Summary
Respiratory syncytial virus (RSV)‐specific CD8+ T cell responses do not protect against reinfection. Activation of mammalian target of rapamycin (mTOR) impairs memory CD8+ T cell differentiation. Our hypothesis was that RSV inhibits the formation of CD8+ T cells memory responses through mTOR activation. To explore this, human and mouse T cells were used. RSV induced mTOR phosphorylation at Ser2448 in CD8 T cells. mTOR activation by RSV was completely inhibited using rapamycin. RSV‐infected children presented higher mTOR gene expression on nasal washes comparing to children infected with metapneumovirus and rhinovirus. In addition, RSV‐infected infants presented a higher frequency of CD8+ pmTORser2448+ T cells in nasal washes compared to RSV‐negative infants. Rapamycin treatment increased the frequency of mouse CD8 RSV‐M282–90 pentamer‐positive T cells and the frequency of RSV‐specific memory T cells precursors. These data demonstrate that RSV is activating mTOR directly in CD8 T cells, indicating a role for mTOR during the course of RSV infection.
Keywords: CD8+ T cells, mTOR, nasal washes, RSV, RSV‐infected infants
Introduction
Respiratory syncytial virus (RSV) is ubiquitous in the environment, and may cause severe infections in infants, immunodeficient patients and elderly people. Lower respiratory tract infection caused by RSV constitutes the most prevalent disease in children during the first years of life 1. RSV infections are estimated to result in approximately 200 000 deaths annually worldwide in children younger than 5 years of age, and an even much larger impact on hospital admissions 2. RSV infection does not result in protective immune responses, and consequently re‐infections are common 3, 4, 5, pointing to a probable deficit in memory responses. It is known that children with deficiencies in cell‐mediated immunity suffer more severe and prolonged RSV disease, suggesting that this is an important mechanism of RSV infection control 6. Also, older individuals who are susceptible to RSV infection presented lower numbers and decreased function of memory RSV‐specific T cells 7. RSV inhibits T cell responses in mice and humans, and several virulent mechanisms have been proposed 8, 9, 10, 11, 12, 13, 14. RSV infection in mice could suppress CD8+ T cell effector activity and memory CD8+ T cell in the respiratory tract 14.
mTOR (mammalian target of rapamycin), a serine–threonine protein kinase, which plays a role in regulating cell growth, metabolism and survival, also controls the transition of CD8+ T cells effectors into memory cells 15, 16. Araki et al. have demonstrated that low doses of rapamycin, an mTOR inhibitor, promoted the generation of memory CD8+ T cells during a virus infection 17. Our hypothesis was that activation of mTOR by RSV is a virulence mechanism employed to decrease CD8+ T cell activity, especially in the memory compartment. The aim of this study was to investigate whether RSV infection modulates mTOR in CD8 T cells. To answer this question, human and mouse T cells were used. Our data demonstrate clearly that RSV induces mTOR phosphorylation at ser2448 directed in CD8 T cells. In addition, mTOR gene expression in nasal washes of RSV‐infected infants is increased significantly compared to non‐infected infants or infants infected with metapnemovirus and rhinovirus, indicating the role of mTOR during RSV infection.
Materials and methods
Virus
RSV A strain (line 19) was provided by Fernando Polack, Fundación Infant, Argentina. The virus was grown in human epithelial type 2 (Hep‐2) cells. Viral plaque‐forming units (PFU) were identified using an RSV F protein‐specific antibody (Millipore, Billerica, MA, USA).
Human study
The present study used peripheral blood mononuclear cells (PBMC) isolated from healthy adults donors aged 20–40 years. Ten ml of blood was collected from donors after they had assigned a written informed consent. This procedure was approved by PUCRS research ethical committee (Protocol CEP 844.206).
In addition, we used nasal washes from infants hospitalized with bronchiolitis (first episode) or from those with recurrent wheezing in the first year of life. These samples came from patients who had up to 72 h of clinical signs or symptoms in the lower respiratory tract (wheezing and/or respiratory distress); infants with other chronic or underlying severe diseases were excluded. All patients underwent data collection and nasopharyngeal wash for identification of respiratory viruses on the first day of hospitalization. RSV presence was tested in the nasal washes using either QuickVue RSV test (Quidel, San Diego, CA, USA) or confirmed by quantitative polymerase chain reaction (qPCR). PUCRS Research Ethics Committee approved this study (Protocol CEP 09/04 678). Parents who agreed to participate in the study signed a written informed consent. Demographic data are shown on Table 1. There was no difference between age and gender distribution among the patients (χ2).
Table 1.
Patients’ characteristics
| Age (months)* | Sex (M/F)† | |
|---|---|---|
| RSV‐positive (n = 18) | 3.9 (s.d. 2·2) | 10/8 |
| RSV‐negative (n = 23) | 5.0 (s.d. 3·3) | 18/5 |
| MPV‐ and rhino‐positive (n = 05) | 3.2 (s.d. 2·3) | 2/3 |
Values are presented as mean [standard deviation (s.d.)], P = 0·03 (one‐way analysis of variance).
Gender distribution, P > 0·05 (χ2). RSV = respiratory syncytial virus; MPV = metapneumovirus; M = male; F = female.
Human cell culture
PBMCs from healthy volunteers were isolated using a Histopaque (Sigma‐Aldrich, St Louis, MO, USA) density gradient. Memory CD8 T cells from PBMC were isolated by magnetic beads purification (CD8a+ memory T cell isolation kit; Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions.
A total of 4 × 105 cells were cultured per well in 96 U‐well plates using RPMI 2% of fetal calf serum (FCS) in the presence of 1 × 102 PFUs viable or ultraviolet (UV)‐inactivated RSV. As a positive control, cells were stimulated with phorbol‐12‐myristate‐13‐acetate (PMA) (Sigma, St Louis, MO, USA). As negative controls, uninfected Hep‐2 cells were used, processed similarly to RSV infected Hep‐2 cells. Those cells were incubated for 15, 30 or 45 min, and at each time‐point there were collections for analysis. To perform some experiments, cells were incubated with 50 µM of PI3K inhibitor (Ly294002) (Sigma Aldrich) or 20 ηg/ml of rapamycin (Cell Signaling, Danvers, MA, USA) for 1 h before the virus incubation. Also, recombinant RSV F protein (Sino Biological Inc., Beijing, China) was used to stimulate PBMCs for 30 min.
Nasal washes were centrifuged at 300 g (1800rpm) the supernatant was remove and washed with 1 ml of phosphate‐buffered saline (PBS) with 15 mM of ethylenediamine tetraacetic acid (EDTA), then RPMI 2% of FCS was added. RSV‐positive samples were incubated with rapamycin for 1 h or with only media as a control.
Human cell staining to flow cytometry
PBMC, CD8 T cell memory or nasal washes cells were incubated with FcBlock (supernatant of 24G2 cells with 5% of human serum) for 20 min before staining. Cells were then fixed with Cytofix buffer (BD Bioscience, San Jose, CA, USA) for 10 min at 37ºC and permeabilized with Perm Buffer III (BD Bioscience) for 20 min on ice. After that, cells were stained either with anti‐phospho‐4EPB1 (T69) phycoerythrin (PE) (clone M34‐273) (BD Bioscience), anti‐phospho‐mTOR (ser2448), antibodies (Millipore Merck, Darmstadt, Germany), for 30 min, followed by staining with cyanin 3 (Cy3)‐conjugated secondary antibody (Chemicon International, Temecula, CA, USA).
Nasal wash cells were also stained with CD8 fluorescein isothiocyanate (FITC) (clone RPA‐T8), CD45RO peridinin clorophyll (PerCP) 5·5 (clone UCHL1) and anti‐granzyme B allophycocyanin (APC) antibody (clone GB11) (all from BD Bioscience). Samples were acquired on flow cytometer fluorescence activated cell sorter (FACS)Canto II (BD Bioscience) and the data were analysed using FlowJo version X·0·.7 software (TreeStar, Ashland, OR, USA).
Immunofluorescence staining
PBMCs, incubated with either the virus or a control, were used to cytospin preparations. The cells on the microscope slides were fixed with acetone and then incubated with FcBlock. Cells were stained with anti‐phospho‐mTOR (ser2448) followed by staining with Cy3‐conjugated secondary antibody (Chemicon International). Nuclear staining was performed with Hoescht 33342 (Invitrogen Corporation, Carlsbad, CA, USA) and slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) for analysis on a fluorescence microscope (Olympus, Tokyo, Japan). Images were acquired using software Cell Sens (Olympus).
Real‐time PCR analysis
Nasal washes were tested for RSV, human rhinovirus (HRV) and human metapneumovirus (HMPV). RNA extraction was performed using TRIzolTM (Life Technologies, Foster City, CA, USA), according to the manufacturer's instructions. cDNA was synthesized using Superscript III kit (Invitrogen™ by Life Technologies) and quantified using Qubit (DNA HS kit; Invitrogen™). The quality of the cDNA for each patient was tested by amplification of β‐actin endogenous gene using specific primers and probes for TaqMan Assay (HuACTB; Applied Biosystems® by Life Technologies), and TaqMan Master Mix (Applied Biosystems®) on StepOne™ (real‐time PCR; Applied Biosystems®). PCR conditions were recommended by TaqMan Master Mix protocol. Samples in which transcription of the β‐actin gene could not be detected were excluded from the study.
mTOR relative expression was performed using specific primers and probes for TaqMan Assay (Hs00234508_m1mTOR) using the β‐actin (HuACTB) and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Hs03929097_g1GAPDH) genes as endogenous controls. Quantification of gene expression was conducted using StepOne™ (real‐time PCR system; Applied Biosystems®). Relative mRNA expression was determined using the delta cycle threshold (Δ‐CT) method. Delta value was calculated by subtracting the CT value for β‐actin or GAPDH from the CT value for mTOR of each of the samples.
Mice studies
Female BALB/c (H‐2d) mice ranging from 8 to 12 weeks old were purchased from Cembe (PUCRS). The mice were housed at the animal facility of Instituto de Pesquisas Biomédicas (IPB‐PUCRS). All animal procedures were performed in accordance with protocols approved by the University Ethics Committee (CEUA Protocol number 11/00268).
Murine bone marrow‐derived dendritic cell (DC) cultures
Murine bone marrow‐derived DCs (BMDCs) were grown from bone marrow in AIM‐V medium (Gibco, Carlsbad, CA, USA) with granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and interleukin (IL)‐4 (Peprotech, Ribeirao Preto, Brazil), as described previously 18.
Murine memory T cell analysis
Murine T cells were purified from splenocytes using Pan T cells magnetic beads (Miltenyi Biotec), following the manufacturer's instructions; 5 × 104 purified T cells were treated with 20 ηg/ml of rapamycin for 1 h. The cells were then co‐cultured with 15 × 103 BMDCs infected with 102 PFU of RSV in AIM‐V medium (Gibco ®, Life Technologies) for 4 days. In order to evaluate the memory T cell precursors, cells were stained with anti‐CD8 PECy7 (clone 53‐6.7), anti‐killer cell lectin‐like receptor G1 (KLRG1), APC, anti‐B220 FITC (clone RA3‐6B2), anti‐CD197 PerCP (clone 4B‐12), anti‐CD127 PECy7 (clone SB/199) and anti‐CD44 FITC (clone IM7) (all from BD Bioscience). RSV M282–90‐specific CD8+ T cell responses were measured by staining the cells with RSV M282–90 pentamers (ProImmune, Oxford, UK) for 1 h at 37ºC. Samples were acquired on a flow cytometer FACS CANTO II (BD Bioscience). Data were analysed using FlowJo software (TreeStar).
Statistical analysis
The analysis of variance (anova) test followed by a Bonferroni post‐test and t‐test were applied to parametric data using GraphPad Prism software (San Diego, CA, USA). Values demonstrated in graphs are mean ± standard deviation (s.d.) and a level of significance of P < 0·05 was established for the analyses.
Results
RSV induced mTOR phosphorylation at Ser2448 on CD8 T cells
To determine whether RSV stimulates mTOR phosphorylation on CD8 T cells, human PBMCs were incubated with RSV for 15 or 30 min. RSV induced mTOR phosphorylation at Ser2448 on CD8 T cells (Fig. 1a,b). To confirm our flow cytometry results, immunocytochemical staining of PBMCs was performed. Figure 1c shows mTOR phosphorylation induced by RSV in red.
Figure 1.
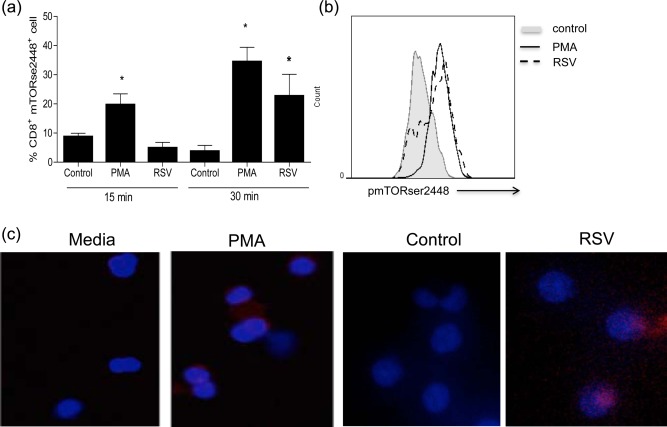
Respiratory syncytial virus (RSV) induced mammalian target of rapamycin (mTOR) phosphorylation on CD8 T cells. Peripheral blood mononuclear cells (PBMCs) (4 × 105/well) were culture in RPMI 2% of fetal calf serum (FCS) with 102 plaque‐forming units (PFU) of RSV, phorbol myristate acetate (PMA) or control [uninfected human epithelial type 2 (Hep‐2) cells processed similarly to RSV‐infected Hep2 cells) for 15 or 30 min, then cells were fixed, permeabilized and stained with anti‐phospho mTOR Ser2448, followed by anti‐rabbit immunoglobulin (Ig)G cyanin 3 (Cy3) and analysed on a flow cytometer or the fluorescence microscope after cytospin and mTOR staining. (a) Graph showing the frequency of CD8+mTORser2448+ cells. (b) Histograms showing mTOR phosphorylation when cells were incubated with RSV compared to control and PMA. (b) Images of mTOR phosphorylation at ser2448 in BMCs incubated with RSV or control (blue = nuclei staining, red = mTORser2448 staining).
mTOR functions within two distinct multi‐protein complexes, mTORC1 and mTORC2. mTORC1 is described to be involved in memory differentiation of antigen‐specific CD8+ T cells 17. Rapamycin specifically inhibits mTORC1, but also inhibits mTORC2 in prolonged treatment. Our results showed that mTOR activation by RSV was inhibited completely by a low dose of rapamycin after 1 h of treatment (Fig. 2a). In order to confirm that RSV modulates mTORC1, 4EBP1 activation, an mTORC1 downstream target, was analysed by flow cytometry after human PBMC incubation with the virus. RSV increased MFI of p4EBP1 2·5‐fold compared to control (Fig. 2c), suggesting that RSV induces mTORC1 activation. Signalling through phosphoinositide 3‐kinase (PI3K)/protein kinase B (AKT) is a common upstream pathway that up‐regulates mTORC1. To verify if RSV modulates mTOR through PI3K/AKT signalling, human PBMCs were incubated with a PI3K inhibitor (LY294002) for 1 h, then RSV was added and after 30 min mTOR phosphorylation was evaluated. Figure 2a data show that mTOR activation induced by RSV was not dependent upon the PI3K/AKT pathway.
Figure 2.
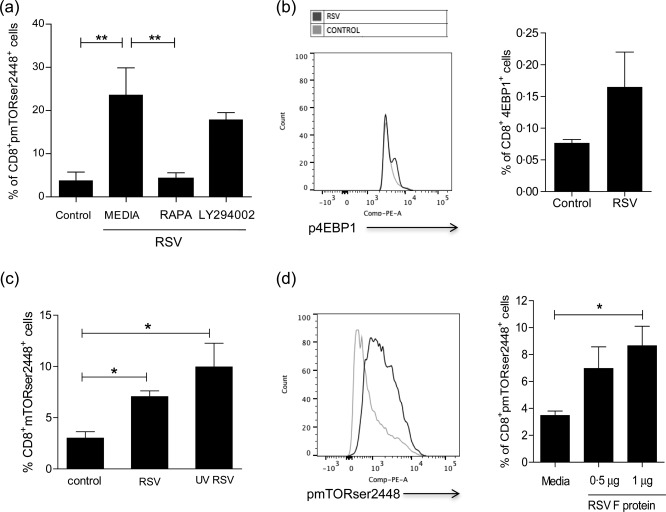
Rapamycin inhibited respiratory syncytial virus (RSV) induction of mammalian target of rapamycin (mTOR) phosphorylation on CD8 T cells. (a) Frequency of CD8+mTORser2448+ cells after peripheral blood mononuclear cell (PBMC) incubation with 20 ηg/ml of rapamycin or 50 µM of phosphoinositide 3‐kinase/protein kinase B (PI3K/AKT) inhibitor (LY294002) 1 h before RSV incubation for 30 min. (b) PBMC were incubated with 102 plaque forming units (PFU), RSV or control for 30 min and stained with anti‐4EPB1. Graph shows mean fluorescence intensity (MFI) of 4BP1 on RSV‐stimulated cells comparing to control. (c) Frequency of CD8+mTORser2448+ cells after PBMC incubation with ultraviolet (UV)‐inactivated RSV, RSV and control for 30 min. (d) Frequency of CD8+mTORser2448+ T cells after incubation with 0·5 μg or 1 μg of RSV F protein and media for 30 min. One‐way analysis of variance (anova) with Bonferroni's multiple comparison post‐test (*P < 0·05 and **P < 0·01).
In order to test whether infective viruses were necessary to induce mTOR phosphorylation in CD8 T cells, RSV was inactivated using UV light for 30 min and incubated with PBMCs for 30 min. Subsequently, PBMC were stained with anti‐CD8 and anti‐mTOR antibodies. UV‐inactivated virus induced mTOR phosphorylation at ser2448 in CD8 T cells (Fig. 2c), suggesting that cell infection is not essential for this process. Therefore, mTOR induction by RSV was not dependent upon virus replication processes, suggesting that this effect might be associated with viral contact to CD8 T cells. Therefore, we tested whether RSV fusion protein, a viral transmembrane surface glycoprotein, is linked with mTOR activation by RSV. Figure 2d shows that the frequency of CD8+pmTORser2448+ cells increased when the cells were incubated with recombinant RSV F protein compared to cells with media only.
We next asked whether RSV could induce mTOR phosphorylation directly in memory CD8 T cells. In order to answer this question, human CD8+CD45RO+CD45RA–CD56–CD57 – memory T cells were purified from PBMCs by depletion of non‐CD8+ T cells and CD8+ T cells with a different phenotype using magnetic isolation. The purified cells were incubated with RSV for 30 min, following mTOR phosphorylation analysis. RSV activated mTOR mediated through phosphorylation on the serine 2448 site directly in memory CD8 T cells, and this effect was inhibited by rapamycin treatment (Fig. 3a,b). Taken together, these data demonstrated clearly that RSV modulated mTOR directly on CD8 T cells in vitro. Consequently, this raises the question of whether this phenomenon also occurs in vivo.
Figure 3.
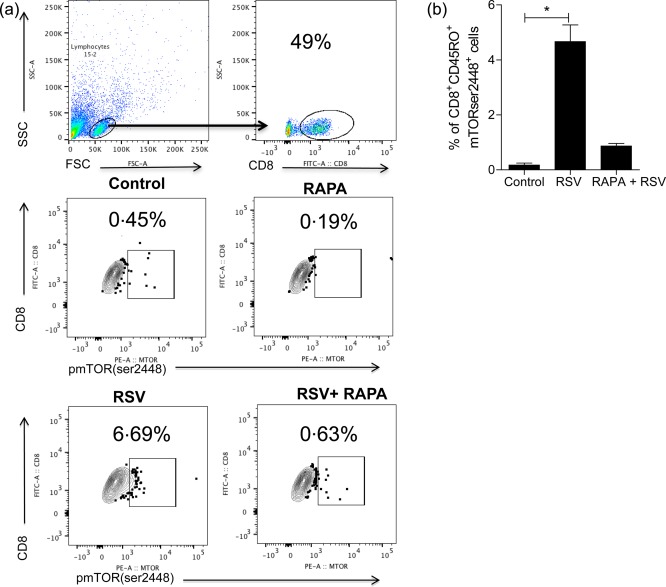
Respiratory syncytial virus (RSV) induced mammalian target of rapamycin (mTOR) directly on CD8 memory T cells. Human CD8+CD45RO+CD45RA–CD56–CD57 – memory T cells were purified from peripheral blood mononuclear cells (PBMCs) by depletion of non‐CD8+ T cells and CD8+ T cells with a different phenotype using magnetic isolation. The purified cells were incubated with RSV for 30 min, following mTOR phosphorylation analysis with or without previous incubation with rapamycin for 1 h. (a) Dot‐plots show the frequency of CD8+mTORser2448+ cells. (b) Graph shows the frequency of CD8+CD45RO+mTORser2448+ cells. One‐way analysis of variance (anova) with Bonferroni's multiple comparison post‐test (*P < 0·05).
RSV‐infected infants presented higher mTOR gene expression on nasal wash cells compared to RSV‐negative infants
To address mTOR levels during RSV infection in vivo, nasal wash samples were collected from infants up to 12 months of age with an acute viral event (bronchiolitis) or those with recurrent wheezing. Presence of RSV, MPV and rhinovirus was confirmed by real‐time PCR. mTOR mRNA relative expression was analysed in RSV‐positive samples (n = 12), MPV‐ or rhinovirus‐positive samples (n = 5) and negative to all (RSV, MPV and rhinovirus) samples (n = 12). Infants who were RSV‐positive (mean 8·0 ± 1·30 s.d.) presented higher mTOR mRNA levels in nasal wash cells compared to infants negative to all virus tested (mean 1·95 ± 3·47 s.d.) (P < 0·0001) (Fig. 4). In addition, RSV‐positive infants showed fivefold higher mTOR gene expression compared to infants infected with rhinovirus or MPV (Fig. 4) (P < 0·001). These results demonstrated that RSV infection in infants up‐regulates mTOR gene expression in a virus‐specific manner. Thus, the next question was whether or not mTOR is activated in CD8 T cells from nasal washes during RSV infection.
Figure 4.
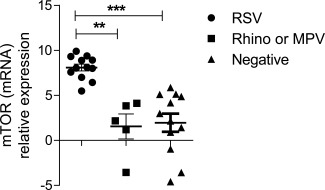
Mammalian target of rapamycin (mTOR) expression on nasal washes of respiratory syncytial virus (RSV)‐infected and non‐infected infants. mTOR gene expression was determined in nasal washes of RSV‐infected infants (n = 12), metapneumovirus (MPV)‐ or rhinovirus‐ infected infants (n = 5) and controls (negative to all viruses tested) (n = 12) using the delta‐cycle threshold (CT) method. The delta value was calculated by subtracting the CT value for the endogenous control gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) from the CT value for mTOR of each sample amplified by real‐time polymerase chain reaction (PCR). One‐way analysis of variance (anova) with Bonferroni's multiple comparison post‐test (**P < 0·001 and ***P < 0·0001).
CD8 T cells from RSV‐infected infants showed higher mTOR phosphorylation compared to RSV‐negative infants
To evaluate whether RSV‐infected infants presented phosphorylated mTOR in CD8+ T cells, nasal wash samples from RSV‐positive infants (n = 7) and RSV‐negative controls (n = 12) were used. RSV infection was confirmed by rapid test before T cell evaluation. RSV‐positive infants presented a significantly higher percentage of CD8+mTORser2448+ T cells compared to the RSV‐negative (Fig. 5a,b) (P = 0·02). These data support that activation of mTOR pathway by RSV is mediated through phosphorylation on the serine 2448 site in CD8 T cells in vivo.
Figure 5.
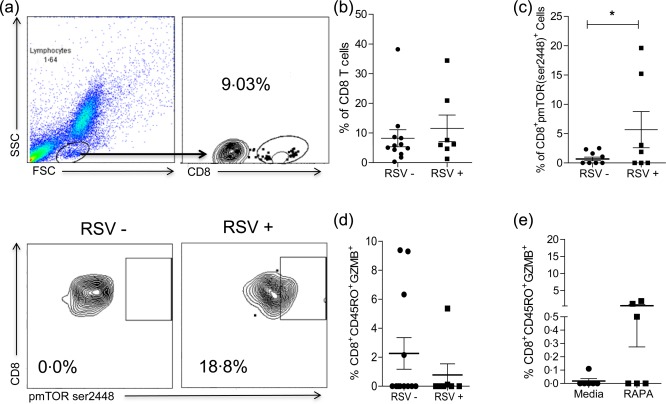
CD8 T cell analysis of nasal washes from respiratory syncytial virus (RSV)‐infected and non‐infected infants. (a) Representative dot‐plots showing the gate strategy of CD8 T cell analysis on nasal washes and dot‐plots showing the frequency of CD8+mammalian target of rapamycin (mTOR)ser2448+ cells on nasal wash of an RSV‐negative (–) (n = 7) and an RSV‐positive (+) (n = 12) infant. (b) Graph shows the frequency of CD8+ T cells. (c) Graph shows the frequency of CD8+mTORser2448+ cells on nasal washes of RSV– and RSV+ infants. (d) Graph shows the frequency of CD8+CD45RO+GZMB+ T cell on nasal washes of RSV – and RSV + infants. (e) Graph shows the frequency of CD8+CD45RO+granzyme B (ZMB)+ T cells from RSV+ infants after 1 h of rapamycin treatment or media only. Unpaired t‐test (*P < 0·05).
RSV‐positive infants also showed that CD8+CD45RO+GZM+ cells decreased in frequency compared to RSV‐negative infants, although this difference was not statistically significant (Fig. 5c) (P = 0·32). When cells from nasal washes of RSV‐positive infants were treated with rapamycin for 1 h, the frequency of CD8+CD45RO+GZMB+ T cells increased compared to those untreated (Fig. 5d) (P = 0·07). These data indicate that rapamycin treatment might have a potential role in rescuing CD8 T cells function on RSV infection.
mTOR inhibition induced RSV‐specific CD8 T cells
mTOR acts intrinsically in antigen‐specific CD8 T cells through the mTORC1 pathway to regulate memory CD8 T cell differentiation 17. In order to evaluate the direct effect of mTOR inhibition on T cells during RSV infection, T cells were purified from splenocytes of naive BALB/c mice using magnetic beads. Purified T cells were treated with rapamycin and co‐cultured with RSV‐infected bone marrow‐derived DCs (BMDC). After 4 days, RSV‐specific CD8 T cells were stained with M282–90 pentamers. The percentage of RSV M289–90‐specific CD8 T cells generated was 10‐fold higher when the cells were treated previously with rapamycin (Fig. 6a) (P = 0·0002). These results demonstrate that mTOR inhibition increased the frequency of RSV‐specific CD8 T cells.
Figure 6.
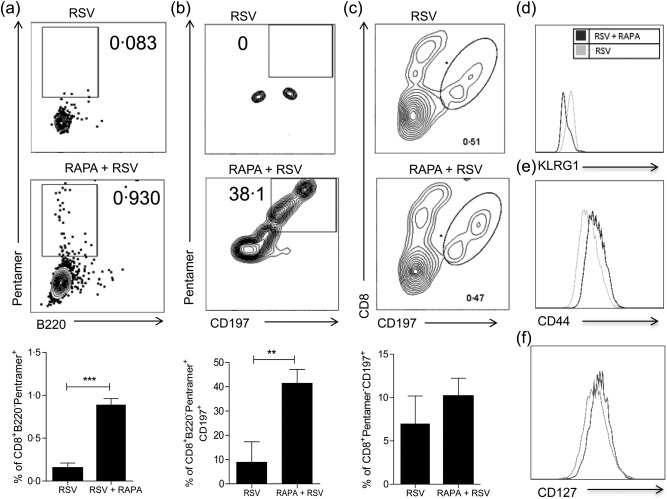
Rapamycin‐treated purified T cell co‐culture with respiratory syncytial virus (RSV)‐infected bone marrow‐derived dendritic cells (BMDCs) better differentiated to memory precursors. T cells were purified from BALB/c splenocytes and treated or not with rapamycin (20 ηg/ml) for 1 h, then 5 × 104 T cells were co‐cultured with 1·5 × 103 of BMDC cells infected with 102 plaque‐forming units (PFU) of RSV for 4 days. (a) Graph shows the frequency of CD8+B220–pentamer+ cells. (b) Graph shows the frequency of CD8+B220–pentamer+CD197+ cells. (c) Graph shows the frequency of CD8+pentamer–CD197+ cells. (d) Histogram shows CD8+B220–pentamer+KLRG1high cells. (e) Histogram shows CD8+CD44+ cells. (f) Histogram shows CD8+CD127+ cells. Unpaired t‐test (*P < 0·05; **P < 0·005; ***P < 0·0005).
Memory precursor effector CD8 T cells (MPECs) are KLRG1–CD127+ 19, and express the chemokine receptor CCR7 (CD197) 20. We evaluated whether mTOR inhibition modulates the expression of memory precursors markers on RSV‐specific CD8 T cells. Figure 6b shows that the percentage of B220‐CD8+pentamer+CD197+ cells increased from 0 to 38·1% with rapamycin treatment. Conversely, rapamycin does not alter the percentage of CD8+pentamer–CD197+ cells (Fig. 6c). These results demonstrated that rapamycin increased CD197 only in RSV‐specific CD8 T cells. CD8+pentamer+KLRG1high cells decreased with rapamycin treatment (Fig. 6d). In addition, CD44+ cells and CD127+ cells increased when incubated with RSV plus rapamycin treatment. These data reveal that mTOR inhibition directly on CD8 T cells is important to RSV‐specific memory precursor differentiation.
Discussion
This study reports that RSV induces mTOR phosphorylation at ser2448 in CD8+ T cells. RSV‐infected infants presented significantly higher mTOR gene expression compared to MPV or rhinovirus‐infected infants and RSV non‐infected infants. Also, RSV‐infected infants had a higher frequency of CD8+mTORser2448+ T cells on nasal washes compared to those who were negative for RSV. These results demonstrate that mTOR plays a role in the context of acute RSV infection. In addition, mTOR inhibition increased the frequency of mouse RSV‐specific CD8 T cells and the expression of memory precursor markers.
mTOR acts within two distinct multi‐protein complexes, mTORC1 and mTORC2, responsible for different physiological functions. While mTORC1 is involved mainly in the regulation of the translation initiation machinery influencing cell growth, proliferation and survival, mTORC2 participates in actin cytoskeleton rearrangements and cell survival 21. Rapamycin exerts its effect on memory differentiation by acting directly on mTORC1 in antigen‐specific CD8+ T cells 17. mTORC1 targets are S6K kinases (p70 ribosomal protein S6 kinase 1 and 2) and 4EBP1 (eIF4E binding protein 1); both proteins are involved in the translation initiation process.
This study showed that mTOR signalling activation by RSV did not require live virus. A direct contact between virus and CD8 T cells must be involved, as RSV F protein was able to induce mTOR phosphorylation. Several cell surface proteins have been identified that interact with the RSV F protein and might function as receptors: intercellular adhesion molecule 1 (ICAM‐1) 22, Toll‐like receptor 4 (TLR‐4) 23 and nucleolin 24. However, which receptor RSV F protein used to activate mTOR still needs to be determined. Some viruses, such as human immunodeficiency virus (HIV) and Epstein–Barr (EBV) virus, have proteins with the ability to induce mTOR. EBV latent membrane protein (LMP1) regulates mTOR signalling through AKT in cells of nasopharyngeal carcinoma 25. In addition, the env protein of the HIV envelope induces the activation of mTOR and S6K, regulating the DC autophagy 26 and the syncytial apoptosis induced by env‐CD4 27. West Nile virus induces mTOR activation to support viral growth 28 and hepatitis C virus activates mTOR, contributing to cell survival 29.
In our study, rapamycin treatment increased granzyme B production in CD8+CD45RO+ T cells from nasal washes of RSV‐infected infants; however, this difference was not statistically significant. One reason for that might be the poor effect of rapamycin in comparison with other mTORC1‐specific targets 30. It was demonstrated recently by Berezhnoy et al. that mTORC1 inhibition by rapamycin enhances antigen‐activated CD8+ T cell persistence, although the cytotoxic effector functions of reactivated memory cells were reduced 30. These authors suggested an aptamer‐targeted siRNA inhibitor of mTORC1 function in CD8+ T cells as a more effective and specific treatment compared to rapamycin 30.
One limitation of our study is the number of cells achieved in the nasal washes. Performing a CD8 T cell cytotoxic assay will be important to prove the role of rapamycin in rescuing CD8 T cell function on RSV infection. However, it is difficult to be performed with a low number of cells. Also, an ideal sample would be from the lower respiratory tract, but this requires invasive procedures which are more difficult to be ethically approved for studies in children. In addition, mTOR expression was not associated with disease severity, but it was not the scope of this study. Further longitudinal studies with RSV‐infected children are necessary to complete this task.
No effective vaccine against RSV is currently available, and the burden of disease urges us to move towards new and creative interventions without the risk taken in previous strategies 31. In order to promote the development of RSV vaccines, several major challenges must be overcome. Understanding the mechanisms linked to the generation of better CD8+ T cell memory responses during the course of RSV infection is a key component for these next steps. Mechanisms for immune evasion are common to many pathogens that have undergone prolonged co‐evolution with their hosts. We suggest that mTOR activation induced by RSV during the infection is associated with a viral immune evasion mechanism from CD8 T cell responses and could be a promising target for future intervention.
Author's contribution
A. P. D. S. undertook the design and performed the experiments, acquisition and analysis of data, interpretation of data, drafted the work and revised it critically. D. N. F., K. E. A. F., M. D. C., J. L. A. F.; R. B. G. and T. F. performed the experiments, acquisition and analysis of data. M.S; R.M; L.A.P., P.M.C.P., C. B. and R. T. S. drafted the work and revised it critically.
Disclosure
All authors have no disclosures to declare.
Acknowledgements
This study was supported by Conselho Nacional de Pesquisa (CNPq) (grant number 477359/2013‐2). Fundação de Amparo a Pesquisa do Rio Grande do Sul (FAPERGS) (grant number 001884‐25.51/13.4). A. P. D. S. received post‐doctoral fellowships from the CAPES/PNPD programme and Brazilian Immunology Society (SBI)/BD Bioscience Award 2011. We thank Rodrigo Godinho for technical assistance.
References
- 1. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD et al Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bueno SM, Gonzalez PA, Pacheco R et al Host immunity during RSV pathogenesis. Int Immunopharmacol 2008; 8:1320–9. [DOI] [PubMed] [Google Scholar]
- 4. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010; 23:74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeVincenzo JP, Wilkinson T, Vaishnaw A et al Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010; 182:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall CB, Powell KR, MacDonald NE et al Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 1986; 315:77–81. [DOI] [PubMed] [Google Scholar]
- 7. Cherukuri A, Patton K, Gasser RA Jr et al Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 2013; 20:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Curr Opin Virol 2013; 3:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schlender J, Walliser G, Fricke J, Conzelmann KK. Respiratory syncytial virus fusion protein mediates inhibition of mitogen‐induced T‐cell proliferation by contact. J Virol 2002; 76:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kotelkin A, Belyakov IM, Yang L, Berzofsky JA, Collins PL, Bukreyev A. The NS2 protein of human respiratory syncytial virus suppresses the cytotoxic T‐cell response as a consequence of suppressing the type I interferon response. J Virol 2006; 80:5958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munir S, Hillyer P, Le Nouen C et al Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLOS Pathog 2011; 7:e1001336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welliver TP, Garofalo RP, Hosakote Y et al Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195:1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Telcian AG, Laza‐Stanca V, Edwards MR et al RSV‐induced bronchial epithelial cell PD‐L1 expression inhibits CD8+ T cell nonspecific antiviral activity. J Infect Dis 2011; 203:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T‐cell effector activity and peripheral CD8+ T‐cell memory in the respiratory tract. Nat Med 2002; 8:54–60. [DOI] [PubMed] [Google Scholar]
- 15. Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T‐bet and eomesodermin. Immunity 2010; 32:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He S, Kato K, Jiang J et al Characterization of the metabolic phenotype of rapamycin‐treated CD8+ T cells with augmented ability to generate long‐lasting memory cells. PLOS ONE 2011; 6:e20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Araki K, Turner AP, Shaffer VO et al mTOR regulates memory CD8 T‐cell differentiation. Nature 2009; 460:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inaba K, Inaba M, Romani N et al Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony‐stimulating factor. J Exp Med 1992; 176:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long‐lived memory cells. Nat Immunol 2003; 4:1191–8. [DOI] [PubMed] [Google Scholar]
- 20. Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol 2010; 185:5315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tchevkina E, Komelkov A. Protein phosphorylation as a key mechanism of mTORC1/2 signaling pathways In: Huang C, ed. Biochemistry, genetics and molecular biology. 2012; 4–50. [Google Scholar]
- 22. Behera AK, Matsuse H, Kumar M, Kong X, Lockey RF, Mohapatra SS. Blocking intercellular adhesion molecule‐1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem Biophys Res Commun 2001; 280:188–95. [DOI] [PubMed] [Google Scholar]
- 23. Haynes LM, Moore DD, Kurt‐Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll‐like receptor 4 in innate immunity to respiratory syncytial virus. J Virol 2001; 75:10730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med 2011; 17:1132–5. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Hu CF, Hou JH et al Epstein–Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J Transl Med 2010; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanchet FP, Moris A, Nikolic DS et al Human immunodeficiency virus‐1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 2010; 32:654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castedo M, Roumier T, Blanco J et al Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV‐1 envelope. EMBO J 2002; 21:4070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shives KD, Beatman EL, Chamanian M, O'Brien C, Hobson‐Peters J, Beckham JD. West Nile virus‐induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression. J Virol 2014; 88:9458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng L, Liang D, Tong W, Li J, Yuan Z. Hepatitis C virus NS5A activates the mammalian target of rapamycin (mTOR) pathway, contributing to cell survival by disrupting the interaction between FK506‐binding protein 38 (FKBP38) and mTOR. J Biol Chem 2010; 285:20870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer‐targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest 2014; 124:188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jorquera PA, Oakley KE, Tripp RA. Advances in and the potential of vaccines for respiratory syncytial virus. Expert Rev Respir Med 2013; 7:411–27. [DOI] [PubMed] [Google Scholar]


