into separatory funnels and allowed to settle for 4 h. Approximately 10 ml of the WAF or CEWAF was collected in autoclaved Hungate tubes for use in the toxicity tests. The toxicity tests were conducted in 24-well plates, and samples of the WAF and CEWAF were diluted with 15 ppt artificial seawater in aliquots of 20% (from 0% to 100%) to determine the 50% lethal concentration for the test organisms (LC50) (14). In each well, 10 rotifers were added, and their viability was scored after 24 and 48 h (we present only the 48-h results). The plates were incubated at 25°C in the dark. The trimmed Spearman-Karber method (41) was used to calculate the 48-h LC50s as implemented in the R package “tsk” (42). Data were trimmed using parameters generated by the package, and data were smoothed when mortality was observed in the controls (total rate of 1.8% across all controls).
All raw data generated from this study are available for download at http://www.joelkostka.net/data/data_files/Overholt_etal_ASM_2013.tar.gz.
RESULTS
Bacterial growth on crude oil and dispersant.
Bacterial growth assays for Acinetobacter sp. COS-3 and Alcanivorax sp. P2S70, hereinafter referred to as Acinetobacter and Alcanivorax, respectively, were conducted using the five treatments detailed above, i.e., crude oil (0.5%, vol/vol), Corexit 9500A-dispersed crude oil (1:50 Corexit/oil ratio), Corexit 9500A, a nonbacterial control, and a noncarbon control. In all treatments studied, Acinetobacter showed higher growth rates and a higher maximum biomass accumulation than Alcanivorax (Fig. 1; see Fig. S2 in the supplemental material).
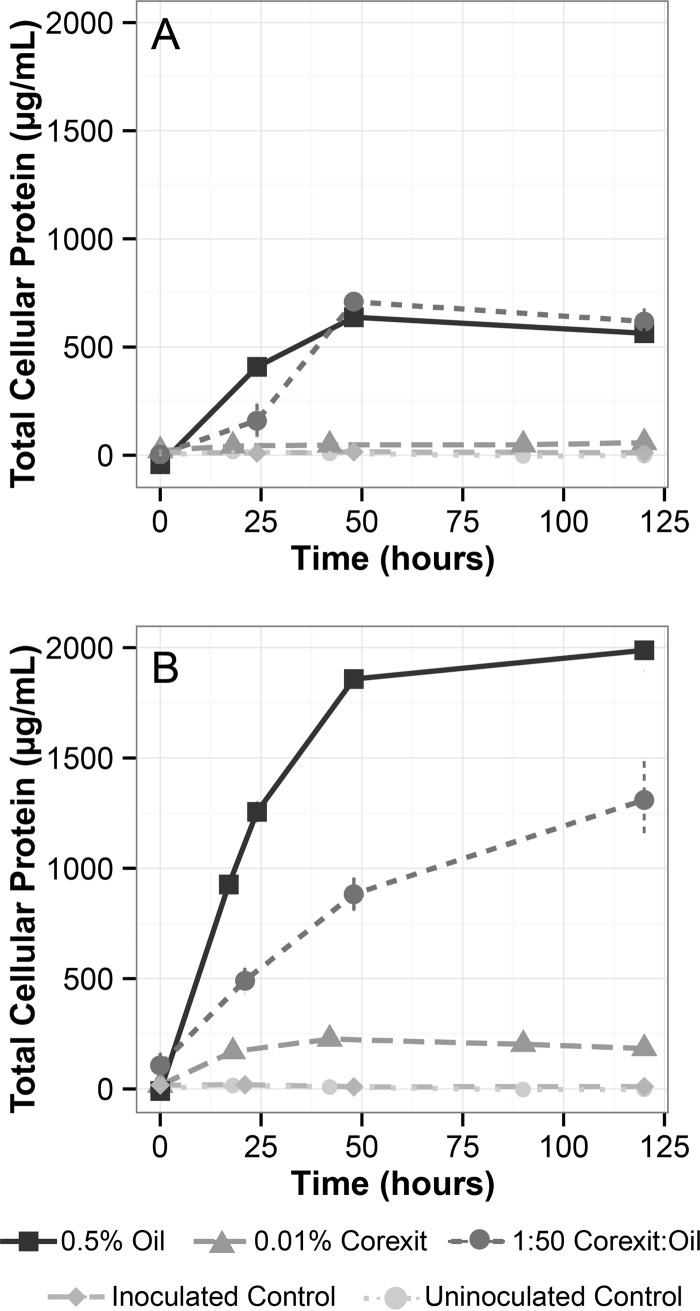
FIG 1.
Bacterial growth curves determined using a Pierce bicinchoninic acid protein assay. Carbon sources are indicated by line type and point shape. (A) Growth of Alcanivorax sp. P2S70; (B) growth of Acinetobacter sp. COS-3. See Fig. S2 in the supplemental material for an expanded view of growth on Corexit alone.
Alcanivorax showed similar maximum growth yields (50-h time point, two-sample t test, t = −1.41; P = 0.23) when grown on dispersed oil (709.7 ± 56.3 μg/ml total protein) and on crude oil alone (637.8 ± 29.9 μg/ml total protein), although a lag was observed in the dispersed-oil treatment (Fig. 1A). Significant growth of Alcanivorax was observed with Corexit as the sole carbon and energy source (58.3 ± 22.2 μg/ml; t = 4.05; P = 0.015) (see Fig. S2 in the supplemental material) in comparison to the growth observed in the inoculated noncarbon control (11.0 ± 3.6 μg/ml), comprising ∼10 times less growth than that with crude oil as the carbon source. Little to no cell protein accumulated with time in either of the control treatments (Fig. 1A; see Fig. S2 in the supplemental material).
Acinetobacter demonstrated maximum growth rates and produced maximum protein when it was grown on crude oil alone (1,988.0 ± 160.5 μg/ml) (Fig. 1B). The dispersed-oil treatment (1,309.9 ± 175.5 μg/ml) produced 34% less protein than crude oil alone and resulted in a lower growth rate (t = 4.98; P = 0.009). Like Alcanivorax, Acinetobacter was capable of growth on Corexit alone (183.6 ± 7.8 μg/ml), although the growth yield was 11% of the protein produced after growth on crude oil alone (see Fig. S2 in the supplemental material). All treatments yielded maximum protein concentrations significantly different from those of the two control treatments (t = −1.41; P = 0.23) (see Table S1 in the supplemental material).
Quantification of oil degradation.
Total petroleum hydrocarbons (TPHs) were extracted from the oil-amended treatments. Oil degradation potential was determined in parallel through comparison of the results of bacterial treatments to those of the uninoculated control. Volatile hydrocarbons are lost during extraction analysis using this method, and thus only n-alkanes with a number of carbon atoms higher than 11 were detected in the control and bacterial treatments, as with the weathered oil washed ashore at Pensacola Beach, FL (43).
Alcanivorax and Acinetobacter showed contrasting patterns in oil degradation capacity in pure culture. Concomitantly with bacterial growth, Alcanivorax degraded or transformed 25% of the TPHs in dispersed oil relative to the level degraded in the uninoculated control treatment (t = −4.45; P = 0.011) (Fig. 2A). In the absence of Corexit (crude oil treatment), no detectable transformation or removal of TPHs was observed in Alcanivorax cultures after 7 days of incubation (t = −0.75; P = 0.49). In contrast, 52% of the TPHs present in the crude oil treatment relative to the uninoculated control treatment were degraded or transformed in Acinetobacter cultures (t = −7.35; P = 0.0019), while only 30% of oil was removed or transformed in the dispersed-oil treatment (n = 2, t = −4.44; P = 0.021 [P = 0.07136 if unequal variances are assumed]) (Fig. 2B). See the supplemental material for a discussion on an outlier removal; Fig. S3 interprets these data with the outlier included.
FIG 2.
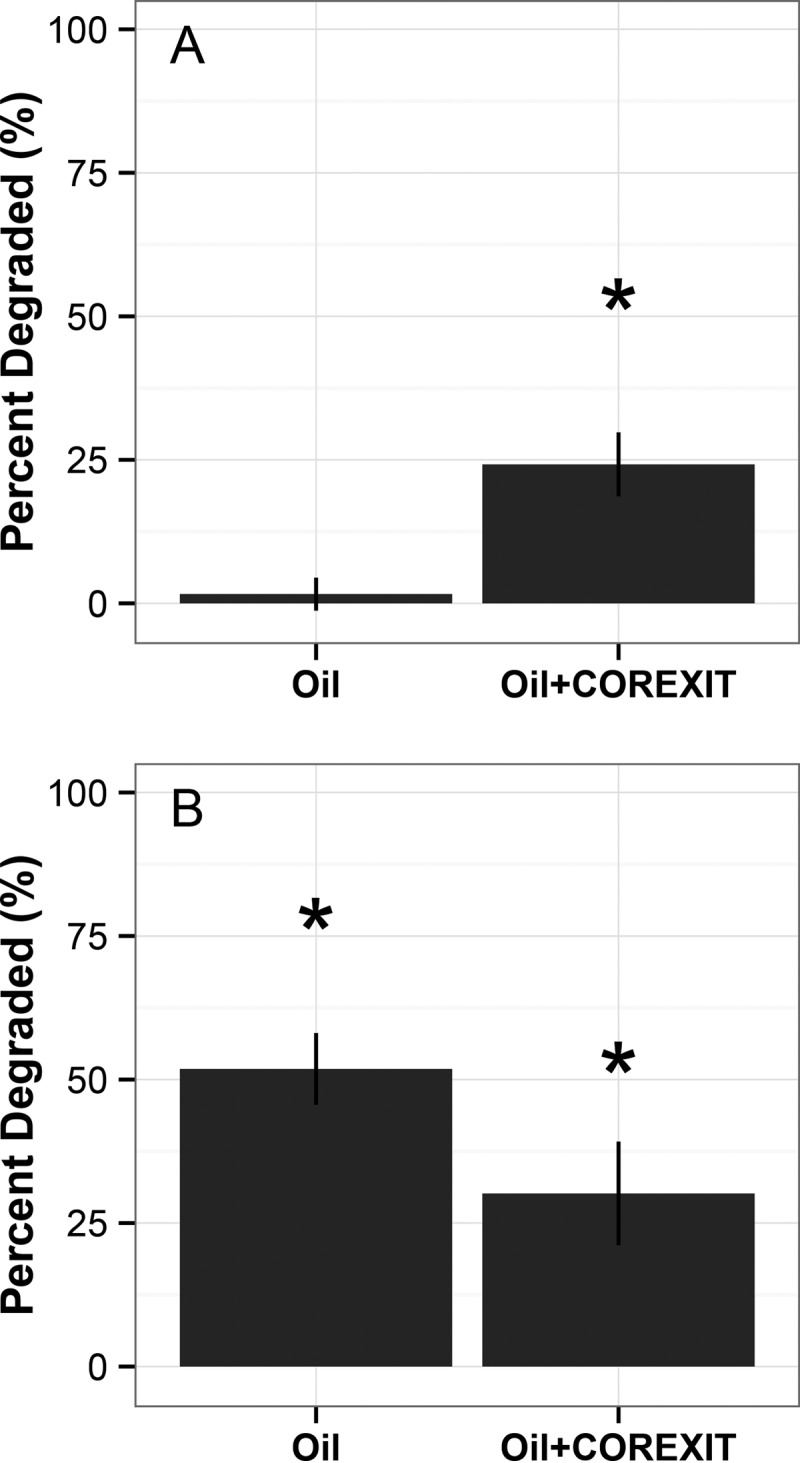
Potential of bacterial strains to degrade or transform petroleum hydrocarbons. Values are relative to those from the uninoculated control and are corrected for extraction efficiency and mass of oil added. TPHs were used by summing areas under each peak as determined by GC-FID. (A) Oil degradation potential for Alcanivorax sp. P2S70; (B) oil degradation potential for Acinetobacter sp. COS-3. An outlier from Acinetobacter was removed; see Fig. S3 for a version of this figure that includes the outlier. Asterisks indicate samples that are significantly different from the control samples. Data shown were collected after 7 days of incubation.
When incubations were extended to 14 days, Acinetobacter transformed or removed 54% and 44% of TPHs in the oil-only and dispersed-oil treatments, respectively (t = −7.0, P = 0.0075, and t = −11.8, P = 0.0024, respectively) (see Fig. S3 in the supplemental material). No significant change was observed in the Alcanivorax cultures between 7 and 14 days of incubation (t = −1.0; P = 0.32).
Quantifying the effects of bacterial treatments on rotifer toxicity.
Three sets of acute-toxicity tests were conducted to quantify the impact of bacterial degradation on the toxicity associated with crude oil, dispersed oil, and Corexit alone within the WAF. All B. manjavacas toxicity tests met the minimum requirement of >90% rotifer survival in control treatments to accept them as valid estimates of toxicity. All toxicity tests consisted of an uninoculated control and treatments of media inoculated with Acinetobacter or Alcanivorax.
All Corexit-only treatments resulted in similar toxicities and statistically indistinguishable LC50s (mean of 80.56 μg Corexit/ml after 48 h [analysis of variance, F = 2.43; P = 0.17]) (Fig. 3A; Table 1). According to EPA standards, these treatments are classified as slightly toxic. The uninoculated crude oil control (WAF) and Alcanivorax crude oil (WAF) treatments were not acutely toxic to B. manjavacas, with 0% mortality observed in all replicates at the tested concentrations (Fig. 3B; Table 1). However, when Acinetobacter was grown on crude oil, acute toxicity was observed, with an LC50 of 94.0% of the WAF (see Fig. S4 in the supplemental material for conversion to micrograms per liter). The uninoculated dispersed-oil control (CEWAF) exhibited significantly more toxicity than either Corexit alone or crude oil alone (WAF) when percentages of dilution were compared (Fig. 3C; Table 1). Bacterial degradation greatly reduced acute toxicity associated with dispersed oil (CEWAF), independently of the strain used (t = −1.53; P = 0.23). No mortality of B. manjavacas was observed in the bacterium-treated dispersed oil when CEWAF was diluted to 40% or greater.
FIG 3.
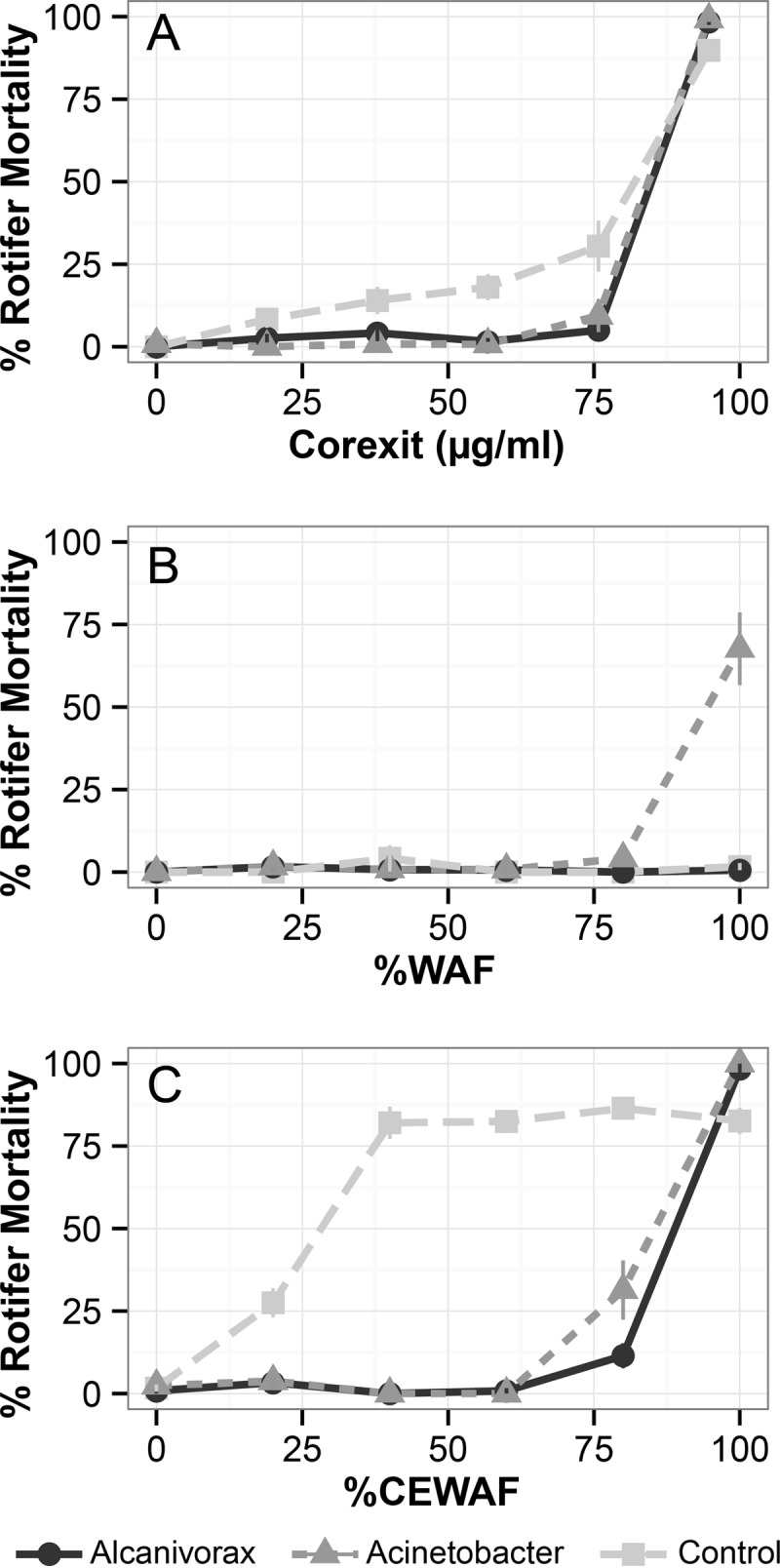
Ecotoxicity of bacterium-treated crude oil and dispersed crude oil and of Corexit only. All toxicity measurements were determined using the marine rotifer Brachionus manjavacas. All controls met the minimum requirement for survival (>90%). (A to C) Toxicity associated with Corexit 9500A alone (A), 0.5% (vol/vol) Macondo surrogate crude oil alone (B), and 1:50 Corexit 9500A-dispersed Macondo crude oil (C).
TABLE 1.
LC50s for all treatments tested in this studyc
| Treatment | LC50 at 48 h | LC50 95% confidence limits | P valuea | % change in LC50b |
|---|---|---|---|---|
| 0.01% Corexit 9500A | ||||
| Uninoculated control | 75.06 μg/ml | 71.33–80.05 μg/ml | ||
| Alcanivorax | 83.8 μg/ml | 81.08–84.70 μg/ml | 0.191 | |
| Acinetobacter | 82.85 μg/ml | 81.93–84.16 μg/ml | 0.251 | |
| 1:50 Corexit 9500A/oil ratio (%CEWAF)d | ||||
| Uninoculated control | 27.74% | 24.90%–28.71% | ||
| Alcanivorax | 87.51% | 85.79–88.53% | 7.3 × 10−6 | 215 |
| Acinetobacter | 83.02% | 81.00–84.60% | 4.5 ×10−6 | 199 |
| 0.5% oil (%WAF)e | ||||
| Uninoculated control | NDt | NDt | ||
| Alcanivorax | NDt | NDt | ||
| Acinetobacter | 94.03% | 92.03%–96.08% |
P values were determined with pairwise two-sample t tests comparing bacterial treatments with the corresponding uninoculated control.
Percent change in LC50 relative to the control LC50. This was calculated only if significant change was observed.
NDt, no detectable toxicity.
Percentage of the chemically enhanced water-accommodated fraction causing 50% rotifer mortality (LC50).
Percentage of the water-accommodated fraction causing 50% rotifer mortality (LC50).
Compound-specific analysis.
To better understand the observed changes in toxicity between crude oil (WAF) and dispersed oil (CEWAF) and the impact of bacterial transformations on both, analyses of specific hydrocarbon compounds present in the WAFs were performed after 7 days of growth.
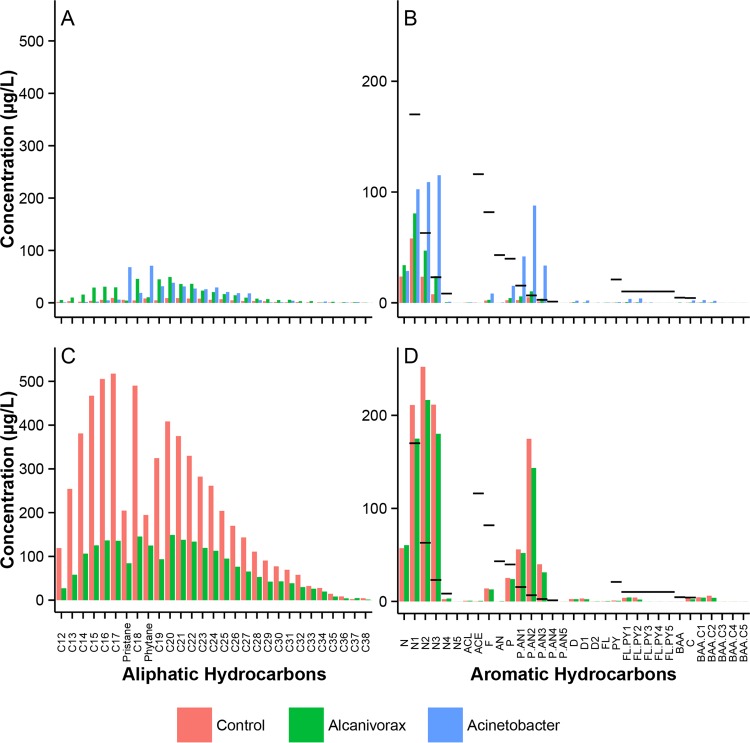
In the nonbacterial control for crude oil treatments, low levels of aliphatic compounds were present in the WAF (sum total, 116 μg/liter; compound maximum, 9.1 μg/liter) (Fig. 4A; see Fig. S5 in the supplemental material). As expected, the aromatic fraction was slightly more soluble, particularly the C1 to C4 naphthalenes, with C2 naphthalene reaching a maximum concentration of 58.1 μg/liter and total aromatics reaching a concentration of 127.2 μg/liter in the WAF (Fig. 4B; see Fig. S5 in the supplemental material). In all inoculated treatments (crude oil and dispersed oil), total aliphatic and aromatic fractions were enriched ∼3-fold relative to those in the uninoculated crude oil treatment (TPHs, 468.2 μg/liter for Alcanivorax and 428.1 μg/liter for Acinetobacter) (see Fig. S5). In the aliphatic fraction, Alcanivorax greatly increased the solubility of all n-alkanes and removed or transformed the branched alkanes (pristane, phytane) into non-GC-amendable forms. In contrast, Acinetobacter solubilized branched alkanes and n-alkanes with numbers of carbon atoms higher than 17 (Fig. 4A). In the aromatic fraction, the Alcanivorax treatment increased total PAH concentrations by 2-fold to 216.3 μg/liter, and the Acinetobacter treatment increased the concentration of aromatic hydrocarbons present in the WAF by 5-fold to 563.5 μg/liter (Fig. 4B). Unlike Alcanivorax, Acinetobacter substantially increased the solubility of PAHs, including the phenanthrenes, fluoranthenes, and benz[a]anthracenes, which were undetectable in all other treatments.
FIG 4.
Detailed analysis of aliphatic (A and C) and aromatic (B and D) hydrocarbons present in the WAF from the crude oil treatment (A and B) or the CEWAF from the dispersed-oil treatment (C and D). Acinetobacter-treated dispersed-oil samples were excluded from this figure due to extraction difficulties in separating EPS from hydrocarbons. EPA acute potency divisor values for aromatic hydrocarbons are indicated on graphs B and D by black bars. Data shown were collected after 7 days of incubation. See the supplemental material for an expanded version of this figure. Target PAHs are as follows: N and NC1-C4, naphthalene and alkylated homologues; ACL, acenaphthylene; ACE, acenaphthene; F, fluorene; D and DC1-C2, dibenzothiophene and alkylated homologues; P, AN, and P/ANC1-C4, phenanthrene, anthracene, and their alkylated homologues; FL, PY, and FL/PYC1-C4, fluoranthene, pyrene, and their alkylated homologues; BAA, C, and BAA/CC1-C4, benz[a]anthracene, chrysene, and their alkylated homologues; BBF, BKF, BAP, DA, and BP/PERC1-C4, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]anthracene, and alkylated homologues; ID, indeno[1,2,3-cd]pyrene; and BGP, benzo[ghi]perylene.
In the nonbacterial control for dispersed-oil treatments (CEWAF), total aliphatic concentrations were 52 times higher than in the corresponding nonbacterial control for crude oil treatments (WAF) (6,128 μg/liter and 116.6 μg/liter, respectively) (Fig. 4C; see Fig. S4 in the supplemental material). Relative to concentrations after the dispersed-oil control treatment, Alcanivorax reduced aliphatic concentrations in the CEWAF by 3-fold, to 2,197 μg/liter. While Acinetobacter greatly decreased the detected aliphatic concentrations, we suspect that this may be the result of biomass sequestration during the oil extraction due to extremely large amounts of extracellular polymeric substance (EPS) in the media (data not shown). Alcanivorax slightly decreased total aromatic hydrocarbons, from 1,076 μg/liter to 924 μg/liter, evenly across all aromatic hydrocarbons analyzed. An expanded version of Fig. 4 can be seen in Fig. S6 in the supplemental material).
DISCUSSION
The use of chemical dispersants is considered one of the main oil spill response tools, as outlined in the National Oil and Hazardous Substance Pollution Contingency Plan (NCP) (44), subpart D §300.310. Dispersants are globally used to mitigate the damage caused by an oil spill, especially in minimizing the impact to near-shore habitats by removing surface oil, diluting oil constituents to below toxic levels, and improving the accessibility of oil-degrading bacteria to hydrocarbons (13, 45). Furthermore, degradation of dispersed-oil constituents in the parts-per-million range are not limited by background nutrient concentrations (46). In order to prevent crude oil released by the DWH oil blowout from reaching sensitive coastal ecosystems, the Unified Area Command (the EPA and the National Incident Commander) approved the use of chemical dispersants according to protocols outlined in the NCP, and ultimately, 1.84 million gallons of Corexit 9500A and Corexit 9527A were applied to the surface and at depth next to the wellhead (1, 47).
One of the primary aims of applying dispersants is to stimulate microbial biodegradation that leads to the removal of petroleum hydrocarbons (13, 45, 48). Past studies have typically focused on quantifying changes to biodegradation based on dispersant application and maximizing microbial community biodegradation activity (see the review by Prince [3] and references therein). Unlike with prior studies, we investigated how specific bacterial populations interact with crude oil and dispersed oil to elucidate the impacts on bacterial growth, biodegradation potential, and ecotoxicity. Here, we tested two strains based on their relevance to the DWH oil spill and different metabolic strategies (27, 49). Alcanivorax is a well-studied obligate hydrocarbonoclastic genus that has a limited carbon substrate range and a cosmopolitan distribution and rapidly responds to oil in the environment (5, 28, 29). Acinetobacter spp. are less-studied generalists with a broad carbon substrate range and a well-known capacity to degrade hydrocarbons in terrestrial and marine ecosystems (26, 27, 30, 50).
Species-specific responses to dispersant application.
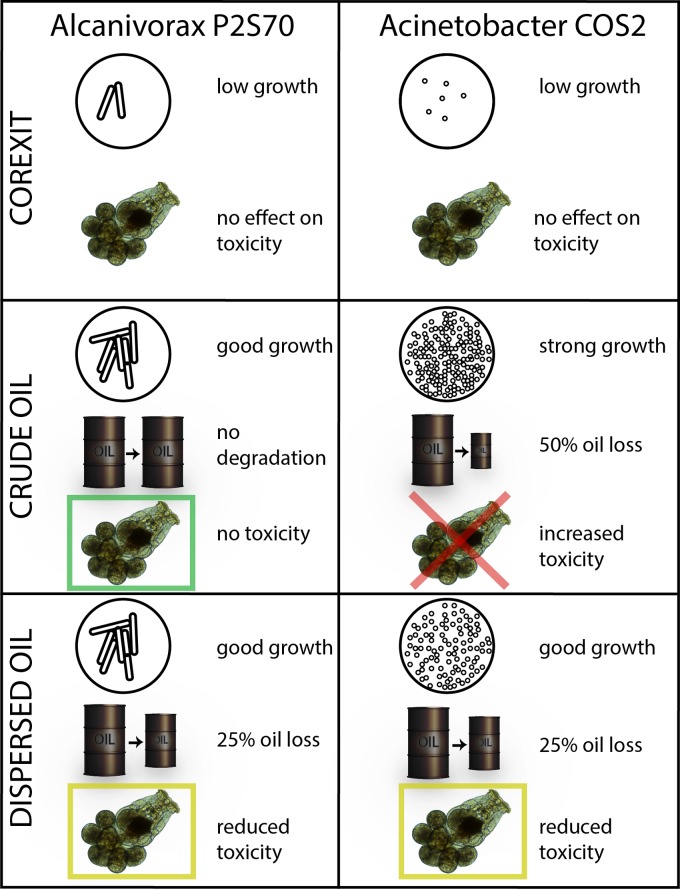
In this study, we found that each strain responded uniquely to dispersed oil versus crude oil (Fig. 5). We further demonstrated that both strains are capable of growth on Corexit 9500A alone, although Acinetobacter appeared to grow far better than Alcanivorax on Corexit alone. These results are corroborated by previous microcosm studies that showed that microbial consortia were capable of rapidly degrading both the hydrocarbon fraction and the dioctyl sodium sulfosuccinate (DOSS) fraction of Corexit 9500A (36, 51, 52). To our knowledge, this is the first report of cultivated strains exhibiting growth using Corexit 9500A as the sole carbon and energy source. Considering our results, it is not appropriate to generalize that hydrocarbon-degrading bacterial populations uniformly respond positively to dispersed oil.
FIG 5.
Strain-specific responses of growth, biodegradation potential, and toxicity as determined by an EPA-approved rotifer assay. Both bacterial strains tested were shown to grow with Corexit as the sole carbon and energy source, and no effect of bacterial transformation was observed on the toxicity of Corexit. Crude oil supported the growth of both bacterial strains, but the extents of biodegradation and their linkage to toxicity showed large variation between the strains. Although both strains showed similar rates of growth, degradation potentials, and linkages to toxicity, each strain responded uniquely to dispersed oil versus crude oil.
While Alcanivorax exhibited similar growth rates on dispersed oil and crude oil, the potential for oil degradation or transformation was significantly higher with the dispersed-oil treatment. It may be that this Alcanivorax strain targets only the short-chain n-alkanes when grown on crude oil (which are not analyzed by this method and would show no evidence of degradation potential) and can access or target a wider range of hydrocarbons when grown on dispersed oil than on crude oil. In crude oil treatments, Alcanivorax was capable of solubilizing many of the aliphatic constituents, which are thought to represent the primary carbon source for this microbial group. Furthermore, two branched-alkane species (pristine and phytane) were present in much lower concentrations than the n-alkane compounds in the crude oil WAF. This observation is significant because it is thought that Alcanivorax strains specialize in the utilization of branched alkanes (29, 53). Alcanivorax weakly solubilized the PAH fraction in the crude oil treatment, primarily the (methyl-)naphthalenes, which were also evident in the control sample. As in the crude oil control, we did not detect a toxicity effect associated with the Alcanivorax WAF (crude oil), which may in part be explained by the low PAH concentrations observed in both, as discussed in more detail below. However, a toxicity effect on B. manjavacas was observed with the dispersed-oil treatment, where concentrations of PAHs and aliphatics were higher than those in the crude oil treatment (Fig. 4).
In contrast to observations with Alcanivorax, the growth of Acinetobacter was inhibited by 37% in the Corexit 9500A-dispersed-oil treatment versus that in the crude oil-only treatment. This growth inhibition corresponded to a 40% reduction in oil degradation potential after 7 days and a 19% reduction after 14 days. Hamdan and Fulmer (54) also reported that Acinetobacter strains isolated from contaminated beach sands were negatively impacted by Corexit. Compared to Alcanivorax, Acinetobacter more effectively solubilized PAHs and n-alkanes in the crude oil treatment. Large increases in naphthalene, phenanthrene, fluoranthene, and benz[a]anthracene concentrations were observed. This potentially explains the toxicity observed in the crude oil Acinetobacter treatment. For the aliphatic fraction, long-chain n-alkanes (with a number of carbon atoms higher than 19) were found at concentrations similar to those of the Alcanivorax WAF (crude oil treatment), branched alkanes were substantially enriched, and shorter-chain alkanes were depleted (Fig. 4A).
Reports of previous field and laboratory evidence of the effects of synthetic dispersants on hydrocarbon-degrading microorganisms are contradictory. While the majority of prior studies showed that dispersants dramatically increased the growth and activity of indigenous hydrocarbon-degrading bacteria relative to the amount of oil that was physically dispersed, a few showed an adverse effect of dispersants on biodegradation (3, 22, 37, 55). Prince et al. (56) concluded that most laboratory studies do not effectively represent how dispersants behave in the environment, where dispersed oil is expected to rapidly diffuse to concentrations of <100 ppm. Studies conducted under conditions nearly identical to in situ dispersed-oil conditions (2.5 ppm oil by volume) revealed rapid oil degradation, little effect of dispersant addition, and no dispersant inhibitory effect on biodegradation. However, these studies should be extended to include the response of the microbial populations and biodegradation capacity, along with ecotoxicity (56, 57).
Impacts of bacterial degradation and transformation on ecotoxicity.
Following the DWH spill, the EPA developed benchmarks for the toxicity of PAHs to marine life (58). Results are reported as an acute potency divisor, which is derived from the 5th percentile of a distribution of acute LC50 values divided by 2. The most solubilized PAHs detected in the WAF or CEWAF of our study were naphthalene (acute potency divisor, 402 μg/liter), C1 to C3 methylnaphthalenes (170, 63, and 23 μg/liter, respectively), and C1 to C3 methylphenanthrenes (15.5, 6.65, and 2.62 μg/liter, respectively). Aromatic constituents in the WAF from the crude oil control and Alcanivorax-treated crude oil samples fell below these benchmarks, for the most part, and were not associated with rotifer toxicity, while with the Acinetobacter crude oil treatment, the undiluted WAF was acutely toxic to rotifers. In this treatment, we observed C2 to C3 naphthalenes and C1 to C3 phenanthrenes in concentrations up to 13 times higher than the EPA limits.
Alcanivorax and uninoculated controls from dispersed-oil treatments had concentrations of substituted naphthalenes and phenanthrenes in the CEWAF up to 21 and 26 times higher, respectively, than the EPA limits. Both treatments were associated with rotifer mortality. However, we observed much lower toxicity in the CEWAF of Alcanivorax-treated dispersed-oil samples than in the uninoculated dispersed-oil control samples. This may be due to much lower levels of aliphatics and slightly lower levels of PAHs in the Alcanivorax treatment. Alternatively, the bacterial biomass may sequester some of the toxic constituents, limiting rotifer exposure. Unfortunately, CEWAF hydrocarbon-specific extractions from Acinetobacter were not successful due to very high levels of EPS, although CEWAF from Acinetobacter-treated dispersed oil showed toxicity similar to that of CEWAF from Alcanivorax-treated dispersed oil.
It is likely that the different responses to dispersed oil exhibited by these two strains can be attributed to the production of different biosurfactants or bioemulsifiers. Acinetobacter spp. are some of the best-studied high-molecular-weight surfactant producers (25). These compounds are thought to be substrate specific, and Acinetobacter radioresistens is known to produce alasan, a bioemulsifier that solubilizes PAHs (59). If our strain of Acinetobacter produces a similar compound, it would explain the toxicity and greater entrainment of PAHs seen in the crude oil WAF treated with Acinetobacter than in the control and the Alcanivorax WAF. In contrast, Alcanivorax strains are better known for producing low-molecular-weight surfactants that lower surface and interfacial tensions and increase hydrocarbon solubility (25). Alcanivorax borkumensis produces 10 different derivatives of glucose lipids (a type of glycolipid) when grown on n-alkanes, and these compounds reduce the surface tension of water from 72 to 29 mN/m (60). Alcanivorax dieselolei produces a lipoprotein (proline lipids) when grown on hexadecane, and this strain reduces the surface tension of water to 29.6 to 32.8 mN/m. This strain did not produce detectable glycolipids (61). The Alcanivorax strain used in this study (P2S70) is not closely related to either A. dieselolei or A. borkumensis (average nucleotide identity [ANI] of 80.2% and 81.6%, respectively), and we cannot speculate on the biosurfactant produced by this strain at this time. However, our results suggest that Alcanivorax P2S70 produces a biosurfactant that increases the entrainment of the aliphatic fraction of crude oil into culture media.
The observed increase in toxicity associated with the uninoculated dispersed oil is consistent with the literature, and numerous studies have shown dispersant-oil mixtures to be significantly more toxic than dispersants alone (14, 19–21, 62). Like Rico-Martínez et al. (14), we saw a synergistic effect of Corexit 9500A, with Macondo crude oil increasing the toxicity to the marine rotifer B. manjavacas relative to Corexit alone or to physically dispersed crude oil. Our results support the conclusion that dispersants increase the toxicity of oil by introducing a larger percentage of oil components into the soluble phase, as much higher concentrations of PAHs and aliphatics are detected when dispersants are present (15).
Conclusions.
Here, we demonstrate that dispersants do not enhance biodegradation to the same degree or perhaps by the same mechanism for each strain of hydrocarbon-degrading bacteria (Fig. 5). This suggests that specific microbial groups interact with oil and dispersed oil through various mechanisms, likely involving biosurfactant/bioemulsifier production and modification of cellular membrane composition, and the outcome of this interaction will determine the efficiency of biodegradation and its overall effect on the ecosystem. Both positive and negative effects of dispersants on biodegradation can be expected. While we show that individual populations demonstrate a unique response to the application of dispersant to crude oil, more research is needed to uncover the mechanisms of interaction at the strain or population level. The transformation of specific compounds must be related to the activities of specific organismal groups, both in the laboratory and in the field. We foresee the ultimate goal of such studies as focusing on the implementation of a predictive model incorporating how specific microbial populations respond to dispersant applications in the environment.
Supplementary Material
ACKNOWLEDGMENTS
We thank N. Zenzola and Q. Miller for their help during the laboratory work.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Funding Statement
This work was made possible in part by a grant from BP/The Gulf of Mexico Research Initiative to the C-IMAGE, C-IMAGE-2, and Deep-C consortia and in part by a National Science Foundation Graduate Research Fellowship (W.A.O.) under grant no. 2013172310.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02379-15.
REFERENCES
- 1.Zukunft PF. 2010. Summary report for sub-sea and sub-surface oil and dispersant detection: sampling and monitoring. DIANE Publishing, Collingdale, PA. [Google Scholar]
- 2.Leahy JG, Colwell RR. 1990. Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince RC. 2010. Bioremediation of marine oil spills, p 2617–2630. In Timmis KN, McGenity TJ, van der Meer JR, de Lorenzo V (ed), Handbook of hydrocarbon and lipid microbiology. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 4.Ramseur JL. 2010. Deepwater Horizon oil spill: the fate of the oil. Congressional Research Service, Washington, DC. [Google Scholar]
- 5.King GM, Kostka JE, Hazen TC, Sobecky PA. 2015. Microbial responses to the Deepwater Horizon oil spill: from coastal wetlands to the deep sea. Ann Rev Mar Sci 7:377–401. doi: 10.1146/annurev-marine-010814-015543. [DOI] [PubMed] [Google Scholar]
- 6.Hazen TC, Dubinsky EA, DeSantis TZ, Andersen GL, Piceno YM, Singh N, Jansson JK, Probst A, Borglin SE, Fortney JL, Stringfellow WT, Bill M, Conrad ME, Tom LM, Chavarria KL, Alusi TR, Lamendella R, Joyner DC, Spier C, Baelum J, Auer M, Zemla ML, Chakraborty R, Sonnenthal EL, D'haeseleer P, Holman H-YN, Osman S, Lu Z, Van Nostrand JD, Deng Y, Zhou J, Mason OU. 2010. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330:204–208. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- 7.Camilli R, Reddy CM, Yoerger DR, Van Mooy BA, Jakuba SMV, Kinsey JC, McIntyre CP, Sylva SP, Maloney JV. 2010. Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science 330:201–204. doi: 10.1126/science.1195223. [DOI] [PubMed] [Google Scholar]
- 8.Mason OU, Hazen TC, Borglin S, Chain PSG, Dubinsky EA, Fortney JL, Han J, Holman H-YN, Hultman J, Lamendella R, Mackelprang R, Malfatti S, Tom LM, Tringe SG, Woyke T, Zhou J, Rubin EM, Jansson JK. 2012. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J 6:1715–1727. doi: 10.1038/ismej.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason OU, Scott NM, Gonzalez A, Robbins-Pianka A, Bælum J, Kimbrel J, Bouskill NJ, Prestat E, Borglin S, Joyner DC, Fortney JL, Jurelevicius D, Stringfellow WT, Alvarez-Cohen L, Hazen TC, Knight R, Gilbert JA, Jansson JK. 2014. Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. ISME J 8:1464–1475. doi: 10.1038/ismej.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentine DL, Kessler JD, Redmond MC, Mendes SD, Heintz MB, Farwell C, Hu L, Kinnaman FS, Yvon-Lewis S, Du M, Chan EW, Garcia Tigreros F, Villanueva CJ. 2010. Propane respiration jump-starts microbial response to a deep oil spill. Science 330:208–211. doi: 10.1126/science.1196830. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Deng Y, Van Nostrand JD, He Z, Voordeckers J, Zhou A, Lee Y-J, Mason OU, Dubinsky EA, Chavarria KL, Tom LM, Fortney JL, Lamendella R, Jansson JK, D'haeseleer P, Hazen TC, Zhou J. 2012. Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J 6:451–460. doi: 10.1038/ismej.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivers AR, Sharma S, Tringe SG, Martin J, Joye SB, Moran MA. 2013. Transcriptional response of bathypelagic marine bacterioplankton to the Deepwater Horizon oil spill. ISME J 7:1–15. doi: 10.1038/ismej.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessard RR, DeMarco G. 2000. The significance of oil spill dispersants. Spill Sci Technol Bull 6:59–68. doi: 10.1016/S1353-2561(99)00061-4. [DOI] [Google Scholar]
- 14.Rico-Martínez R, Snell TW, Shearer TL. 2013. Synergistic toxicity of Macondo crude oil and dispersant Corexit 9500® to the Brachionus plicatilis species complex (Rotifera). Environ Pollut 173:5–10. doi: 10.1016/j.envpol.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 15.George-Ares A, Clark JR. 2000. Aquatic toxicity of two Corexit dispersants. Chemosphere 40:897–906. doi: 10.1016/S0045-6535(99)00498-1. [DOI] [PubMed] [Google Scholar]
- 16.Dahms HU, Hagiwara A, Lee JS. 2011. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat Toxicol 101:1–12. doi: 10.1016/j.aquatox.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Snell TW, Janssen CR. 1995. Rotifers in ecotoxicology: a review. Hydrobiologia 313-314:231–247. [Google Scholar]
- 18.US Coast Guard, US Environmental Protection Agency. 2010. Dispersant monitoring and assessment directive for subsurface dispersant application. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 19.Hemmer MJ, Barron MG, Greene RM. 2011. Comparative toxicity of eight oil dispersants, Louisiana sweet crude oil (LSC), and chemically dispersed LSC to two aquatic test species. Environ Toxicol Chem 30:2244–2252. doi: 10.1002/etc.619. [DOI] [PubMed] [Google Scholar]
- 20.Ballou TG, Hess SC, Dodge RE, Knap AH, Sleeter TD. 1989. Effects of untreated and chemically-dispersed oil on tropical marine communities: a long-term field experiment. Int Oil Spill Conf Proc 1989:447–454. doi: 10.7901/2169-3358-1989-1-447. [DOI] [Google Scholar]
- 21.Shafir S, Van Rijn J, Rinkevich B. 2007. Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ Sci Technol 41:5571–5574. doi: 10.1021/es0704582. [DOI] [PubMed] [Google Scholar]
- 22.Mulkins-Phillips GJ, Stewart JE. 1974. Effect of four dispersants on biodegradation and growth of bacteria on crude oil. Appl Microbiol 28:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. 2010. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 24.Ron EZ, Rosenberg E. 2002. Biosurfactants and oil bioremediation. Curr Opin Biotechnol 13:249–252. doi: 10.1016/S0958-1669(02)00316-6. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg E, Ron EZ. 2013. Biosurfactants, p 545–577. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 26.Overholt WA, Green SJ, Marks KP, Venkatraman R, Prakash O, Kostka JE. 2013. Draft genome sequences for oil-degrading bacterial strains from beach sands impacted by the Deepwater Horizon oil spill. Genome Announc 1:pii=e01015-13. doi: 10.1128/genomeA.01015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M. 2011. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl Environ Microbiol 77:7962–7974. doi: 10.1128/AEM.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Head IM, Jones DM, Röling WFM. 2006. Marine microorganisms make a meal of oil. Nat Rev Microbiol 4:173–182. doi: 10.1038/nrmicro1348. [DOI] [PubMed] [Google Scholar]
- 29.Yakimov MM, Timmis KN, Golyshin PN. 2007. Obligate oil-degrading marine bacteria. Curr Opin Biotechnol 18:257–266. doi: 10.1016/j.copbio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y-S, Jung J, Jeon C, Park W. 2011. Acinetobacter oleivorans sp. nov. is capable of adhering to and growing on diesel-oil. J Microbiol 49:29–34. doi: 10.1007/s12275-011-0315-y. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Yu XZ, Wu SC, Cheung KC, Tam NFY, Qian PY, Wong MH. 2006. Interactions of rice (Oryza sativa L.) and PAH-degrading bacteria (Acinetobacter sp.) on enhanced dissipation of spiked phenanthrene and pyrene in waterlogged soil. Sci Total Environ 372:1–11. doi: 10.1016/j.scitotenv.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Di Cello F, Pepi M, Baldi F, Fani R. 1997. Molecular characterization of an n-alkane-degrading bacterial community and identification of a new species, Acinetobacter venetianus. Res Microbiol 148:237–249. doi: 10.1016/S0923-2508(97)85244-8. [DOI] [PubMed] [Google Scholar]
- 33.Widdel F. 2010. Cultivation of anaerobic microorganisms with hydrocarbons as growth substrates, p 3787–3798. In Timmis KN, McGenity TJ, van der Meer JR, de Lorenzo V (ed), Handbook of hydrocarbon and lipid microbiology. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 34.Gardiner WW, Word JQ, Word JD, Perkins RA, Mcfarlin KM, Hester BW, Word LS, Ray CM. 2013. The acute toxicity of chemically and physically dispersed crude oil to key arctic species under arctic conditions during the open water season. Environ Toxicol Chem 32:2284–2300. doi: 10.1002/etc.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson JA, Kuhl AJ, Anderson AN. 2014. Toxicity of oil and dispersed oil on juvenile mud crabs, Rhithropanopeus harrisii. Bull Environ Contam Toxicol 92:375–380. doi: 10.1007/s00128-014-1216-7. [DOI] [PubMed] [Google Scholar]
- 36.Campo P, Venosa AD, Suidan MT. 2013. Biodegradability of Corexit 9500 and dispersed South Louisiana crude oil at 5 and 25°C. Environ Sci Technol 47:1960–1967. doi: 10.1021/es303881h. [DOI] [PubMed] [Google Scholar]
- 37.Swannell RPJ, Daniel F. 1999. Effect of dispersants on oil biodegradation under simulated marine conditions. Int Oil Spill Conf Proc 1999:169–176. doi: 10.7901/2169-3358-1999-1-169. [DOI] [Google Scholar]
- 38.Pelz O, Brown J, Huddleston M, Rand G, Gardinali P, Stubblefield W, Benkinney MT, Ahnell A. 2011. Selection of a surrogate MC252 oil as a reference material for future aquatic toxicity tests and other studies, poster Soc Environ Toxicol Chem Meet, Boston, MA http://gulfresearchinitiative.org/wp-content/uploads/2012/05/Surrogate-Oil-selection-Paper-at-SETAC.pdf. [Google Scholar]
- 39.US Environmental Protection Agency. 2014. COREXIT® EC9500A. Emergency response. US Environmental Protection Agency, Washington, DC: http://www2.epa.gov/emergency-response/corexitr-ec9500a. [Google Scholar]
- 40.Singer MM, Aurand D, Bragin GE, Clark JR, Coelho GM, Sowby ML, Tjeerdema RS. 2000. Standardization of the preparation and quantitation of water-accommodated fractions of petroleum for toxicity testing. Mar Pollut Bull 40:1007–1016. doi: 10.1016/S0025-326X(00)00045-X. [DOI] [Google Scholar]
- 41.Hamilton MA. 1977. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719. doi: 10.1021/es60130a004. [DOI] [Google Scholar]
- 42.Stone BR. 2012. tsk: trimmed Spearman-Karber method. R package version 1.1. https://r-forge.r-project.org/projects/tskarber/. [Google Scholar]
- 43.Gros J, Reddy CM, Aeppli C, Nelson RK, Carmichael CA, Arey JS. 2014. Resolving biodegradation patterns of persistent saturated hydrocarbons in weathered oil samples from the Deepwater Horizon disaster. Environ Sci Technol 48:1628–1637. doi: 10.1021/es4042836. [DOI] [PubMed] [Google Scholar]
- 44.National Oil and Hazardous Substances Pollution Contigency Plan (NCP). 1994. National Oil and Hazardous Substances Pollution Contigency Plan (NCP). Code of Federal Regulations, title 40, chapter I, subchapter J, part 300, 59 FR 47416 http://www.ecfr.gov/cgi-bin/text-idx?SID=f7cd5cf77fe84292d78d1ac1edf92a95&mc=true&node=pt40.28.300&rgn=div5. [Google Scholar]
- 45.Chapman H, Purnell K, Law RJ, Kirby MF. 2007. The use of chemical dispersants to combat oil spills at sea: a review of practice and research needs in Europe. Mar Pollut Bull 54:827–838. doi: 10.1016/j.marpolbul.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 46.National Research Council. 2005. Oil spill dispersants: efficacy and effects. National Academies Press, Washington, DC. [Google Scholar]
- 47.Allen TW. 2010. National Incident Commander's report: MC252 Deepwater Horizon. US Coast Guard National Incident Command, Washington, DC. [Google Scholar]
- 48.Lubchenco J, McNutt MK, Dreyfus G, Murawski SA, Kennedy DM, Anastas PT, Chu S, Hunter T. 2012. Science in support of the Deepwater Horizon response. Proc Natl Acad Sci U S A 109:20212–20221. doi: 10.1073/pnas.1204729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Liu J. 2013. Evaluating bacterial community structures in oil collected from the sea surface and sediment in the northern Gulf of Mexico after the Deepwater Horizon oil spill. Microbiologyopen 2:492–504. doi: 10.1002/mbo3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaneechoutte M, Tjernberg I, Baldi F, Pepi M, Fani R, Sullivan ER, van der Toorn J, Dijkshoorn L. 1999. Oil-degrading Acinetobacter strain RAG-1 and strains described as “Acinetobacter venetianus sp. nov.” belong to the same genomic species. Res Microbiol 150:69–73. doi: 10.1016/S0923-2508(99)80047-3. [DOI] [PubMed] [Google Scholar]
- 51.Baelum J, Borglin S, Chakraborty R, Fortney JL, Lamendella R, Mason OU, Auer M, Zemla M, Bill M, Conrad ME, Malfatti SA, Tringe SG, Holman H-Y, Hazen TC, Jansson JK. 2012. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ Microbiol 14:2405–2416. doi: 10.1111/j.1462-2920.2012.02780.x. [DOI] [PubMed] [Google Scholar]
- 52.Chakraborty R, Borglin SE, Dubinsky EA, Andersen GL, Hazen TC. 2012. Microbial response to the MC-252 oil and Corexit 9500 in the Gulf of Mexico. Front Microbiol 3:357. doi: 10.3389/fmicb.2012.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yakimov MM, Golyshin PN, Lang S, Moore ERB, Abraham W, Lunsdorf H, Timmis KN. 1998. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Bacteriol 48(Pt 2):339–348. doi: 10.1099/00207713-48-2-339. [DOI] [PubMed] [Google Scholar]
- 54.Hamdan L, Fulmer P. 2011. Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill. Aquat Microb Ecol 63:101–109. doi: 10.3354/ame01482. [DOI] [Google Scholar]
- 55.Varadaraj R, Robbins ML, Bock J, Pace S, Macdonald D. 1995. Dispersion and biodegradation of oil spills on water. Int Oil Spill Conf Proc 1995:101–106. doi: 10.7901/2169-3358-1995-1-101. [DOI] [Google Scholar]
- 56.Prince RC, McFarlin KM, Butler JD, Febbo EJ, Wang FCY, Nedwed TJ. 2013. The primary biodegradation of dispersed crude oil in the sea. Chemosphere 90:521–526. doi: 10.1016/j.chemosphere.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Prince RC, Butler JD. 2014. A protocol for assessing the effectiveness of oil spill dispersants in stimulating the biodegradation of oil. Environ Sci Pollut Res Int 21:9506–9510. doi: 10.1007/s11356-013-2053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US Environmental Protection Agency. 2015. Correction of Deepwater Horizon acute screening benchmarks for aquatic life. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 59.Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E. 1999. Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol 65:2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abraham WR, Meyer H, Yakimov M. 1998. Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim Biophys Acta 1393:57–62. doi: 10.1016/S0005-2760(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 61.Qiao N, Shao Z. 2010. Isolation and characterization of a novel biosurfactant produced by hydrocarbon-degrading bacterium Alcanivorax dieselolei B-5. J Appl Microbiol 108:1207–1216. doi: 10.1111/j.1365-2672.2009.04513.x. [DOI] [PubMed] [Google Scholar]
- 62.Goodbody-Gringley G, Wetzel DL, Gillon D, Pulster E, Miller A, Ritchie KB. 2013. Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit® 9500, to coral larvae. PLoS One 8: e45574. doi: 10.1371/journal.pone.0045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-R LM, Overholt WA, Hagan C, Huettel M, Kostka JE, Konstantinidis KT. 2015. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. ISME J 9:1928–1940. doi: 10.1038/ismej.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.