Summary
In progressive immunoglobulin (Ig)A nephropathy (IgAN), cyclophosphamide pulse therapy (CyP), high‐dose intravenous immunoglobulins (IVIg) and mycophenolic acid (MPA) have been used to stop progressive loss of renal function, but disease progression may occur after the end of the initial treatment. Here, we report the long‐term follow‐up of patients with progressive IgAN with MPA as maintenance therapy after CyP (CyP‐MPA). In a median observation time of 6·2 years, we analysed the slopes of the loss of renal function of 47 patients with biopsy‐proven IgAN and treated with CyP. Thirty‐one patients with further progression were treated with MPA maintenance for a median time of 5·2 years. Follow‐up was compared with symptomatic therapy and IVIg as historically matched control groups. Median loss of renal function was reduced significantly from 0·9 ml/min to 0·1 ml/min per month with CyP (P < 0·05), and with MPA in patients with a relapse from −0·4 ml/min to −0·1 ml/min per month (P < 0·05) until the end of the study. Proteinuria decreased significantly from 1·6 g/l to 1·0 g/l after CyP, and during MPA treatment to 0·6 g/l (P = 0·001 Friedman test). Median renal survival time was in patients with CyP 10·5 years (range = 3·2–17·8), with CyP‐MPA 10·7 years (range = 8·3–13·1), with IVIg 4·7 years (range = 2·6–6·6), and in untreated patients 1·2 years (range = 0·8–1·6; log‐rank test P < 0·01). In patients with progressive IgAN, our long‐term follow‐up observation indicates that sequential CyP‐MPA therapy maintains renal survival significantly.
Keywords: cyclophosphamide, IgA nephropathy, immunosuppression, long‐term follow‐up, mycophenolic acid
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide, and at least 50% of affected patients have a progressive clinical course with loss of renal function after 10–20 years 1.
Four steps in the autoimmunopathophysiology will be of interest, and all might be influenced by immunosuppressive therapy: (i) aberrant glycosylated immunoglobulin (Ig)A1 by the B cells, (ii) a soluble FcαRI (CD89) that initially develops IgA1 circulating immune complexes (IC), (iii) transferrin receptor (CD71) that mediates mesangial deposition; and (iiii) at least the inflammatory response of the mesangium. Corticosteroids, cyclophosphamide (CyP), high‐dose intravenous immunoglobulins (IVIg) and mycophenolic acid will be able to modulate this autoimmune cascade in patients with rapid loss of renal function.
Clearly, the pathomechanism implicates a long life mesangial deposition of immunocomplexes in the mesangium, an impaired clearance of IgA1 molecules due to aberrant glycosylation and nephron loss by inflammation 2, 3. Therefore, immunosuppressive therapy is effective in prolonging renal survival by systemic and local effects on the immune system 4, 5, 6, 7, 8, 9, 10. In patients with stable disease – without progression in linear regression analysis of serum creatinine [or estimated glomerular filtration rate (eGFR)] – only supportive therapy with ACE inhibitors (ACEI), cholesterol‐lowering and fish oil were proposed in the former Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines for glomerulonephritis (2012). Recently, in a large retrospective long‐term study, the clear benefit of long‐term use of low‐dose corticosteroids was demonstrated even in patients with mild to moderate proteinuria and stable renal function 4. However, the use of cyclophosphamide, high‐dose steroids, intravenous immunoglobulines (IVIg), azathioprine and mycophenolate mofetil is well proven in progressive IgAN. The concept of sequential therapy was also used in two randomized controlled trials (RCTs) and other studies 11, 12.
The aim of our study was to conduct a proof‐of‐concept method in patients with progressive IgA and relapse after cyclophosphamide with a less toxic sequential therapy with no repeated receipt of cyclophosphamide or high‐dose corticosteroids. Here, we report an extended long‐term follow‐up with 6·2 years (range = 0·6–14·8) of this sequential maintenance therapy with mycophenolic acid (MPA) in patients with further progressive IgAN after cyclophosphamide pulse induction therapy (CyP) 8, 13, 14. Furthermore, we evaluate the established risk factors after the running‐in phase with ACE inhibitors (ACEI) or angiotensin receptor blockers (ARB) and CyP on the outcome of our patients.
Materials and methods
Patients, selection criteria and treatment protocol
Forty‐seven patients with biopsy‐proven IgAN and progressive loss of renal function while on ACEI and/or ARB treatment were treated with CyP, and 31 with further progression received MPA as maintenance therapy, between October 1987 and July 2010 in the University Hospital of Ulm, Ulm, Germany 8, 13, 14. The renal biopsies were performed in all patients during initial clinical evaluation and were classified according to Lee et al. 15, 16, Haas et al. 17 and after the Oxford classification 18, 19. The percentage of crescents and tubular atrophy was scored as mild or severe, as defined by the proportion of affected glomerula and tubuli below or exceeding 50%. Six renal biopsies were performed outside our hospital and were not included (Table 1).
Table 1.
Histological grading and classification in patients with progressive immunoglobulin (Ig)A nephropathy (IgAN) after Lee et al. 15, Haas et al. 17 and the Oxford classification 18, 19, 25.
| CyP (%) | CyP‐MPA (%) | P‐value | ||
|---|---|---|---|---|
| Lee | I | 0 | 0 | – |
| II | 7 | 4 | 0·71 | |
| III | 33 | 48 | 0·18 | |
| IV | 60 | 48 | 0·33 | |
| Haas | I | 7 | 4 | 0·71 |
| II | 27 | 40 | 0·39 | |
| III | 4 | 52 | 0·46 | |
| IV | 27 | 4 | 0·04 | |
| Oxford | M0/1 | 13/87 | 32/68 | 0·27 |
| E0/1 | 13/87 | 36/64 | 0·16 | |
| S0/1 | 13/87 | 36/64 | 0·16 | |
| T0/1/2 | 7/40/53 | 8/56/36 | 0·53 | |
| Crescents | 0/1 | 100/0 | 96/4 | 0·63 |
| Tubular atrophy | 0/1 | 33/67 | 33/67 | 0·47 |
The percentage of crescents and tubular atrophy was scored as mild or severe as defined by proportion of affected glomerula and tubuli below or exceeding 50%. There was no significant difference in the distribution of the frequency, except Haas IV, in χ2 test between the cyclophosphamide pulses (CyP)‐mono group and the CyP‐mycophenolic acid (MPA) group.
CyP therapy consisted of six pulses of cyclophosphamide adapted to leucocytes, e.g. neutrophil count [750–1000 mg/m2, intravenously (i.v.)] and 50 mg prednisolone, 8 mg ondansetrone and 800 mg i.v. mesna given over a period of 6 months, as described previously in a subgroup of 21 patients 8. We preferred intravenous cyclophosphamide pulses (CyP) because CyP have shown superiority in safety 20 and less toxicity 20, 21 by short‐term acrolein bladder exposure with fewer cumulative doses 22, equally 23 or with better efficacy 20, 21, as proven in other autoimmune diseases. Disease progression after CyP during ACEI or ARB therapy was defined as an increase in serum creatinine by more than 10% within 3 months and/or as proteinuria > 0·7 g/l measured on two occasions within 2 weeks.
Oral prednisolone was reduced stepwise from a starting daily dose of 20 mg to 5 mg daily until CyP 6, and continued with 5 mg until the end of the study.
MPA was administered orally continuously, with a starting dose of free mycophenolic acid (Myfortic©; Novartis, Basel, Switzerland) 360 mg twice per day or mycophenolate mofetil (CellCept©; Hoffmann LaRoche, Basel, Switzerland) 500 mg twice per day.
Six male patients [mean age 46 years, standard deviation (s.d.) 3 years] with biopsy‐proven IgAN were included as a historically matched patient group treated with high‐dose immunoglobulin therapy (IVIg, 2 g/kg body weight, i.v.) divided over 2 days during a period of 6 months between December 1997 and March 2002 in the University Hospital Ulm 10, and eight patients as historical controls with biopsy‐proven IgAN who were not treated with immunosuppression or tonsillectomy, but all received ACEI 8, 10.
Exclusion criteria were age < 18 years, serum creatinine > 4·5 mg/dl (>400 µmol/l), kidney size <9 cm in ultrasound, rapidly progressive, and extracapillary proliferative forms of IgAN with crescents more than 75% of affected glomerula, secondary mesangioproliferative IgA nephropathies due to systemic diseases [e.g. systemic lupus erythematosus (SLE), Schoenlein–Henoch purpura, acute or chronic infections (including human immunodeficiency virus, hepatitis B and C virus)], carcinoma, leucocyte counts < 3·0/nl, platelet counts < 80/nl, gastrointestinal bleeding, haemolytic anaemia, pregnancy, lactation or women with childbearing potential.
The study protocol (no. 390/13) was approved by the Institutional Review Board Medical Faculty of the University of Ulm, and informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki 8, 13, 14.
Study parameters
Serum creatinine, proteinuria, serum protein, white blood cell and platelet count, haemoglobin, total cholesterol, liver enzymes, blood pressure and body weight [respectively, body weight index (BMI)] were measured before, with and after CyP and the course of renal function was assessed by linear regression analysis by estimated GFR values (eGFR) according to National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF‐KDOQI) 2002.
Proteinuria was obtained from spontaneous morning urine (g/l). We discounted the 24 h sample urine in the ambulant setting due to high error probability, and therefore we used the second morning's spot urine at every recall and in the hospital before the pulse began. In patients travelling long distances, spot urine was the only feasible sample method, because nowadays physicians do not use always the recommended urine protein or albumin/creatinine ratio. In order to compare all patients and to have a high sample size, in this study we decided to use urine protein excretion in g/l. However, our variable proteinuria represents an intraindividual course with a sample size >10 with each period.
Statistical methods
Linear regression analysis of the time‐dependent course of eGFR values and inverse serum creatinine was performed 8, 14, 24. Non‐parametric tests to compare group values (Wilcoxon's test, Friedman's test and the Mann–Whitney U‐test) were performed as indicated. Renal survival until the end of the study or end‐stage renal disease (ESRD) was estimated by Kaplan–Meier analysis and log‐rank test. Cox regression analysis was used to calculate the proportional ratios of the categorical variables times from CyP‐end to MPA‐start, age, smoker status, BMI, arterial hypertension, serum creatinine, loss of renal function per month, eGFR and proteinuria before and with therapy using the ‘enter’ method. Haemodialysis, eGFR < 10 ml/min per 1·73 m2 or serum creatinine > 620 µmol/l were defined as event ful.
Statistical significance for all tests was set at a level of P < 0·05. No adjustment for multiple comparisons was made, so significant results are interpreted in an exploratory manner. Statistical analysis was performed using the spss version 21.0 software package (SPSS Inc., Chicago, IL, USA). If not indicated otherwise, data are given as median values with the minimum and maximum.
Results
Study population
Forty‐seven patients with a mean age of 54 ± 13 years (all male, all Caucasian) were treated with CyP induction therapy (CyP). Thirty‐one of these patients (s.d. 55 ± 14 years) received maintenance therapy with MPA (CyP‐MPA). Patient baseline characteristics at the start of CyP induction are shown in Table 2.
Table 2.
Baseline patient characteristics in 47 male Caucasian patients with advanced progressive immunoglobulin (Ig)A nephropathy (IgAN) at the beginning of cyclophosphamide pulses (CyP) induction therapy.
| CyP mono n = 16 | CyP‐MPA n = 31 | Significance P‐value | |
|---|---|---|---|
| Age (years) | 52 (29–70) | 55 (24–77) | 0·369* |
| Body mass index (kg/m2) | 25 (20–31) | 27 (19–33) | 0·144* |
| Systolic blood pressure (mmHg) | 140 (120–160) | 140 (110–215) | 0·267* |
| Diastolic blood pressure (mmHg) | 90 (70–105) | 90 (60–110) | 0·682* |
| Serum creatinine (µmol/l) | 226 (143–477) | 248 (146–398) | 0·670* |
| eGFR (ml/min per 1·73 m2) | 27 (12–54) | 25 (14–45) | 0·912* |
| Proteinuria (g/l) | 1·5 (0·4–3·5) | 1·7 (0·12–5·2) | 0·722* |
| ACEI | n = 10 (63%) | n = 18 (58%) | 0·769** |
| ARB | n = 3 (19%) | n = 8 (25%) | 0·588** |
| ACEI + ARB | n = 3 (19%) | n = 5 (16%) | 0·821** |
Mann–Whitney U‐test was used in continuous variables.
**χ2 test for dichotomous traits. eGFR = Estimated glomerular filtration rate according KDOQI guidelines 2002 (MDRD2); ACEI = ACE inhibitor; ARB = AT1 receptor blocker; MPA= mycophenolic acid.
There were no significant differences in loss of renal function, eGFR, proteinuria, serum protein, body mass index, smoking status and age between both intervention groups before MPA therapy. The overall observation time from renal biopsy to the end of the study or ESRD was 6·2 years (range = 0·6–14·8). In the case of disease progression, MPA therapy was started 5 months (range = 0–71) after completion of CyP and was given subsequently for a median of 27 months (range = 2–114).
Course of renal function on linear regression analysis
The course of renal function on linear regression during therapy episodes is shown in Fig. 1 for both groups. The loss of renal function improved significantly with CyP therapy from −1.3 ml/min per month (range = −16·6 to −0·6) to 0·1 ml/min per month (range = −3·3 to +4·1; Wilcoxon's test, P = 0·001, Fig. 2a).
Figure 1.
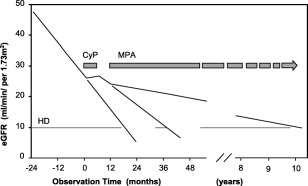
Median course of renal function in linear regression analysis [estimated glomerular filtration rate (ΔGFR)] in 47 patients with progressive immunoglobulin (Ig)A nephropathy (IgAN) treated with cyclophosphamide pulses (CyP) and sequential maintenance therapy with mycophenolic acid (CyP‐MPA, n = 31). The dotted lines indicate the extrapolated course of renal function without immunosuppressive therapy. HD denotes haemodialysis.
Figure 2.
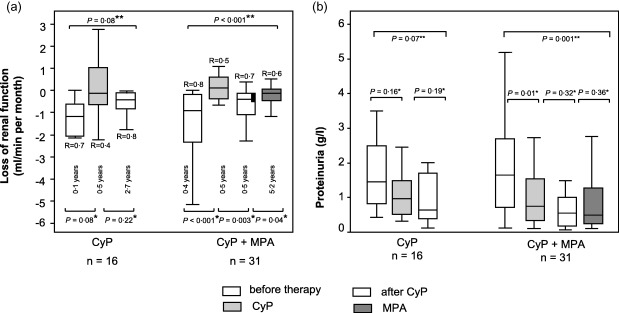
Box‐plots of median loss of renal function in linear regression analysis [estimated glomerular filtration rate (ΔGFR)] (a) and proteinuria (b) before (white) and with cyclophosphamide pulses (CyP) (grey), after CyP (dotted) and facultative sequential mycophenolic acid (MPA) maintenance therapy (dark grey) in patients with progressive immunoglobulin (Ig)A nephropathy (IgAN). The several time‐periods are given below the boxes. Box‐plots show the median, interquartile range and outliers [not significant (n.s.) = P > 0·05; R = regression coefficient; *Wilcoxon's signed‐rank test; **Friedman's test].
In the CyP mono group there was no significant decrease of renal function after CyP (P = 0·22 Wilcoxon's signed‐rank test, P = 0·08 Friedman's test), and further MMF/MPA therapy was not performed (Fig. 2a, left‐hand boxes).
In the MPA/MMF group there was a significant decrease of renal function after CyP from −0·4 to −0.1 ml/min per month (P = 0·003 Wilcoxon's signed‐rank test, P < 0·001 Friedman's test; Fig. 2a, right‐hand boxes). In patients with sequential MPA treatment, a further loss of renal function was reduced significantly after 6 months with MPA. The loss of renal function improved to −0·1 ml/min per month (range = −2·3 to 1·6; Wilcoxon's test, P < 0·05; Fig. 2a) and sustained without significant relapse while on MPA maintenance therapy (Friedman's test, P < 0·001).
ACEI, ARB and proteinuria
At the patient's first referral, diagnosis in renal biopsy or elevated arterial blood pressure and/or proteinuria, all patients were treated continuously with an ACEI or ARB with a median interval of 0·1 months (range = 0–61) prior to initial CyP therapy, and median 0·94 years (range = 0·6–6·5) prior to MPA treatment. ACEI or ARB medication was continued for a median of 5·2 years during the entire study period (range = 0·8–12·1).
Before CyP induction, eight patients had a nephrotic range proteinuria (> 3 g/l), with CyP three patients, and after ending CyP four patients. While on MPA, only one patient had a nephrotic range proteinuria. The median value of proteinuria before CyP therapy was 1·6 g/l (range = 0·1–5·1), and decreased significantly with CyP therapy to 1·0 g/l (range = 0·3–3·3; Wilcoxon's test, P < 0·05; Fig. 2b). During MPA therapy proteinuria decreased further, but not significantly, to 0·6 g/l (range = 0·1–3·9; Wilcoxon's test, P = 0·36).
Systolic and diastolic blood pressure could be adjusted at a stable level (130/90 mm Hg) in the running‐in and treatment periods without significant alteration during follow‐up (Fig. 3).
Figure 3.
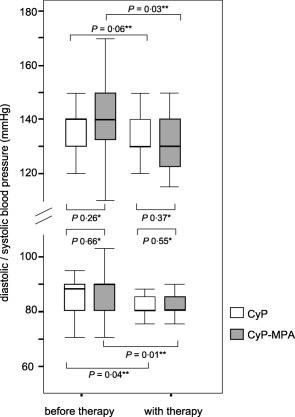
Median systolic and diastolic blood pressure before and with therapy. There was no significant difference between the cyclophosphamide pulses (CyP) mono and the CyP/mycophenolic acid (MPA) group (Mann–Whitney U‐test, P > 0·05) without and with therapy. Box‐plots show the median, interquartile range and outliers (P‐values by *Mann–Whitney U‐test and **Friedman's test).
Renal survival in Kaplan–Meier analysis
The median renal survival time was 10·5 years (range = 3.2–17·8) in patients with CyP, 10·7 years (range = 8·3–13·1) with CyP‐MPA, 4·7 years (range = 2·6–6·6) with IVIg and 1·2 years in untreated patients (range = 0·8–1·6; log‐rank test P < 0·01; Fig. 4). There was no difference between the renal survival time in patients with CyP and patients who received CyP‐MPA with further progression after CyP, according to our rescue protocol with MPA (log‐rank test, P = 0·58).
Figure 4.
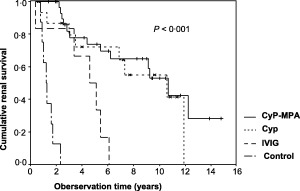
Cumulative renal survival in patients with progressive immunoglobulin (Ig)A nephropathy (IgAN) with cyclophosphamide pulse therapy (CyP), mycophenolic acid (MPA), high‐dose intravenous immunoglobulins (IVIg) and without immunosuppressive therapy (control) in Kaplan–Meier analysis. Loss of renal function was defined by estimated glomerular filtration rate (ΔGFR) < 10 min/min per 1·73 m2 or serum creatinine > 620 µmol/l (P < 0·001 log‐rank test).
However, there were no significant differences in baseline characteristics between all patients (age, proteinuria, progression of renal function and blood pressure; Mann–Whitney U‐test), and ACEI or ARB treatment (χ2 test; P > 0·05).
Renal risk factors in Cox regression analysis
Before therapy, a median blood pressure of 140/90 mmHg was observed. After starting CyP induction, blood pressure decreased in all patients to 130/90 mmHg and was sustained at this level during follow‐up. Blood pressure was not statistically different before and after MPA therapy.
The median cholesterol level before therapy was 5·7 mmol/l (range = 2·4–8·9) and there was a significant reduction with CyP to 4·9 mmol/l (range = 2·4–7·7), and with MPA to 4·9 mmol/l (range = 3·0–7·4; Wilcoxon's test, P < 0·05).
The study population had a median body mass index of 26 kg/m2 (range = 19–34) and showed no significant differences during the therapy episodes (Mann–Whitney U‐test, P > 0·05).
The influence of the established renal risk factors while on sequential therapy was analysed by Cox regression. As shown in Fig. 5, only BMI, serum creatinine and eGFR were correlated significantly with renal outcome (P < 0·05). Kaplan–Meier analysis for BMI, eGFR and serum creatinine showed a reduction of renal survival at BMI ≥26 kg/m2 of 2·6 years (5‐year survival rate 60·6 versus 80·3%). Patients with eGFR ≤ 25 ml/min per 1·73 m2 showed an estimated median dialysis‐free time of 9·1 versus 12·1 years (5‐year survival rate 61·4 versus 80·8%) and with serum creatinine ≥ 250 µmol/l a median dialysis‐free time of 6·2 versus 12·1 years (5‐year survival rate 63·4 versus 94·0%). Time from CyP‐end and MPA‐start and proteinuria were not correlated significantly with renal survival before and with MPA.
Figure 5.
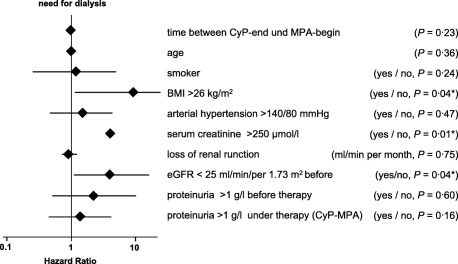
Hazard ratios and 95% confidence intervals of renal risk factors for end‐stage renal disease in univariate Cox‐regression analysis (logarithmic scale). *Body mass index (BMI) ≥ 26 kg/m2, serum creatinine ≥ 250 μmol/l and estimated glomerular filtration rate (ΔGFR) ≤ 25 ml/min per 1·73 m2 at the beginning pointed to a significant influence on renal survival.
Renal histology in several classifications and grading systems 15, 17, 18, 25 and the percentage of crescents or tubular atrophy was not correlated significantly (P > 0·05) with the clinical outcome parameters of loss of renal function (ΔGFR), renal survival time, proteinuria and frequency of ESRD.
Side effects
In patients with CyP, one patient suffered from an ambulant acquired mycoplasma pneumonia that was treated successfully with clarithromycin 8, 13, 14. No haemorrhagic cystitis, severe leucopenia, anaemia or thrombocytopenia (> 2 grade ‘Common Terminology Criteria of Adverse Events Version 4·0’, CTCAE) occurred 26.
In patients with IVIg, one patient with severely impaired renal function (eGFR 16·5 ml/min per 1·73 m2) developed an intercurrent acute kidney injury immediately after the first sucrose‐containing IVIg‐pulse, due presumably to osmotic nephrosis 27. After renal function recovered (eGFR 11.7 ml/min per 1·73 m2), he was switched to a sucrose‐free formulation of IVIg.
No specific side effects, e.g. hypertension, impaired glucose tolerance, cataracts, etc., of corticosteroids were noted during the induction or maintenance periods. There was no significant weight gain (P = 0·47) during the study period. Vitamin D [calcitriol (Rocaltrol®; Roche Pharma AG, Grenzach‐Whylen, Germany) 0·25 µg/day] was prescribed in all patients receiving corticosteroids (prednisolone).
In a subsequent publication, we also demonstrated all pharmacokinetic/pharmacodynamic aspects of the differences between MMF and MPA in a subgroup of our patients, with no sign of less exposure or inosine 5′‐monophosphate dehydrogenase suppression in comparison to other studies 28. In four patients a temporary leucocyte count nadir was between 3·9 and 3·0 × 10 9/l after a median of 15 months, according to grade 1 of Common Terminology Criteria for Adverse Events (CTCAE) 26. In one patient, MMF (2 × 500/day) was switched to MPA (2 × 360 mg/day). There was no patient with prolonged thrombocytopenia or anaemia. One patient suffered temporarily from gastrointestinal disorders under MPA. The diagnosis of a papillary thyroid carcinoma in one patient required the termination of MPA for initiation of the appropriate chemotherapy.
In all other patients, CyP, MPA, IVIg (sucrose‐free formulation) and prednisolone were well tolerated 8, 10, 14.
Discussion
After the first description by Berger in the 1960s, the pathomechanism and treatment of IgAN 29 still remain under discussion and, as shown in a large retrospective study, patients will probably benefit from long‐term immunosuppression 4. The disappearance of IgAN in transplanted kidneys in patients without IgAN after renal transplantation 30, the de‐novo appearance of IgAN in patients without IgAN after bone marrow transplantation 31 and IgAN remission in patients after bone marrow transplantation 32 suggests a systemic origin and sustained B cell defect in the altered glucosylated IgA 2, 3, 13, 33, according to the progressive loss of renal function, and in the presence of established risk factors is an immunosuppressive therapy to compromise B cells and inhibit secondary inflammation induced by mesangial IgA deposits 4, 5, 6, 8, 9, 10, 11, 14, 34, 35, 36.
In this study, patients without sequential therapy and reaching the so‐called ‘point of no return’ (serum creatinine > 2·5–3 mg/dl/220–265 μmol/l) progressed to end‐stage renal failure 37. However, with sequential therapy, only 53% of our patients progressed to end‐stage renal failure.
Baseline therapy with ACEI/ARB, arterial hypertension and proteinuria
In this study, we included patients with obvious progressive renal function and all patients were treated with ACEI or ARBs, so that the potential confounding of ACEI or ARB treatment effects on renal function or proteinuria may be largely excluded. After the running‐in phase, blood pressure was 140/90 mmHg and decreased in all patients to a sustained basis of 130/90 mmHg during follow‐up, comparable to other studies 11, 38.
Arterial hypertension and proteinuria were lowered significantly with ACEI or ARB by a reduction of intraglomerular hydraulic pressure and interstitial sclerosis 39, with a secondary effect on the loss of renal function due to nephrosclerosis 40. In general, the beneficial effect of ACEI or ARB in preventing renal fibrosis is clearly needed in all patients with IgAN 41. In a recent study, treatment with only ACEI or ARB in comparison to ACEI or ARB augmented with corticosteroids was inferior, according to the renal survival time 4, 36. However, the different interindividual course of IgAN with rapid progressive forms and long‐term renal survivors could not be reduced simply on the treatable secondary risk factors of proteinuria and arterial hypertension. Obviously in patients with rapid loss of renal function, symptomatic therapy with ACEI or ARB systemic immunosuppressive therapy will be essential for renal survival 6, 8, 10, 11, 14, 34.
Proteinuria above 1 g/day before therapy was identified as a renal risk factor in several studies 42, 43. Steroids 44, cyclophosphamide 5, 6, 8, 11 and MPA 14, 45, 46 have proved effective in lowering proteinuria. In our study, there was a significant reduction of the proteinuria after lowering of blood pressure and the prescription of ACEI/ARB with CyP from 1·6 to 1·0 g/l and with MPA to 0·6 g/l (Friedman's test). However, in Cox regression analysis, proteinuria > 1·0 g/day before CyP and with CyP/MPA could not be identified as an independent renal risk factor. The amount of proteinuria probably denotes a surrogate for intraglomerular pressure lowered by ACEI/ARB and for permeability decreased by the systemic anti‐inflammatory therapy CyP/MPA.
Induction therapy – cyclophosphamide, IVIg, rituximab and steroids
Immunosuppressive therapy will decrease the systemic amount of altered IgA and attenuate the local inflammatory process by the deposited IgA in the mesangium. Cyclophosphamide is a highly potent cytotoxic agent used frequently for cytoreductive induction therapy in autoimmune disease by depletion and inhibition of T and B lymphocytes, but its long‐term use is limited due to high cumulative toxicity 47. Cylophosphamide has been shown to be beneficial in combination with steroids 11. Corticosteroids display anti‐inflammatory effects and induce apoptosis 48. These effects may responsible for a reduction of proliferative lesions, glomerular sclerosis and tubular fibrosis in IgAN with superior renal survival compared with patients receiving only ARB/ACEI 4, 36, 44. In contrast to other studies 5, 6, 11, 34, 44 that showed long‐term beneficial effects with high‐dose steroid regimens, we used only low‐dose corticosteroids (5–7·5 mg prednisone/day). However, the majority of our patients (67%, 31 of 47) showed further disease activity 4 months on average after CyP.
A possibly less toxic alternative is IVIg pulse therapy 9, 10, e.g. for patients with pregnancy or high cumulative doses of cyclophosphamide, with comparable effects in the reduction of ΔGFR from −1·05 ml/min per month to −0·15 ml/min per month (P = 0·024) and proteinuria (from 2·4 g/l to 1·0 g/l, P = 0·015) 8, 10, 13, 14. However, 3 years after IVIg, further loss of renal function was observed. In Kaplan–Meier analysis survival time was only 4·7 years with IVIg versus 10·5 with CyP/MPA. After IVIg, maintenance therapy will probably be needed with prednisolone and/or MMF/MPA with prednisolone.
Rituximab, which acts by inhibiting CD20‐mediated B cell proliferation and differentiation, is effective in the treatment of acute vasculitis in combination with steroids. In patients with mild renal impairment (proteinuria 1·0 g/day; serum creatinine 1·1 mg/dl), rituximab given as a single dose showed no effect on proteinuria or serum creatinine after 6 months 49. In patients with acute nephrotic syndrome with recurrent IgAN after kidney transplantation, rituximab displayed a beneficial effect on proteinuria 50. Long‐term treatment is necessary in patients with primary chronic IgAN, but adverse effects, infections, solid tumours, progressive multi‐focal leukoencephalopathy, pulmonary complications and malignancies, costs and application modus should be considered.
MPA in maintenance therapy
MPA inhibits B and T cell proliferation specifically and has been shown to suppress immunoglobulin and cytokine secretion of B cells 51, induce apoptosis of activated T lymphocytes 52 and inhibit the migration of lymphocytes and antigen presentation by dendritic cells 53. However, other important inflammatory responses are not influenced by MPA as the expression of activation markers of inflammation, including CD25 and CD69 54. MPA was used with different outcomes in several studies with 13, 14 and without cytotoxic induction therapy 46, 55, 56, 57; however, the patients included in these studies were not comparable regarding risk factors, e.g. renal progression in linear regression analysis, proteinuria and age 46, 55, 56, 57. This might explain why a study in 21 IgAN patients with mild renal impairment without induction therapy found no significant effect of MPA monotherapy on renal function and proteinuria after 3 years 56. In another trial, there was no effect of MPA on renal function in 17 patients with moderate renal impairment in the absence of an induction therapy 57. After steroid pulses, less specific azathioprine for 6 months in non‐progressive IgAN patients provided no additional benefit to steroids alone after a 5‐year follow‐up, but more side effects have been described 12. However, a decrease in proteinuria was observed in two randomized controlled trials in 31 patients 45 and in 16 patients 46. The arguments raised by some nephrologists, that there is a difference between the response to MMF/MPA between Asians and Europeans due to genetic aspects, should be proved; detailed information of ethnicity has been given in only one study 14. However, genetic or pharmacogenetic aspects have been not been explored in published studies of IgAN patients with supportive or immunosuppressive studies. Both drugs have been used successfully worldwide in autoimmune diseases and in transplantation using the same doses. In a subsequent publication, in our patients we also demonstrated all pharmacokinetic/pharmacodynamic aspects of the differences between MMF and MPA, with no sign of less exposure or inosine 5′‐monophosphate dehydrogenase suppression in comparison to other studies 28. Genetic variants of the uridine diphosphate‐glucoronyltransferases may enlarge the drug exposition of MMF in the area under the curve and will be responsible for more side effects 58, but larger trials in MMF patients with worldwide examination of this defect have not been published.
In our study, sequential MPA maintenance therapy was effective in such patients in reducing the further loss of renal function (ΔGFR) from −0·4 to −0·1 ml/min per month, with even a trend in reduction of proteinuria from 1·0 to 0·6 g/l. The full effect of MPA on renal function was observed after an average time lag of 6 months and in reduction of proteinuria after 5 months. Maintenance therapy with MPA and low‐dose corticosteroids consolidates the clinical outcome after induction therapy with CyP or steroids, while reducing side effects and the cumulative toxicity of cyclophosphamide and corticosteroids 8, 13, 14, 54, 59, 60, 61.
In summary, our data suggest that MPA preceded by immunosuppressive induction therapy seems to be effective to slow the loss of renal function and improves proteinuria in patients with advanced progressive IgAN.
Disclosure
The authors have no disclosures.
Acknowledgement
We gratefully thank Mrs Brigitte Gaule‐Scheffler for her support and collecting the patient's records.
References
- 1. Lee H, Hwang JH, Paik JH et al Long‐term prognosis of clinically early IgA nephropathy is not always favorable. BMC Nephrol 2014; 15:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hiki Y, Horii A, Iwase H et al O‐linked oligosaccharide on IgA1 hinge region in IgA nephropathy. Fundamental study for precise structure and possible role. Contrib Nephrol 1995; 111:73–84. [PubMed] [Google Scholar]
- 3. Moura IC, Arcos‐Fajardo M, Sadaka C et al Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 2004; 15:622–34. [DOI] [PubMed] [Google Scholar]
- 4. Tesar V, Troyanov S, Bellur S et al Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol 2015; 26:2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harper L, Ferreira MA, Howie AJ et al Treatment of vasculitic IgA nephropathy. J Nephrol 2000; 13:360–6. [PubMed] [Google Scholar]
- 6. Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 2003; 18:1321–9. [DOI] [PubMed] [Google Scholar]
- 7. Ballardie FW. IgA nephropathy treatment 25 years on: can we halt progression? The evidence base. Nephrol Dial Transplant 2004; 19:1041–6. [DOI] [PubMed] [Google Scholar]
- 8. Rasche FM, Klotz CH, Czock D et al Cyclophosphamide pulse therapy in advanced progressive IgA nephropathy. Nephron Clin Pract 2003; 93:131–6. [DOI] [PubMed] [Google Scholar]
- 9. Rostoker G, Desvaux‐Belghiti D, Pilatte Y et al High‐dose immunoglobulin therapy for severe IgA nephropathy and Henoch–Schonlein purpura. Ann Intern Med 1994; 120:476–84. [DOI] [PubMed] [Google Scholar]
- 10. Rasche FM, Keller F, Lepper PM et al High‐dose intravenous immunoglobulin pulse therapy in patients with progressive immunoglobulin A nephropathy: a long‐term follow‐up. Clin Exp Immunol 2006; 146:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol 2002; 13:142–8. [DOI] [PubMed] [Google Scholar]
- 12. Pozzi C, Andrulli S, Pani A et al Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 2010; 21:1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasche FM, Keller F, von Muller L et al Sequential immunosuppressive therapy in progressive IgA nephropathy. Contrib Nephrol 2007; 157:109–13. [DOI] [PubMed] [Google Scholar]
- 14. Rasche FM, Keller F, von Muller L et al Mycophenolic acid therapy after cyclophosphamide pulses in progressive IgA nephropathy. J Nephrol 2006; 19:465–72. [PubMed] [Google Scholar]
- 15. Lee HS, Lee MS, Lee SM et al Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee's glomerular grading system. Nephrol Dial Transplant 2005; 20:342–8. [DOI] [PubMed] [Google Scholar]
- 16. Lee SM, Rao VM, Franklin WA et al IgA nephropathy: morphologic predictors of progressive renal disease. Hum Pathol 1982; 13:314–22. [DOI] [PubMed] [Google Scholar]
- 17. Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis 1997; 29:829–42. [DOI] [PubMed] [Google Scholar]
- 18. Cattran DC, Coppo R, Cook HT et al The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76:534–45. [DOI] [PubMed] [Google Scholar]
- 19. Roberts IS, Cook HT, Troyanov S et al The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009; 76:546–56. [DOI] [PubMed] [Google Scholar]
- 20. Haubitz M, Schellong S, Gobel U et al Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody‐associated vasculitis and renal involvement: a prospective, randomized study. Arthritis Rheum 1998; 41:1835–44. [DOI] [PubMed] [Google Scholar]
- 21. Guillevin L, Cordier JF, Lhote F et al A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis Rheum 1997; 40:2187–98. [DOI] [PubMed] [Google Scholar]
- 22. Rasche FM, Klotz CH, Czock D et al Cyclophosphamide pulse therapy in advanced progressive IgA nephropathy. Nephron Clin Pract 2003; 93:c131–6. [DOI] [PubMed] [Google Scholar]
- 23. Boumpas DT, Austin HA 3rd, Vaughn EM et al Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet 1992; 340:741–5 [DOI] [PubMed] [Google Scholar]
- 24. Mitch WE, Walser M, Buffington GA et al A simple method of estimating progression of chronic renal failure. Lancet 1976; 2:1326–8. [DOI] [PubMed] [Google Scholar]
- 25. Roberts IS. Oxford classification of immunoglobulin A nephropathy: an update. Curr Opin Nephrol Hypertens 2013; 22:281–6. [DOI] [PubMed] [Google Scholar]
- 26. Chen AP, Setser A, Anadkat MJ et al Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events version 4.0. J Am Acad Dermatol 2012; 67:1025–39. [DOI] [PubMed] [Google Scholar]
- 27. Hansen‐Schmidt S, Silomon J, Keller F. Osmotic nephrosis due to high‐dose immunoglobulin therapy containing sucrose (but not with glycine) in a patient with immunoglobulin A nephritis. Am J Kidney Dis 1996; 28:451–3. [DOI] [PubMed] [Google Scholar]
- 28. Czock D, Rasche FM, Carius A et al Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric‐coated mycophenolate versus mycophenolate mofetil in patients with progressive IgA nephritis. J Clin Pharmacol 2007; 47:850–9. [DOI] [PubMed] [Google Scholar]
- 29. Berger J, Hinglais N. Les dépôts intercapillaires d'IgA–IgG [IgA‐IgG intercapillary deposits]. J Urol Nephrol (Paris) 1968; 74:694–5. [PubMed] [Google Scholar]
- 30. Berger J, Yaneva H, Nabarra B et al Recurrence of mesangial deposition of IgA after renal transplantation. Kidney Int 1975; 7:232–41. [DOI] [PubMed] [Google Scholar]
- 31. Hu SL, Colvin GA, Rifai A et al Glomerulonephritis after hematopoietic cell transplantation: IgA nephropathy with increased excretion of galactose‐deficient IgA1. Nephrol Dial Transplant 2010; 25:1708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imasawa T, Nagasawa R, Utsunomiya Y et al Bone marrow transplantation attenuates murine IgA nephropathy: role of a stem cell disorder. Kidney Int 1999; 56:1809–17. [DOI] [PubMed] [Google Scholar]
- 33. Andre PM, Le Pogamp P, Chevet D. Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal 1990; 4:115–9. [DOI] [PubMed] [Google Scholar]
- 34. Roccatello D, Ferro M, Cesano G et al Steroid and cyclophosphamide in IgA nephropathy. Nephrol Dial Transplant 2000; 15:833–5. [DOI] [PubMed] [Google Scholar]
- 35. McIntyre CW, Fluck RJ, Lambie SH. Steroid and cyclophosphamide therapy for IgA nephropathy associated with crescenteric change: an effective treatment. Clin Nephrol 2001; 56:193–8. [PubMed] [Google Scholar]
- 36. Lv J, Xu D, Perkovic V et al Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol 2012; 23:1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scholl U, Wastl U, Risler T et al The ‘point of no return’ and the rate of progression in the natural history of IgA nephritis. Clin Nephrol 1999; 52:285–92. [PubMed] [Google Scholar]
- 38. Pozzi C, Andrulli S, Del Vecchio L et al Corticosteroid effectiveness in IgA nephropathy: long‐term results of a randomized, controlled trial. J Am Soc Nephrol 2004; 15:157–63. [DOI] [PubMed] [Google Scholar]
- 39. Maschio G, Cagnoli L, Claroni F et al ACE inhibition reduces proteinuria in normotensive patients with IgA nephropathy: a multicentre, randomized, placebo‐controlled study. Nephrol Dial Transplant 1994; 9:265–9. [PubMed] [Google Scholar]
- 40. Rekola S, Bergstrand A, Bucht H. Deterioration rate in hypertensive IgA nephropathy: comparison of a converting enzyme inhibitor and beta‐blocking agents. Nephron 1991; 59:57–60. [DOI] [PubMed] [Google Scholar]
- 41. Praga M, Gutierrez E, Gonzalez E et al Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol 2003; 14:1578–83. [DOI] [PubMed] [Google Scholar]
- 42. Berthoux F, Mohey H, Laurent B et al Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 2011; 22:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasche FM, Schwarz A, Keller F. Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol 1999; 51:147–52. [PubMed] [Google Scholar]
- 44. Pozzi C, Bolasco PG, Fogazzi GB et al Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 1999; 353:883–7. [DOI] [PubMed] [Google Scholar]
- 45. Chen X, Chen P, Cai G et al [A randomized control trial of mycophenolate mofetil treatment in severe IgA nephropathy] [in Chinese]. Zhonghua Yi Xue Za Zhi 2002; 82:796–801. [PubMed] [Google Scholar]
- 46. Tang S, Leung JC, Chan LY et al Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int 2005; 68:802–12. [DOI] [PubMed] [Google Scholar]
- 47. Austin HA 3rd, Klippel JH, Balow JE et al Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 1986; 314:614–9. [DOI] [PubMed] [Google Scholar]
- 48. Czock D, Keller F, Rasche FM et al Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005; 44:61–98. [DOI] [PubMed] [Google Scholar]
- 49. Sugiura H, Takei T, Itabashi M et al Effect of single‐dose rituximab on primary glomerular diseases. Nephron Clin Pract 2011; 117:c98–105. [DOI] [PubMed] [Google Scholar]
- 50. Otsuka Y, Takeda A, Horike K et al Early recurrence of active IgA nephropathy after kidney transplantation. Nephrology (Carlton) 2014; 19:45–8. [DOI] [PubMed] [Google Scholar]
- 51. Jonsson CA, Carlsten H. Mycophenolic acid inhibits inosine 5'‐monophosphate dehydrogenase and suppresses immunoglobulin and cytokine production of B cells. Int Immunopharmacol 2003; 3:31–7. [DOI] [PubMed] [Google Scholar]
- 52. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000; 47:85–118. [DOI] [PubMed] [Google Scholar]
- 53. Colic M, Stojic‐Vukanic Z, Pavlovic B et al Mycophenolate mofetil inhibits differentiation, maturation and allostimulatory function of human monocyte‐derived dendritic cells. Clin Exp Immunol 2003; 134:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Izeradjene K, Revillard JP. Apoptosis of superantigen‐activated T cells induced by mycophenolate mofetil treatment. Transplantation 2001; 71:118–25. [DOI] [PubMed] [Google Scholar]
- 55. Maes B, Evenepoel P, Kuypers D et al A prospective placebo‐controlled randomized single centre study of mycophenolate mofetil treatment for IgA nephropathy: lack of clinical efficacy after two years (Abstract). J Am Soc Nephrol 2001; 12:114A. 11134257 [Google Scholar]
- 56. Maes BD, Oyen R, Claes K et al Mycophenolate mofetil in IgA nephropathy: results of a 3‐year prospective placebo‐controlled randomized study. Kidney Int 2004; 65:1842–9. [DOI] [PubMed] [Google Scholar]
- 57. Frisch G, Lin J, Rosenstock J et al Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double‐blind randomized controlled trial. Nephrol Dial Transplant 2005; 20:2139–45. [DOI] [PubMed] [Google Scholar]
- 58. Kuypers DR, de Jonge H, Naesens M et al Effects of CYP3A5 and MDR1 single nucleotide polymorphisms on drug interactions between tacrolimus and fluconazole in renal allograft recipients. Pharmacogenet Genomics 2008; 18:861–8. [DOI] [PubMed] [Google Scholar]
- 59. Contreras G, Pardo V, Leclercq B et al Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–80. [DOI] [PubMed] [Google Scholar]
- 60. Langford CA, Talar‐Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of Wegener's granulomatosis. Arthritis Rheum 2004; 51:278–83. [DOI] [PubMed] [Google Scholar]
- 61. Pesavento TE, Bay WH, Agarwal G et al Mycophenolate therapy in frequently relapsing minimal change disease that has failed cyclophosphamide therapy. Am J Kidney Dis 2004; 43:e3–6. [DOI] [PubMed] [Google Scholar]


