Summary
Active complement mediators play a key role in graft‐versus‐host diseases, but little attention has been given to the angiogenic balance and complement modulation during allograft acceptance. The complement cascade releases the powerful proinflammatory mediators C3a and C5a anaphylatoxins, C3b, C5b opsonins and terminal membrane attack complex into tissues, which are deleterious if unchecked. Blocking complement mediators has been considered to be a promising approach in the modern drug discovery plan, and a significant number of therapeutic alternatives have been developed to dampen complement activation and protect host cells. Numerous immune cells, especially macrophages, develop both anaphylatoxin and opsonin receptors on their cell surface and their binding affects the macrophage phenotype and their angiogenic properties. This review discusses the mechanism that complement contributes to angiogenic injury, and the development of future therapeutic targets by antagonizing activated complement mediators to preserve microvasculature in rejecting the transplanted organ.
Keywords: allograft rejection, angiogenesis, complement inhibition, complement‐mediated injury
Introduction
The complement system is an effector of innate immune response with the ability to enhance antibody‐mediated removal of foreign antigens and cellular debris, as well as the ability to initiate local inflammatory responses 1, 2 that destroy pathogens and recruit cellular repair mechanisms. Angiogenesis is an example of the reparative role of immune inflammation with pathological significance in ischaemic and inflammatory diseases, including coronary artery disease, acute myocardial infarction and transplant allograft rejection 3, 4. The process of angiogenesis is regulated tightly by an equilibrium between angiogenic activators and inhibitors 5. An imbalance in the expression of angiogenic activators compared to inhibitors, as occurs in the case of tumours and wound healing, results in a pro‐angiogenic state termed the ‘angiogenic switch‘ 6. The formation of new blood vessels is structured in a tissue‐specific manner and is key for tissue growth and repair 7, providing cells with the nutrition and oxygen necessary for normal physiological activities. It is recognized that the complement system in its capacity to modulate angiogenesis and microvascular rejection, may play a vital role in the long‐term health of the transplanted allograft.
The complement system regulates both the innate and adaptive immune systems through the classical pathway, the lectin pathway and the alternative pathway with the activation of complement proteins secreted from various cells. In humans, hepatocytes provide the primary source of C3 8, which is also expressed to a lesser degree by macrophages 9, fibroblasts 10, vascular endothelial cells 11, renal tubular epithelium 12, astroglia 13 and adipocytes 14.
The complement system consists of plasma and membrane‐bound proteins 15, which are activated through three different pathways: the classical pathway, the lectin pathway and the alternative pathway. The classical pathway can be activated by the binding of C1q directly to the surface of pathogen, and also during an adaptive immune response by the binding of C1q to antigen‐antibody immune complexes, and is thus a crucial link between the effector mechanisms of innate and adaptive immune responses 16, 17, 18. The mannose binding lectin (MBL) pathway is initiated by binding of the MBL, a serum protein, to mannose‐containing carbohydrates on bacteria or viruses. In addition, the alternative pathway can be activated by the spontaneous conversion of C3 to an active protease 19. All three pathways converge in the activation of the pivotal complement molecule, C3, and the generation of C3 convertase. C3 convertase, in turn, cleaves C3 into C3a and C3b. The C3b molecule then combines with C3 convertase to form the C4bC2aC3b complex in classical and lectin pathways, and to the formation of the C3bBbC3b complex in the alternate pathway. Both C4bC2aC3b and C3bBbC3b complexes are C5 convertases, which cleave C5 into C5a and C5b 2, 20, 21. The generated C5a can then function as a potent anaphylatoxin at the site of production, while C5b participates in the assembly of the membrane attack complex (C5b‐9 or MAC) 22. Finally, MAC complex can initiate cell lysis, and in sublytic quantities can lead to cell activation (Fig. 1). Complement mediators such as C3a and C5a and C3b, C1q and MBL modulate macrophage responses during inflammation. Furthermore, C3a, C5a and MAC initiate the inflammatory response, while C1q suppresses the inflammatory response and up‐regulate clearance of apoptotic cells with a M2 macrophage polarization 23, 24, 25, which promote the recruitment of the leucocytes involved in the tissue repair and remodelling process 26 through the secretion of proangiogenic growth factors such as vascular endothelial cell growth factor (VEGF), placental growth factor (PLGF), stromal cell‐derived factor (SDF)‐1 and fibroblast growth factor (FGF)‐2 27, 28 (Figs 1 and 2).
Figure 1.
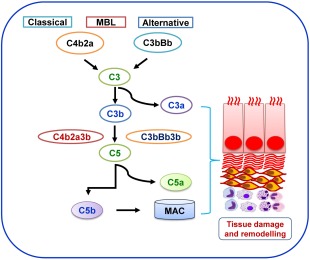
Overview of complement system: highlights activation of classical, mannose‐binding lectin (MBL) and alternate pathway leading to tissue remodelling during the inflammatory condition.
Figure 2.
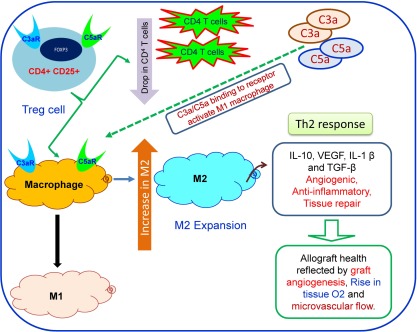
Immune regulation of tissue repair: the model illustrates cross‐talk between regulatory T cells, complement activators and macrophages during tissue repair and angiogenesis in transplants. Binding of C3a and C5a to respective receptors on macrophages will polarize M1 macrophage and cause the T helper type 1 (Th1) response. Inhibition/blocking of both complements will shift in M2 macrophage and Th2 responses.
In addition to its involvement in complement activation, C3 and its degradation products promote phagocytosis, activate inflammatory responses against pathogens and regulate adaptive immunity, but its uncontrolled activation may also result in host cell damage 29. In particular, complement components, and their activated fragments C3a and C5a, synchronize the magnitude of adaptive immune responses via ligation of their respective receptors expressed on antigen‐presenting cells (APCs) and T lymphocytes 30, 31, 32, 33. In what follows, we will focus on the role that C3, C5 and C1q in play in angiogenesis and summarize the evidence underlying the use of complement inhibitors in animal studies and in human clinical trials. Recently, the role of MBL pathway was demonstrated in a mouse renal ischaemia–reperfusion injury (IRI) model and clinically in post‐transplant acute renal failure. A strong co‐localization was seen between MBL‐A, MBL‐C and C6 deposition, which indicates the role of MBLs in renal complement activation 34. Furthermore, MBL‐A and MBL‐C knock‐out mice were protected from kidney injury, and reconstitution of knock‐out mice with recombinant human MBL restored renal damage after IRI, confirming the role of the MBL pathway in renal IRI. MBL‐mediated renal IRI appears to be governed by direct splicing of C3 by Mannose binding protein (MBSP1) and not through C4, as C4–/– mice were not protected against renal IRI 35.
Angiogenesis and the role of the inflammatory environment
Angiogenesis is a complex process that involves the degradation of the vascular membrane and the extracellular matrix, as well as endothelial cell proliferation and migration 36. Angiogenesis is a distinctive feature of many disorders, and also occurs during cell‐mediated immune responses and chronic inflammatory diseases 37 such as arthritis 38, chronic asthma 39 and in transplant allograft rejection 27, 40, 41. The equilibrium between the production of pro‐ versus anti‐angiogenic factors in the course of the immune response will result in vascular repair or injury, a process that may be critically important to the health of the transplanted graft 42, 43. Using a murine orthotopic tracheal transplant model, we have demonstrated distinct roles for CD4+ and CD8+ T cells, as well as antibody‐mediated complement activation in allograft microvascular health. CD4+ T cells are central in the initiation of the transplant alloimmune response and play an important role in donor graft angiogenesis 40. CD4+ T cells have also been linked to angiogenesis in cancer 44, 45 and in skin 46, corneal 47, 48 and heart transplantation 49. CD8+ T cells are required for allograft neovascularization following CD4+‐mediated microvascular rejection. Similar to the role of CD4+ T cells, antibody‐mediated complement activation is independently sufficient to induce allograft microvascular rejection 20, 40. Blocking complement, using the C3 inhibitor, complement receptor 2 complement‐inhibitory protein (CR2‐Crry), synergizes with CD4+‐depletion to prevent transplant ischaemia and chronic allograft rejection 40. CR2‐Crry is a fusion protein of the iC3b/C3dg‐binding fragment of mouse CR2 attached to a mouse Crry. CR2 is a member of the C3‐binding protein family, and its natural ligands are cleavage products of C3 that become deposited at sites of complement activation. A benefit of this therapy is that it only attaches to deposited long‐lived C3 cleavage fragments, iC3b, C3dg and C3d and does not affect circulating C3; because of this property, therapy does not increase susceptibility to infection 50. CR2‐Crry is a well‐characterized complement inhibitor 50, 51, 52, 53, 54 which has demonstrated benefit in preclinical models of acute lung injury following intestinal ischaemia–reperfusion injury 50, spinal cord injury 52, ischaemic stroke 51, arthritis 53 and autoimmune renal disease 54. Taken together, these findings suggest that treating acute rejection with complement inhibition while avoiding CD8+ T cell depletion could prevent the onset of chronic rejection 2, 20, 40.
It is now understood that both activated T cells 55 and macrophages 56 can secrete angiogenesis factors, specifically VEGF, which is a vital promoter of the leucocyte‐induced reaction 4, 57. This cross‐talk between cell‐mediated immune response and macrophages secreting transforming growth factor (TGF)‐β and VEGF have resulted in the paradigm shift to explain why chronic inflammatory disorders are characterized by both angiogenesis and fibrosis 58. Therefore, the pro‐angiogenesis phase may arise as a result of cytokine‐ and cell‐mediated responses that increase local VEGF release. Babu et al. demonstrated a similar neovascularization reaction in an orthotopic tracheal model of transplantation of acute allograft rejection 41. Angiogenesis‐promoting factors such as VEGF are over‐expressed in all models of chronic inflammation, and their increased expression is associated with disease progression 36, 37. In a rat renal transplantation model, blockade of angiogenesis factors, including VEGF–VEGF receptor (VEGFR) interaction, attenuated the progression of the chronic allograft nephropathy 59. Inflammatory mediators stimulate resident cells to produce VEGF and promote the process of angiogenesis and tissue damage, typified by choroidal neovascularization in age‐related macular degeneration and joint destruction inrheumatoid arthritis, respectively 60, 61, 62. Under certain conditions, however, inflammatory cells may counteract VEGF‐induced angiogenesis by secreting soluble VEGFR (sVEGR)‐1, sequestering VEGF 63, 64. VEGF can serve as a potent leucocyte chemoattractant via direct interactions with its receptors expressed on subsets of monocyte/macrophages and T cells 65. In addition, VEGF induces the expression of E‐selectin, intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1) 66 and proinflammatory chemokines such as CXCL10/IP‐10 and monocyte chemoattractant protein‐1 (MCP‐1) 67. During the acute inflammation phase, leucocytes and platelets induce and deliver angiogenic factors into the inflammation site, mediate the proliferation of vascular endothelial cells and facilitate the recruitment of endothelial progenitor cells 4. Several angiogenic mediators stimulate the process of angiogenesis by acting on different vascular cells including pericytes and vascular endothelial cells. Pericytes are cells of mesenchymal origin that are best characterized by the expression of α‐smooth muscle actin (SMA) 68, desmin 69, CD248 70, neural/glial antigen 2 (NG‐2) 71 and platelet‐derived growth factor receptor‐β (PDGFR‐β) 72 and participate in the expansion and stabilization of vascular networks. VEGF and pericytes play a key role in the initiation and progression of blood vessel formation 5, 6 with the contribution of PDGF, TGF‐β, fibroblast growth factor and angiopoietins 7, 8. During inflammation, pericytes contribute to the building of matrix through myofibroblast populations, while the detachment of pericytes from the microvasculature through antibody‐mediated complement activation contributes to the microvasculature loss and subsequent hypoxia 73, 74, 75 (Fig. 3).
Figure 3.
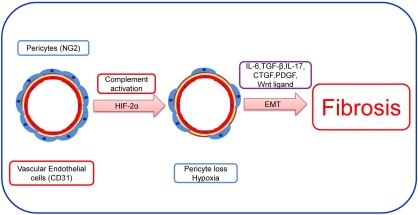
Inflammation and fibrosis: loss of pericyte during inflammation and/or local hypoxia in microvessel. Under normal conditions homeostatic repair occurs under the influence of vascular endothelial growth factor (VEGF). In contrast, during the chronic inflammatory condition, the pericyte loss facilitates epithelial–mesenchymal transition (EMT) and transforming growth factor (TGF)‐β, interleukin (IL)‐17 and connective tissue growth factor (CTGF) release, which in turn results in fibrosis and scarring of tissue.
Complement mediators and angiogenic shift
The role of the complement system in regulating microvascular health and in tissue remodelling is well recognized in multiple pathologies, most notably in the microvascular damage that occurs in IRI 76. However, the mechanisms underlying the regulation of angiogenesis by the complement system are incompletely understood 77, 78. The vascular connections that supply almost all tissues serve to nourish cells with micronutrients and oxygen and enables them to expel metabolic toxic wastes. In physiological circumstances, angiogenesis occurs mainly during embryo development, wound healing and in response to ovulation 79, 80. However, complement‐mediated pathological angiogenesis, or the abnormal rapid proliferation of blood vessels, is observed in a number of diseases, including cancer, IRI, transplant rejection, cardiovascular, retinopathy, wound healing and cerebral ischaemia 40, 81, 82, 83. Angiogenesis is the compensatory physiological adaptation to tissue ischaemia, serving as an adaptive response to increase oxygen delivery to tissues through the activation of VEGF signalling 84.
During acute inflammation, complement anaphylatoxins C3a and C5a mediate changes in microvascular flow, permeability, leucocyte extravasation and migration that contribute to tissue damage after IRI 2, 40. Complement deposition in the septal vasculature of lung transplants is highest in patients with chronic lung transplant rejection 85. The close correlation between C3d and C4d complement deposition with chronic allograft dysfunction may indicate that humoral rejection is an important contributor to poor outcomes after lung transplantation 85, 86. The role of complement driven angiogenesis has also been reported in an orthotopic mouse tracheal model of transplantation 2, 40; both C3–/– transplant recipients and mice treated with CR2‐Crry demonstrated improved recovery of the microvasculature and tissue oxygenation 2, 40 (Fig. 4). In addition, C3–/– recipient mice exhibited more dilated and leaky microvessels compared to controls 2, 40. Similarly, in a mouse model of retinopathy of prematurity, an increased neovascularization in C3−/− mice was observed. These studies highlight the importance of complement in regulating angiogenesis 77. In addition to the role of C3 in neovascularization, C5a and C1q also play a crucial role in angiogenesis, with increased neovascularization observed in C5aR−/− mice 87. In a wound‐healing model, the application of C1q resulted in an increased permeability, proliferation and chemotaxis of endothelial cells, indicating the proangiogenic activity of C1q 88. A role for other complement modulators has been reported in various pathological conditions and in a number of organ models. It has been well shown that other complement fragments C1 (including C1q, C1r and C1s), C2, C3, C4, C6 and complement factor B also play a key role during organ transplantation 89. A number of murine studies have investigated the interaction between complement components and adaptive immunity 15. These findings highlight a potential role for the C3a receptor (C3aR) and the C5a receptor in APCs and T cell activation and differentiation via the expression of co‐stimulatory molecules CD28 and CD40L 90, 91, 92. In addition, the role of C1q, as an essential trigger of the classical pathway in T helper type 1 (Th1) cell activation and in modulating both innate and adoptive immunity, has been shown in different transplant models 89. However, in studies of allergic asthma, it was shown that anaphylatoxin receptors exert key immunoregulatory functions that stimulate or suppress asthma exacerbations through dendritic and T cell interactions and Teffector cell function. Further, immunomodulation of C5aR and C3aR in pulmonary dendritic cells suggest that C3aR signalling augments airway inflammation and the Th2 response through regulation of protective C5aR signalling 93, 94. Recent studies show that the expression of C3a and C5a on both T cells and APCs, and direct binding of complement cleavage products to receptors on T cells and APCs, influence T cell differentiation, expansion and survival of CD4+ T helper cells, suggesting that complement deficiency or blockade can potentially attenuate T cell‐mediated autoimmunity and delay allograft rejection 95. Furthermore, it has been demonstrated that C5aR–/– dendritic cells promote induction of regulatory T cells (Tregs) and Th17 and hence C5aR activation in dendritic cells provides a key innate immune signal that control differentiation of naive T cells into Treg Th1 and Th17 96. Interestingly, expression and signalling through both C3aR and C5aR on nTreg cells has been reported to inhibit Treg cell function 1, 97. However, it was demonstrated that blocking/or genetic deficiency of C3aR/C5aR on nTreg cells enhanced their in‐vitro and in‐vivo suppressive activity and prolonged allogeneic skin graft survival 98. Additional studies demonstrated that C3aR/C5aR deficiency/or blockade stimulates murine iTreg cells, stabilizes forkhead box protein 3 (FoxP3) gene expression, prevents iTreg conversion to IFN‐γ/TNF‐α‐producing Teffector cells and thereby limit graft‐versus‐host disease 99, 100. Liu et al. reported an antagonistic effect between CD4+CD25– T cells and CD4+CD25+ Treg cells on macrophage polarization 101, which demonstrated that the differentiation of M2 macrophage is induced by CD4+CD25+ Treg cells, whereas the M1 macrophages can be induced by CD4+CD25– Teffector cells 102.
Figure 4.
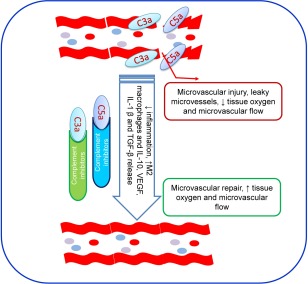
Targeted complement blocking: model illustrates how targeted blocking will promote microvascular repair through vascular endothelial growth factor (VEGF) signalling and rescue allograft.
In an in‐vitro human chroroid retinopathy model treatment with a C5aR antagonist promoted neovascularization, whereas treatment with a C5a agonist reduced neovascularization, supporting the angioinhibitory role of C5a and C5aR 87. Treatment of C3−/− mice with C3a and C5a mitigated the aforementioned enhanced neovascularization effect observed in C3−/− mice, suggesting that C3a and C5a are capable of inhibiting hypoxia‐driven retinal neovascularization 77, 87. Macrophages express receptors for activated C3 and C5 and respond to activated C3a and C5a at the site of local inflammation to mediate tissue angiogenesis 57, 58, 59, 60, 76. Macrophages also modulate inflammation and adaptive immunity, promote cell proliferation through the release of growth factors, including ornithine and polyamines 76, and promote angiogenesis, tissue remodelling and tissue repair 103, 104, 105. M2 macrophages promote tumour angiogenesis at distinct phases of malignant progression of gastric, mammary 106, lung 107 and liver carcinomas 108. C5a induces a proinflammatory (M1) phenotype resulting in the secretion of inflammatory cytokines 22, 109, 110, and is associated with an anti‐angiogenic state 111. The absence or inhibition of C3 and C5 favours anti‐inflammatory macrophage (M2) polarization and the release of IL‐10, VEGF and TGF‐β to promote angiogenesis and tissue repair 112. In contrast, both C3a and C5a anaphylatoxins, when bound to their respective receptors on M1 macrophages 113, and many other cells including airway epithelium, increased mRNA expression of IL‐6, TNF‐α, sVEGFR1 and decreased IL‐10, resulting in an anti‐angiogenic response. Increased sVEGFR1 secretion from monocytes/macrophages also inhibits angiogenesis 114, 115. M2 macrophages play a decisive anti‐inflammatory role by secretion of IL‐10, IL‐1β, and TGF‐β in tissue repair, promoting angiogenesis through VEGF secretion 116 (Fig. 2). Active complement proteins, C3a and C5a, control macrophage‐mediated angiogenesis in tissue 114, 115, 117. C3a and C5a generated from complement cascade have potential effects on macrophage‐mediated angiogenesis inhibition. Both C3a and C5a anaphylatoxins bind to their respective C3aR and C5aR on monocytes/macrophages 113 and many other cells, including airway epithelium, causing an anti‐angiogenic response that results in an increased expression of IL‐6, TNF‐α, sVEGFR1 and decreased expression of IL‐10 mRNA. Increased sVEGFR1 secretion from monocytes/macrophages inhibits angiogenesis in different disease models 114, 115. Several in‐vitro studies also confirmed that the C3‐activated mesenchymal stem cells may constitute a naturally occurring repair mechanism used under pathophysiological conditions to improve tissue repair through the production of angiogenic factors 118. Historically, C3a and C5a have been identified as proinflammatory mediators; however, stem cell studies now suggest that the true biological role of C3a may not be that of an inflammatory mediator, but rather a wound‐healing factor 118.
Discussion
In last two decades, attenuating complement activation during allograft transplantation has been an area of research interest, and the multiple molecular signals that govern tissue injury and repair, immune response and angiogenesis continue to be the focus of intense investigation. Numerous molecules capable of modulating upstream complement activation and specific inhibitors of the end‐products of the complement cascade have been investigated with varying degrees of success both in animal models and in clinical studies 98, 119, 120, 121. To date, two complement inhibitors are undergoing human clinical trials with demonstrated effectiveness in IRI and potential utility in the prevention of tissue fibrosis and chronic tissue injury 122. As mentioned previously, complement mediates a wide array of functions, including tissue remodelling and angiogenesis 40, 62, 78. Because of the role that complement plays in major inflammatory diseases, different complement activators have been targeted to dampen inflammation through the specific complement inhibitors (Figs 1 and 2). In a clinical trial of lung transplantation, the soluble complement receptor type 1 (sCR1) TP‐10 attenuated IRI by inactivating C3a and C5a convertases 123, and sCR1 has been tested in many in‐vitro and in‐vivo experimental models and shown to block interactions with C3b, as well as to serve as a co‐factor for the inactivation of C3b 124, 125. Eculizumab is a humanized monoclonal antibody that inhibits complement factor C5, preventing formation of the activated form C5a and the MAC 9 (C5b‐9). In renal transplantation, eculizumab has demonstrated efficacy for the treatment of antibody‐mediated rejection and in the prevention of atypical haemolytic uraemic syndrome post‐transplant 119, 126, 127. As described earlier, C5a and signalling through the C5aR play key roles in mediating IRI and the dysregulated inflammatory response in the brain‐dead donor 128. In addition, efficacy of eculizumab in the prevention of antibody‐mediated response in renal transplant recipients has been investigated, which predicts an active role of complement in adaptive immune responses during allograft rejection 127, 129. In a murine model of myocardial IRI, the efficacy of blocking the complement cascade with CR2‐Crry was equivalent to the inhibition of only the alternative pathway (CR2‐fH), except for the expression of certain inflammatory markers 120, 130. Alternatively, C5a could intervene in other pathways that lead to graft injury, and of particular interest is its potential to augment the alloimmune response 20, 120, 130. Complement inhibitor of C5a, Spiegelmer NOX‐D19 (NOXXON Pharma, Berlin, Germany) has been shown to reduce airway rejection in a mouse model of orthotopic tracheal transplantation 20 and another C5a inhibitor, Spiegelmer NOX‐D20, has been reported to prolong survival and to reduce liver and kidney graft failure, inflammatory cytokines and vascular leakage 131 (Table 1).
Table 1.
Complement‐based therapeutic targets and their potential applications in organ transplantation.
| Molecular mediators | Mechanism of action | Experimental/clinical studies |
|---|---|---|
| Eculizumab | C5, abrogate terminal complement activation | Human (ClinicalTrials.gov Identifiers, NCT02013037, NCT01327573, NCT02113891) |
| C1‐INH (Berinert) (CINRYZE) | Prevent complement initiation via the classical and lectin pathways | Human (ClinicalTrials.gov Identifiers: NCT02134314, NCT01134510, NCT01035593, NCT01147302) |
| TP‐10 (sCR1) | Inactivate C3a and C5a convertases | Human (a randomized, placebo‐controlled trial in lung transplantation) |
| CR2‐FH | Inhibits alternative complement pathway | Mice (heart transplants) |
| CR2‐Crry | Inhibits all complement pathways | Mice (heart and trachea transplants) |
| NOX‐D19, NOX‐D20, NOX‐D21 | Specifically inhibits complement component C5a | Mice (trachea transplants) |
| Y‐CVF | A C3 inhibitor | Non‐human primates |
Activation of the complement pathway can be controlled at various points to attenuate complement‐mediated tissue injury. It is now well accepted that complement mediators play a key role in the pathogenesis of renal IRI and in the mechanisms resulting in tissue damage in the deceased kidney organ donor 132. Current evidence suggests that MAC (C5b‐9) assembly and the generation of anaphylatoxins C3a and C5a are responsible for eliciting proinflammatory responses injurious to ischaemic tissue and antibody‐mediated rejection 133. Complement therapy with sCR1 decreased intestinal myeloperoxidase activity and mucosal injury significantly in a rat model of intestinal IRI 134. sCR1 administration also restored protection of the rat liver from IRI 135. In addition, sCR1 has been shown to prevent acute rejection and to extend kidney allograft survival in renal transplantation 124, 125. In clinical trials, sCR1 has been shown to have significant beneficial effects in rescuing the allograft in myocardial IRI 134. In addition, treatments with TP‐10 (a sCR1) decreased mortality and myocardial infarction in high‐risk male patients undergoing cardiopulmonary bypass during cardiac surgery 136, 137 (Table 1). Amsterdam et al. were the first to show the efficacy of blocking of C5a in a pig model of myocardial infarction, which also inhibited neutrophil cytotoxicity 138. In animal models, treatment with a monoclonal antibody against C5 decreased myocardial IRI and prevented late inflammation and apoptosis in renal IRI 139, 140, 141. Similarly, C5aR antagonist treatment in renal IRI decreased kidney injury and improved graft function 76, 141. Complement‐mediated injury can also be attenuated by silencing C3 or C5, using a small interfering RNA (siRNA) approach 142.
Studies have also employed C1 esterase inhibitors (C1‐INH) that irreversibly bind and inactivate complement proteases such as C1r, C1s and mannan‐binding lectin‐associated serine protease‐2 (MASP‐2) 143, as well as many other mediators of relevance to IRI and antibody‐mediated rejection, including coagulation and cell migration 132. A recombinant form of C1‐INH (rhC1‐INH) has been approved by the US Food and Drug Administration for the treatment of hereditary angioedema 144. Treatment with a C1‐INH diminished renal IRI in a pig model, suggesting its potential to prevent delayed allograft dysfunction in renal transplantation 145. Rodent IRI models of myocardial, hepatic, intestinal and neurological injury corroborate this finding 145. Furthermore, pretreatment with recombinant C1‐INH was shown to lessen fibrosis in pigs subjected to renal IRI 146 and had a beneficial effect for the treatment of acute antibody‐mediated rejection in a baboon model of kidney transplantation 147. C1‐INH therapy has also been evaluated in lung transplant recipients exhibiting early signs of primary graft dysfunction (PGD), and a study reported that C1‐INH treatment improved the 1‐year survival and reduced length of intensive care unit stay when compared to patients with early signs of PGD without treatment 148. Other complement inhibitors under testing include factor D inhibitors, which inhibit both alterative complement pathway activation, as well as opsonization, and could potentially serve as a new therapeutic approach to rescue tissue injury following intestinal ischaemia–reperfusion during inflammation 149, 150.
The complement cascade plays a vital role in anti‐microbial defence, removal of immune complexes and apoptotic cells 22. The complement system facilitates the innate immune response against pathogens and also contributes to alloimmune‐mediated rejection of transplants through cytotoxic and lytic effects, but an increasing number of studies are revealing that the complement cascade enables a remarkable array of proliferative events 151. Complement proteins facilitate angiogenesis and cellular proliferation and induce dysregulation of angiogenic factors through different pathways 22. The alternative pathway plays a key role in causing tissue injury in a variety of inflammatory and ischaemic conditions. It can serve as an amplification loop for the classical or lectin pathways, and also can be activated independently through a spontaneous ‘tickover’ process 149. Complement activation has been shown to play a key role in inflammation and IRI after transplantation 2. Clinically, complement inhibition can be performed both by inhibiting all complement pathways as well as by blocking individual pathways. Evidence of the activated complement mediators in cellular proliferation and diseases of chronic inflammation has suggested a potentially deleterious role in abnormal cellular growth 132. The complement mediators have been linked to multiple interactions with the vascular endothelium, leading to expression of adhesion molecules, leucocyte recruitment, secretion of proinflammatory cytokines and chemokines and increased vascular permeability 152. Targeted complement inhibition is now established as a therapy to repair tissue injury in animal models and in clinical trials of transplant recipients. Recent studies show that direct binding of complement cleavage products to receptors on T cells can influence functional differentiation of CD4+ T helper cells and even tilt the balance between tolerance and inflammatory responses. This is probably relevant for graft survival in transplantation and must be taken into consideration, as treatment strategies based on complement manipulation are entering clinical use 1, 153, 154. This therapeutic achievement in the area of transplant immunology, along with the key development of pharmacological molecules that block human complement components and receptors 155, 156, now permits testing of the concept that targeting complement in organ transplant recipients will improve long‐term graft survival and patient outcomes. Recent research suggests a central role for complement mediators in the modulation of tissue repair and the progression of fibrosis in models of acute kidney injury, which highlights the complement cascade as a target in the investigation of the specific mechanisms that govern adaptive and abnormal tissue repair. However, more investigation is required to understand fully the effect of complement on the microvasculature during transplantation. The advancements of targeted treatment schemes that lessen the requirement for traditional immunosuppression of transplant recipients and that also have the ability to reduce tissue injury and fibrosis is of crucial importance to increase the limited organ donor pool and improve transplant outcomes.
Disclosure
The authors declare that they have no disclosures.
Author's contributions
M. A. K. was involved in writing, compiling the manuscript and in revising it critically for publication standards; J. H. contributed significantly on literature and critical suggestions to reshape the manuscript; A. M. A. and D. C. B. suggested the manuscript idea and compilation. All authors read and approved the final manuscript.
Acknowledgement
The authors would like to thank Dr Suhail Akhtar (sakhtar@luriechildrens.org) Stanley Manne Children's Research Institute, Chicago, USA, for critically reading this manuscript.
References
- 1. Cravedi P, van der Touw W, Heeger PS. Complement regulation of T‐cell alloimmunity. Semin Nephrol 2013; 33:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan MA, Nicolls MR. Complement‐mediated microvascular injury leads to chronic rejection. Adv Exp Med Biol 2013; 734:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffioen A, Molema WG. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000; 52:237–68. [PubMed] [Google Scholar]
- 4. Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol 2006; 17:932–42. [DOI] [PubMed] [Google Scholar]
- 5. Pollina EA, Legesse‐Miller A, Haley EM, Goodpaster T, Randolph‐Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle 2008; 7:2056–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag 2006; 2:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol 1998; 153:1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramadori G, Rasokat H, Burger R, Meyer Zum Buschenfelde KH, Bitter‐Suermann D. Quantitative determination of complement components produced by purified hepatocytes. Clin Exp Immunol 1984; 55:189–96. [PMC free article] [PubMed] [Google Scholar]
- 9. Mogilenko DA, Kudriavtsev IV, Trulioff AS et al Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll‐like receptor 4 activation in human macrophages. J Biol Chem 2012; 287:5954–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz Y, Strunk RC. Synovial fibroblast‐like cells synthesize seven proteins of the complement system. Arthritis Rheum 1988; 31:1365–70. [DOI] [PubMed] [Google Scholar]
- 11. Warren HB, Pantazis P, Davies PF. The third component of complement is transcribed and secreted by cultured human endothelial cells. Am J Pathol 1987; 129:9–13. [PMC free article] [PubMed] [Google Scholar]
- 12. Zahedi R, Braun M, Wetsel RA et al The C5a receptor is expressed by human renal proximal tubular epithelial cells. Clin Exp Immunol 2000; 121:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levi‐Strauss M, Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol 1987; 139:2361–6. [PubMed] [Google Scholar]
- 14. Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem 1992; 267:12736–41. [PubMed] [Google Scholar]
- 15. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol 2009; 46:2753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20:34–50. [DOI] [PubMed] [Google Scholar]
- 17. Toapanta FR, Ross TM. Complement‐mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol Res 2006; 36:197–210. [DOI] [PubMed] [Google Scholar]
- 18. Wills‐Karp M. Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc 2007; 4:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol Res 2005; 33:103–12. [DOI] [PubMed] [Google Scholar]
- 20. Khan MA, Maasch C, Vater A et al Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci USA 2013; 110:6061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan MA, Nicolls MR, Surguladze B, Saadoun I. Complement components as potential therapeutic targets for asthma treatment. Respir Med 2014; 108:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010; 11:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krych‐Goldberg M, Atkinson JP. Structure–function relationships of complement receptor type 1. Immunol Rev 2001; 180:112–22. [DOI] [PubMed] [Google Scholar]
- 24. Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 2012; 32:463–88. [DOI] [PubMed] [Google Scholar]
- 25. Mills CD, Lenz LL, Ley K. Macrophages at the fork in the road to health or disease. Front Immunol 2015; 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchetti V, Yanes O, Aguilar E et al Differential macrophage polarization promotes tissue remodeling and repair in a model of ischemic retinopathy. Sci Rep 2011; 1:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang X, Khan MA, Tian W et al Adenovirus‐mediated HIF‐1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest 2011; 121:2336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang X, Hsu JL, Tian W et al Tie2‐dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus . J Mol Med 2013; 91:1081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seya T, Hara T, Matsumoto M, Sugita Y, Akedo H. Complement‐mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J Exp Med 1990; 172:1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa K, Tamari M, Shao C et al Variations in the C3, C3a receptor, and C5 genes affect susceptibility to bronchial asthma. Hum Genet 2004; 115:295–301. [DOI] [PubMed] [Google Scholar]
- 31. Humbles AA, Lu B, Nilsson CA et al A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature 2000; 406:998–1001. [DOI] [PubMed] [Google Scholar]
- 32. Kohl J, Wills‐Karp M. A dual role for complement in allergic asthma. Curr Opin Pharmacol 2007; 7:283–9. [DOI] [PubMed] [Google Scholar]
- 33. Banda NK, Hyatt S, Antonioli AH et al Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody‐induced arthritis in mice. J Immunol 2012; 188:1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Vries B, Walter SJ, Peutz‐Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose‐binding lectin‐pathway is involved in complement activation in the course of renal ischemia–reperfusion injury. Am J Pathol 2004; 165:1677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moller‐Kristensen M, Wang W, Ruseva M et al Mannan‐binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol 2005; 61:426–34. [DOI] [PubMed] [Google Scholar]
- 36. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438:967–74. [DOI] [PubMed] [Google Scholar]
- 37. Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol 2001; 158:2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walsh DA, Haywood L. Angiogenesis: a therapeutic target in arthritis. Curr Opin Investig Drugs 2001; 2:1054–63. [PubMed] [Google Scholar]
- 39. Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 2010; 65:946–58. [DOI] [PubMed] [Google Scholar]
- 40. Khan MA, Jiang X, Dhillon G et al CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants. Circ Res 2011; 109:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babu AN, Murakawa T, Thurman JM et al Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest 2007; 117:3774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Granger DN, Senchenkova E. Inflammation and the microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 43. Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res 2010; 31:158–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol 2011; 3:a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev 2011; 25:2559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaminski M, Auerbach R. Angiogenesis induction by CD‐4 positive lymphocytes. Proc Soc Exp Biol Med 1988; 188:440–3. [DOI] [PubMed] [Google Scholar]
- 47. Torres PF, De Vos AF, van der Gaag R, Martins B, Kijlstra A. Cytokine mRNA expression during experimental corneal allograft rejection. Exp Eye Res 1996; 63:453–61. [DOI] [PubMed] [Google Scholar]
- 48. Qazi Y, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol 2013; 2013:006:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ho EK, Vlad G, Vasilescu ER et al Pre‐ and posttransplantation allosensitization in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol 2011; 72:5–10. [DOI] [PubMed] [Google Scholar]
- 50. Atkinson C, Song H, Lu B et al Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest 2005; 115:2444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Atkinson C, Zhu H, Qiao F et al Complement‐dependent P‐selectin expression and injury following ischemic stroke. J Immunol 2006; 177:7266–74. [DOI] [PubMed] [Google Scholar]
- 52. Qiao F, Atkinson C, Song H, Pannu R, Singh I, Tomlinson S. Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am J Pathol 2006; 169:1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Song H, Qiao F, Atkinson C, Holers VM, Tomlinson S. A complement C3 inhibitor specifically targeted to sites of complement activation effectively ameliorates collagen‐induced arthritis in DBA/1J mice. J Immunol 2007; 179:7860–7. [DOI] [PubMed] [Google Scholar]
- 54. Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low‐dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol 2008; 180:1231–8. [DOI] [PubMed] [Google Scholar]
- 55. Mor F, Quintana FJ, Cohen IR. Angiogenesis‐inflammation cross‐talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol 2004; 172:4618–23. [DOI] [PubMed] [Google Scholar]
- 56. Polverini PJ. Role of the macrophage in angiogenesis‐dependent diseases. EXS 1997; 79:11–28. [DOI] [PubMed] [Google Scholar]
- 57. Giraudo E, Primo L, Audero E et al Tumor necrosis factor‐alpha regulates expression of vascular endothelial growth factor receptor‐2 and of its co‐receptor neuropilin‐1 in human vascular endothelial cells. J Biol Chem 1998; 273:22128–35. [DOI] [PubMed] [Google Scholar]
- 58. Booth AJ, Bishop DK. TGF‐beta, IL‐6, IL‐17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection. Immunotherapy 2010; 2:511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malmstrom NK, Kallio EA, Rintala JM et al Vascular endothelial growth factor in chronic rat allograft nephropathy. Transpl Immunol 2008; 19:136–44. [DOI] [PubMed] [Google Scholar]
- 60. De Bandt M, Ben Mahdi MH, Ollivier V et al Blockade of vascular endothelial growth factor receptor I (VEGF‐RI), but not VEGF‐RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol 2003; 171:4853–9. [DOI] [PubMed] [Google Scholar]
- 61. Nozaki M, Raisler B, Sakurai JE et al Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA 2006; 103:2328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bora PS, Sohn JH, Cruz JM et al Role of complement and complement membrane attack complex in laser‐induced choroidal neovascularization. J Immunol 2005; 174:491–7. [DOI] [PubMed] [Google Scholar]
- 63. Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB. GM‐CSF induces expression of soluble VEGF receptor‐1 from human monocytes and inhibits angiogenesis in mice. Immunity 2004; 21:831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rajakumar A, Michael HM, Rajakumar PA et al Extra‐placental expression of vascular endothelial growth factor receptor‐1, (Flt‐1) and soluble Flt‐1 (sFlt‐1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 2005; 26:563–73. [DOI] [PubMed] [Google Scholar]
- 65. Suzuki H, Onishi H, Wada J et al VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol 2010; 40:197–203. [DOI] [PubMed] [Google Scholar]
- 66. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin through nuclear factor‐kappa B activation in endothelial cells. J Biol Chem 2001; 276:7614–20. [DOI] [PubMed] [Google Scholar]
- 67. Boulday G, Haskova Z, Reinders ME, Pal S, Briscoe DM. Vascular endothelial growth factor‐induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN‐gamma‐inducible protein of 10 kDa in vitro and in vivo . J Immunol 2006; 176:3098–107. [DOI] [PubMed] [Google Scholar]
- 68. Diaz‐Flores L, Gutierrez R, Madrid JF et al Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009; 24:909–69. [DOI] [PubMed] [Google Scholar]
- 69. Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res 2010; 180:103–14. [DOI] [PubMed] [Google Scholar]
- 70. Simonavicius N, Robertson D, Bax DA, Jones C, Huijbers IJ, Isacke CM. Endosialin (CD248) is a marker of tumor‐associated pericytes in high‐grade glioma. Mod Pathol 2008; 21:308–15. [DOI] [PubMed] [Google Scholar]
- 71. Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis 2003; 6:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tomkowicz B, Rybinski K, Sebeck D et al Endosialin/TEM‐1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther 2010; 9:908–15. [DOI] [PubMed] [Google Scholar]
- 73. Kawakami T, Mimura I, Shoji K, Tanaka T, Nangaku M. Hypoxia and fibrosis in chronic kidney disease: crossing at pericytes. Kidney Int Suppl 2014; 4:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Keskin D, Kim J, Cooke VG et al Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin‐2. Cell Rep 2015; 10:1066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li Y, Smith D, Li Q et al Antibody‐mediated retinal pericyte injury: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci 2012; 53:5520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 2007; 171:715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Langer HF, Chung KJ, Orlova VV et al Complement‐mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood 2010; 116:4395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Campa C, Costagliola C, Incorvaia C et al Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. Mediators of Inflammation 2010; 2010:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Detmar M, Brown LF, Schon MP et al Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998; 111:1–6. [DOI] [PubMed] [Google Scholar]
- 80. Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillières Best Pract Res Clin Obstet Gynaecol 2000; 14:883–900. [DOI] [PubMed] [Google Scholar]
- 81. Lou YL, Guo F, Liu F et al miR‐210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem 2012; 370:45–51. [DOI] [PubMed] [Google Scholar]
- 82. Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 2011; 27:563–84. [DOI] [PubMed] [Google Scholar]
- 83. Sweigard JH, Yanai R, Gaissert P et al The alternative complement pathway regulates pathological angiogenesis in the retina. FASEB J 2014; 28:3171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. White F, Nakatani C, Nimmo YL, Bloor CM. Compensatory angiogenesis during progressive right ventricular hypertrophy. Am J Cardiovasc Pathol 1992; 4:51–68. [PubMed] [Google Scholar]
- 85. Magro CM, Abbas AE, Seilstad K, Pope‐Harman AL, Nadasdy T, Ross Jr P. C3d and the septal microvasculature as a predictor of chronic lung allograft dysfunction. Hum Immunol 2006; 67:274–83. [DOI] [PubMed] [Google Scholar]
- 86. Westall GP, Snell GI. Antibody‐mediated rejection in lung transplantation: fable, spin, or fact? Transplantation 2014; 98:927–30. [DOI] [PubMed] [Google Scholar]
- 87. Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM‐1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci 2010; 51:5336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bossi F, Tripodo C, Rizzi L et al C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci USA 2014; 111:4209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Naesens M, Li L, Ying L et al Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol 2009; 20:1839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sacks S, Lee Q, Wong W, Zhou W. The role of complement in regulating the alloresponse. Curr Opin Organ Transplant 2009; 14:10–5. [DOI] [PubMed] [Google Scholar]
- 91. Sacks SH, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol 2003; 15:487–92. [DOI] [PubMed] [Google Scholar]
- 92. Strainic MG, Liu J, Huang D et al Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008; 28:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang X, Lewkowich IP, Kohl G, Clark JR, Wills‐Karp M, Kohl J. A protective role for C5a in the development of allergic asthma associated with altered levels of B7‐H1 and B7‐DC on plasmacytoid dendritic cells. J Immunol 2009; 182:5123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang X, Schmudde I, Laumonnier Y et al A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol 2010; 185:6741–52. [DOI] [PubMed] [Google Scholar]
- 95. Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell‐expressed C5aR to enhance effector T‐cell expansion by limiting antigen‐induced apoptosis. Blood 2008; 112:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weaver Jr DJ, Reis ES, Pandey MK et al C5a receptor‐deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol 2010; 40:710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell‐derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant 2013; 13:2530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cravedi P, Heeger PS. Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest 2014; 124:2348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. van der Touw W, Cravedi P, Kwan WH, Paz‐Artal E, Merad M, Heeger PS. Cutting edge: receptors for C3a and C5a modulate stability of alloantigen‐reactive induced regulatory T cells. J Immunol 2013; 190:5921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF‐beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol 2013; 14:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu G, Ma H, Qiu L et al Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol 2011; 89:130–42. [DOI] [PubMed] [Google Scholar]
- 102. Yu X, Li H, Ren X. Interaction between regulatory T cells and cancer stem cells. Int J Cancer 2012; 131:1491–8. [DOI] [PubMed] [Google Scholar]
- 103. Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol 1994; 55:410–22. [DOI] [PubMed] [Google Scholar]
- 104. Kobayashi S, Nagaura T, Kimura I, Kimura M. Interferon‐gamma‐activated macrophages enhance angiogenesis from endothelial cells of rat aorta. Immunopharmacology 1994; 27:23–30. [DOI] [PubMed] [Google Scholar]
- 105. Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol 2011; 55:495–503. [DOI] [PubMed] [Google Scholar]
- 106. Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 2008; 84:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Redente EF, Dwyer‐Nield LD, Merrick DT et al Tumor progression stage and anatomical site regulate tumor‐associated macrophage and bone marrow‐derived monocyte polarization. Am J Pathol 2010; 176:2972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schrader J, Herkel J. Chronic liver inflammation dominated by interferon‐gamma can prevent hepatocarcinogenesis. OncoImmunology 2012; 1:222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gordon S, Taylor PR . Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953–64. [DOI] [PubMed] [Google Scholar]
- 110. Schindler R, Gelfand JA, Dinarello CA . Recombinant C5a stimulates transcription rather than translation of interleukin‐1 (IL‐1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL‐1 itself. Blood 1990; 76:1631–8. [PubMed] [Google Scholar]
- 111. Ferguson TA, Apte RS . Angiogenesis in eye disease: immunity gained or immunity lost? Semin Immunopathol 2008; 30:111–9. [DOI] [PubMed] [Google Scholar]
- 112. Tidball JG . Mechanisms of muscle injury, repair, and regeneration. Compr Physiol 2011; 1:2029–62. [DOI] [PubMed] [Google Scholar]
- 113. Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 2012; 35:369–89. [DOI] [PubMed] [Google Scholar]
- 114. Rutkowski MJ, Sughrue ME, Kane AJ, Ahn BJ, Fang S, Parsa AT. The complement cascade as a mediator of tissue growth and regeneration. Inflamm Res 2010; 59:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Fang S, Parsa AT . Complement and the central nervous system: emerging roles in development, protection and regeneration. Immunol Cell Biol 2010; 88:781–6. [DOI] [PubMed] [Google Scholar]
- 116. Kyurkchiev D, Bochev I, Ivanova‐Todorova E et al Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 2014; 6:552–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res 2010; 8:1453–65. [DOI] [PubMed] [Google Scholar]
- 118. Crisan M, Yap S, Casteilla L et al A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3:301–13. [DOI] [PubMed] [Google Scholar]
- 119. Stegall MD, Diwan T, Raghavaiah S et al Terminal complement inhibition decreases antibody‐mediated rejection in sensitized renal transplant recipients. Am J Transplant 2011; 11:2405–13. [DOI] [PubMed] [Google Scholar]
- 120. Atkinson C, He S, Morris K et al Targeted complement inhibitors protect against posttransplant cardiac ischemia and reperfusion injury and reveal an important role for the alternative pathway of complement activation. J Immunol 2010; 185:7007–13. [DOI] [PubMed] [Google Scholar]
- 121. Keshavjee S, Davis RD, Zamora MR, de Perrot M, Patterson GA. A randomized, placebo‐controlled trial of complement inhibition in ischemia‐reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg 2005; 129:423–8. [DOI] [PubMed] [Google Scholar]
- 122. Swain SM, Kim SB, Cortes J et al Pertuzumab, trastuzumab, and docetaxel for HER2‐positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol 2013; 14:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev 1998; 50:59–87. [PubMed] [Google Scholar]
- 124. Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH. Effects of complement inhibition with soluble complement receptor‐1 on vascular injury and inflammation during renal allograft rejection in the rat. Am J Pathol 1996; 149:2055–66. [PMC free article] [PubMed] [Google Scholar]
- 125. Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH. Allograft immune response with sCR1 intervention. Transpl Immunol 1996; 4:72–5. [DOI] [PubMed] [Google Scholar]
- 126. Jordan SC, Reinsmoen N, Peng A et al Advances in diagnosing and managing antibody‐mediated rejection. Pediatr Nephrol 2010; 25:2035–45; quiz 2045–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody‐mediated rejection in kidney transplantation. Nat Rev Nephrol 2012; 8:670–8. [DOI] [PubMed] [Google Scholar]
- 128. de Vries B, Kohl J, Leclercq WK et al Complement factor C5a mediates renal ischemia‐reperfusion injury independent from neutrophils. J Immunol 2003; 170:3883–9. [DOI] [PubMed] [Google Scholar]
- 129. Jordan SC, Reinsmoen N, Lai CH, Vo A. Novel immunotherapeutic approaches to improve rates and outcomes of transplantation in sensitized renal allograft recipients. Discov Med 2012; 13:235–45. [PubMed] [Google Scholar]
- 130. Elvington A, Atkinson C, Zhu H et al The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J Immunol 2012; 189:4640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hoehlig K, Maasch C, Shushakova N et al A novel C5a‐neutralizing mirror‐image (l‐)aptamer prevents organ failure and improves survival in experimental sepsis. Mol Ther 2013; 21:2236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Danobeitia JS, Djamali A, Fernandez LA. The role of complement in the pathogenesis of renal ischemia‐reperfusion injury and fibrosis. Fibrogenesis Tissue Repair 2014; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jiang X, Sung YK, Tian W, Qian J, Semenza GL, Nicolls MR. Graft microvascular disease in solid organ transplantation. J Mol Med 2014; 92:797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am J Pathol 2003; 162:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lehmann TG, Koeppel TA, Kirschfink M et al Complement inhibition by soluble complement receptor type 1 improves microcirculation after rat liver transplantation. Transplantation 1998; 66:717–22. [DOI] [PubMed] [Google Scholar]
- 136. Damman J, Schuurs TA, Ploeg RJ, Seelen MA. Complement and renal transplantation: from donor to recipient. Transplantation 2008; 85:923–7. [DOI] [PubMed] [Google Scholar]
- 137. Li JS, Jaggers J, Anderson PA. The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev Cardiovasc Ther 2006; 4:649–54. [DOI] [PubMed] [Google Scholar]
- 138. Amsterdam EA, Stahl GL, Pan HL, Rendig SV, Fletcher MP, Longhurst JC. Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol 1995; 268:H448–57. [DOI] [PubMed] [Google Scholar]
- 139. Vakeva A, Meri S. Complement activation and regulator expression after anoxic injury of human endothelial cells. APMIS 1998; 106:1149–56. [DOI] [PubMed] [Google Scholar]
- 140. Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti‐C5 therapy. Circulation 1998; 97:2259–67. [DOI] [PubMed] [Google Scholar]
- 141. Thurman JM, Royer PA, Ljubanovic D et al Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol 2006; 17:707–15. [DOI] [PubMed] [Google Scholar]
- 142. Zheng X, Zhang X, Sun H et al Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation 2006; 82:1781–6. [DOI] [PubMed] [Google Scholar]
- 143. Davis AE III, Lu F, Mejia P. C1 inhibitor, a multi‐functional serine protease inhibitor. Thromb Haemost 2010; 104:886–93. [DOI] [PubMed] [Google Scholar]
- 144. Frank MM. Recombinant and plasma‐purified human c1 inhibitor for the treatment of hereditary angioedema. World Allergy Organ J 2010; 3:S29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Castellano G, Melchiorre R, Loverre A et al Therapeutic targeting of classical and lectin pathways of complement protects from ischemia–reperfusion‐induced renal damage. Am J Pathol 2010; 176:1648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Curci C, Castellano G, Stasi A et al Endothelial‐to‐mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transplant 2014; 29:799–808. [DOI] [PubMed] [Google Scholar]
- 147. Tillou X, Poirier N, Le Bas‐Bernardet S et al Recombinant human C1‐inhibitor prevents acute antibody‐mediated rejection in alloimmunized baboons. Kidney Int 2010; 78:152–9. [DOI] [PubMed] [Google Scholar]
- 148. Sommer W, Tudorache I, Kuhn C et al C1‐esterase‐inhibitor for primary graft dysfunction in lung transplantation. Transplantation 2014; 97:1185–91. [DOI] [PubMed] [Google Scholar]
- 149. Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol 2008; 181:8068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stahl GL, Xu Y, Hao L et al Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol 2003; 162:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol 2003; 40:109–23. [DOI] [PubMed] [Google Scholar]
- 152. Fischetti F, Tedesco F. Cross‐talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity 2006; 39:417–28. [DOI] [PubMed] [Google Scholar]
- 153. Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity. Immunol Res 2012; 54:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pekkarinen PT, Vaali K, Junnikkala S et al A functional complement system is required for normal T helper cell differentiation. Immunobiology 2011; 216:737–43. [DOI] [PubMed] [Google Scholar]
- 155. Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia‐reperfusion damage. Ann Med 2012; 44:205–17. [DOI] [PubMed] [Google Scholar]
- 156. Chen G, Chen S, Chen X. Role of complement and perspectives for intervention in transplantation. Immunobiology 2013; 218:817–27. [DOI] [PubMed] [Google Scholar]


