Summary
Vitamin A supports the induction of immunoglobulin (Ig)A responses at mucosal surfaces in mice, but much less is known about the influence of vitamins on antibody isotype expression in humans. To address this knowledge gap, we examined 46 residual blood samples from adults and children, some of whom were experiencing influenza virus infections of the respiratory tract. Assays were performed for retinol binding protein (RBP, a surrogate for vitamin A), vitamin D (a related vitamin) and antibody isotypes. Results showed that all but two tested samples exhibited RBP and/or vitamin D insufficiencies or deficiencies. Vitamin D correlated with blood IgM and IgG3, while RBP correlated with IgG4 and IgA. RBP also correlated positively with age and with influenza virus‐specific antibody neutralization titres. Individuals with low blood RBP levels exhibited the highest frequencies of over‐expressed cytokines and growth factors in nasal wash samples, an indication of inflamed mucosal tissues. While cause–effect relationships were not discerned, results support a hypothesis that vitamins directly influence B cell isotype expression in humans, and by so doing may help protect mucosal surfaces from respiratory viral disease.
Keywords: antibodies, B cell, cytokines, host–pathogen interactions, viral
Introduction
The World Health Organization estimates that 250 million preschool children are deficient for vitamin A and that 1 billion people worldwide are deficient for vitamin D. Each of these deficiencies, now known to affect populations in both developed and developing countries, associate with a wide variety of health‐care concerns, including vulnerability to respiratory pathogens 1, 2, 3, 4.
Murine studies demonstrate that vitamins A and D are critical for healthy immune responses at mucosal surfaces 5. The vitamins are inter‐related as they differentially bind heterodimeric receptors (e.g. RAR‐RXR; VDR‐RXR) that, in turn, influence the expression of immune response genes. Immunoglobulin (Ig)A, a first line of defence against mucosal pathogens, is particularly dependent upon vitamin A in mice 6, 7, 8, 9. IgA is well suited for protection of the upper respiratory tract mucosa, because it can be shuttled to the lumen by transcytosis through epithelial cells, chaperoned by the poly‐Ig receptor. Following transcytosis, secretory component is cleaved so that IgA can be released in soluble form into mucosal secretions or tethered onto the airway lining. The plethora of IgA functions include antigen binding, virus neutralization, antibody‐dependent cell‐mediated cytotoxicity and modulation of cytokine release by innate immune effectors 10. In transfer studies, polymeric IgA traffics to the upper respiratory tract airway more effectively than IgG1 11, 12, 13. Early capture and destruction of pathogens in the upper respiratory tract by antibodies can prevent trafficking to the lung and thereby prevent serious lower respiratory tract disease.
Given that: (i) vitamin deficiencies correlate with IgA reduction in mice, (ii) IgA serves as a first line of defence against mucosal pathogens and (iii) humans with vitamin deficiencies are vulnerable to mucosal infections, we sought to define direct correlations between vitamin levels and antibody isotype patterns in humans. Results show that human blood levels of vitamins A and D correlate with antibody isotypes and isotype ratios.
Materials and methods
Sample source
Blood samples were collected from two sources in Memphis, TN (Table 1). First, residual plasma were available from a previously described, Institutional Review Board‐approved study of adults and children with acute influenza virus infections (index cases) and their asymptomatic household contacts (n = 21) 14. Informed consent was received from all study participants. This source will be referred to as the ‘Influenza Virus Study’. Secondly, adult sera (n = 25) were available from the Tennessee Blood Services (TBS) in Memphis, TN. All personal identifiers were removed from samples prior to use.
Table 1.
Human blood samples tested for vitamins, antibodies and nasal wash cytokines
| Label | Age (years) | Contact/index flu type (day) | RBP (ng/ml) | Vit D (ng/ml) | IgG1‡ | IgA‡ | Flu Nab titre | +Nasal Cyt>2X |
|---|---|---|---|---|---|---|---|---|
| Influenza Virus Study | ||||||||
| F5028C21 | 7 | FP contact‐H1 (7) | 5933 | 1 | 110 | 20 | 20 | 9 |
| F5030C29 | 22 | Neg contact (0) | 9698 | 16 | 289 | 50 | <10 | 3 |
| F5006C05 | 19 | Neg contact (24) | 11 952 | 6 | 453 | 154 | 10 | 4 |
| F5001* | 12 | Index‐H1 (0) | 15 228 | 24 | 568 | 84 | <10 | 32 |
| F5004 | 29 | Index‐H1 (0) | 17 670 | 1 | 462 | 104 | <10 | 29 |
| F5012C10 | 19 | Neg contact (0) | 18 463 | 8 | 433 | 87 | 40 | 11 |
| F5020C17 | 70 | FP contact‐H1 (0) | 18 623 | 6 | 409 | 122 | 10 | 10 |
| F5011 | 30 | Index‐H1 (0) | 18 862 | 6 | 366 | 66 | <10 | 13 |
| F5021† | 12 | Index‐H1(0) | 19 101 | 14 | 514 | 86 | <10 | 25 |
| F5001* | 12 | Index‐H1 (25) | 19 821 | 22 | 674 | 76 | 10 | 25 |
| F5013C10 | 21 | CV contact‐BA (0) | 20 383 | 10 | 421 | 92 | 40 | 10 |
| F5009C08 | 30 | CV contact‐H1 (0) | 20 463 | 6 | 348 | 89 | 10 | 9 |
| F5015C14 | 26 | Neg contact (0) | 21 999 | 27 | 223 | 43 | 40 | 5 |
| F5014 | 8 | Index‐H1 (0) | 22 732 | n/a | 594 | 41 | <10 | 4 |
| F5021† | 12 | Index‐H1 (32) | 25 286 | 14 | 519 | 79 | 80 | 8 |
| F5018C17 | 25 | Neg contact (0) | 28 740 | 13 | 340 | 96 | 80 | 9 |
| F5002C01 | 30 | Neg contact (0) | 30 373 | 8 | 842 | 322 | 80 | 4 |
| F5040C39 | 33 | FP contact‐H1 (0) | 35 770 | 10 | 369 | 137 | 20 | 6 |
| F5022C21 | 32 | CV contact–H1 (0) | 35 861 | 1 | 260 | 147 | 40 | 0 |
| F5019C17 | 43 | Neg contact (0) | 50 676 | 17 | 170 | 99 | 10 | 3 |
| F5052C51 | 38 | Neg contact (0) | 56 431 | 9 | 476 | 175 | 40 | 2 |
| Tennessee Blood Services samples | ||||||||
| R242680 | 27 | – | 17 071 | 6 | 414 | 91 | n.d. | n.d. |
| R275699 | 45 | – | 21 428 | 20 | 667 | 283 | n.d. | n.d. |
| R275708 | 22 | – | 21 428 | 14 | 355 | 69 | n.d. | n.d. |
| R242682 | 38 | – | 22 370 | 11 | 401 | 52 | n.d. | n.d. |
| R275696 | 37 | – | 22 375 | 16 | 369 | 127 | n.d. | n.d. |
| R275693 | 32 | – | 22 440 | 26 | 505 | 133 | n.d. | n.d. |
| R242676 | 28 | – | 22 605 | 19 | 563 | 131 | n.d. | n.d. |
| R275704 | 22 | – | 23 088 | 24 | 224 | 28 | n.d. | n.d. |
| R242688 | 30 | – | 24 591 | 9 | 525 | 157 | n.d. | n.d. |
| R242683 | 39 | – | 25 238 | 6 | 358 | 55 | n.d. | n.d. |
| R275703 | 31 | – | 26 678 | 19 | 517 | 157 | n.d. | n.d. |
| R275709 | 56 | – | 27 342 | 15 | 610 | 287 | n.d. | n.d. |
| R242675 | 27 | – | 27 632 | 13 | 1031 | 104 | n.d. | n.d. |
| R275700 | 63 | – | 28 454 | 36 | 607 | 81 | n.d. | n.d. |
| R275702 | 44 | – | 28 488 | 28 | 305 | 113 | n.d. | n.d. |
| R242674 | 58 | – | 31 871 | 16 | 387 | 245 | n.d. | n.d. |
| R242677 | 40 | – | 32 049 | 19 | 921 | 196 | n.d. | n.d. |
| R275695 | 45 | – | 32 132 | 28 | 511 | 186 | n.d. | n.d. |
| R242678 | 59 | – | 32 859 | 14 | 313 | 243 | n.d. | n.d. |
| R275698 | 41 | – | 33 779 | 11 | 270 | 56 | n.d. | n.d. |
| R275697 | 39 | – | 34 035 | 45 | 484 | 148 | n.d. | n.d. |
| R275705 | 33 | – | 37 487 | 18 | 358 | 133 | n.d. | n.d. |
| R275694 | 39 | – | 42 198 | 27 | 329 | 66 | n.d. | n.d. |
| R242679 | 56 | – | 55 550 | 20 | 640 | 197 | n.d. | n.d. |
| R275692 | 48 | – | 65 146 | 14 | 337 | 317 | n.d. | n.d. |
Retinol bonding protein (RBP) and vitamin D levels were tested for 21 plasma samples from the Influenza Virus Study and 25 serum samples from the Tennessee Blood Services (Memphis, TN) Column 3 indicates the type of sample, type of virus infection and day of analysis. ‘Index’ was the person from a household who first received (on day 0) an influenza virus diagnosis (index case). ‘FP contact’ was a household contact of the index case who was also diagnosed on day 0 with an influenza virus infection. ‘CV contact’ was a household contact who was diagnosed with an influenza virus infection at some point after day 0, usually within a few days. ‘Neg contact’ was a household member who was never diagnosed with an influenza virus infection. The strain of influenza virus infection is noted (‘H1’ for H1N1 virus, ‘BA’ for apparent co‐infection with influenza B and influenza A viruses) as well as the study day that the sample was collected post‐enrolment. The day of collection is indicated relative to the day of index case entry into the study. ‘Flu NAb titre’ is the serum neutralizing antibody titre against influenza virus. Areas with dark background mark samples with vitamin A sufficiencies. Vitamin D values were scored as ‘1’ if vitamins were below detection in the enzyme‐linked immunosorbent assay (ELISA). +The numbers of cytokines, chemokines or growth factors per sample that scored more than twice the lowest detectable value in the series. When numbers were ≥10, they are shown in bold type. *†Two individuals were tested twice, once on day 0 and again approximately 1 month later. ‡Antibody isotype values (ng/ml) were from samples diluted 1 : 16 000, as per the manufacturer's instructions (Milliplex MAP human isotyping magnetic bead panel HGAMMAG‐301K); n/a = no available value. For statistical analyses, neutralization values of < 10 were given a score of 5; n.d. = not done or not applicable.
Vitamin tests
RBP was tested by enzyme‐linked immunosorbent assay (ELISA) [R&D Systems (Minneapolis, MN, USA) human RBP4 Quantikine ELISA kit] and used as a surrogate for retinol due to its superior stability profile (retinol is sensitive to light and temperature, factors that may confound analyses of residual blood samples). RBP shuttles retinol through the blood, generally existing at a 1 : 1 molar ratio 15. Vitamin D was tested in the Pathology Department at St Jude using the Roche Elecsys Vitamin D ELISA (Roche, Indianapolis, IN, USA) that measures 25‐hydroxylated metabolites of cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Cut‐offs for vitamin deficiencies and insufficiencies were based on Institute of Medicine standards and practice guidelines of the US Endocrine Society (vitamin A deficiencies: ≤0·7 μM or ≤16 000 ng/ml RBP; vitamin A insufficiencies: >0·7 to ≤1·05 μM or >16 000 to ≤22 000 ng/ml RBP; vitamin D deficiencies: <20 ng/ml 25(OH)D; vitamin D insufficiencies: ≥20 to <30 ng/ml 25(OH)D). When vitamin D levels were below detection, they were given a value of 1.00 for numerical analyses. For one sample, the vitamin D level was not determined.
Cytokines, growth factors and antibody tests
Cytokines and growth factors were tested in nasal washes (NW) and plasma samples from individuals in the Influenza Virus Study using a Milliplex MAP human cytokine/chemokine immunoassay (Millipore, St Charles, MO, USA) in a Luminex®xMAP™ system according to the manufacturer's protocol. The following cytokines/chemokines and growth factors were measured: epidermal growth factor (EGF), eotaxin, fibroblast growth factor (FGF)‐2, Fms‐like tyrosine kinase 3 (Flt‐3) ligand, fractalkine, granulocyte–colony‐stimulating factor (G‐CSF), granulocyte–macrophage colony‐stimulating factor (GM‐CSF), growth regulated oncogene (GRO), interferon (IFN)‐α2, IFN‐γ, interleukin (IL)‐10, IL‐12(p40), IL‐12(p70), IL‐13, IL‐15, IL‐17, IL‐1Rα, IL‐1α, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐9, IFN‐γ‐induced protein 10 (IP‐10), monocyte chemoattractant protein (MCP)‐1, MCP‐3, macrophage‐derived chemokine (MDC) (CCL22), macrophage inflammatory protein 1 (MIP‐1)α, MIP‐1β, transforming growth factor (TGF)‐α, tumour necrosis factor (TNF)‐α, TNF‐β, vascular endothelial growth factor (VEGF), sCD40L and sIL‐2Rα. Three additional factors were measured using NW: platelet‐derived growth factor (PDGF)‐AA, PDGF‐AB/BB and regulated upon activation normal T cell expressed and secreted (RANTES). If a sample scored below or above the standard curve, it was assigned the curve's lowest or highest detectable value respectively for graphing and statistical evaluations.
Antibody isotypes were tested from blood samples from both the Influenza Virus Study and TBS. The Milliplex® MAP human isotyping magnetic bead panel‐isotyping multiplex assay (HGAMMAG‐301K) was used. Blood samples were diluted 1 : 16 000 prior to isotype assays as per the manufacturer's instructions; concentrations are reported for the diluted samples. If a sample scored below or above the standard curve, it was respectively assigned the curve's lowest or highest detectable value for graphing and statistical evaluations.
Microneutralization assay
Plasma were tested against the A(H1N1)pdm09 strain in the reverse genetic 6 + 2 backbone (rg‐A/Tennessee/1‐560/2009), the circulating H1N1 strain. Briefly, plasma were heat‐inactivated at 56°C and serially diluted with a starting dilution of 1 : 10. Diluted samples were incubated with 100 tissue culture infectious doses‐50 (TCID50) of the virus for 1 h at 37°C. After incubation, the plasma–virus mixture was transferred to Madin Darby canine kidney (MDCK) cells preseeded in a 96‐well plate. After 3 days, 50 µl of culture supernatant were tested for the presence of virus using a haemagglutination assay with 0·5% turkey red blood cells. The antibody titre was defined as the reciprocal of the highest dilution that resulted in inhibition of virus infection. A score of 5 was given for statistical evaluations if neutralizing activity was not detected.
Statistical methods
Spearman's rank correlation was applied to evaluate the correlation of RBP and vitamin D with antibody isotypes, neutralizing antibodies and cytokines/chemokines/growth factors. To assess the impact of age on correlations of interest, Spearman's rank correlation coefficients were estimated by dividing the patient sample into young and old groups at the median age (31 years for the whole population and 25 years for the Influenza Virus Study). The Wilcoxon–Mann–Whitney test was used to compare cytokine levels between RBP sufficient and insufficient/deficient individuals. Statistical analyses did not adjust for multiple comparisons; a P‐value of 0·05 or less was considered significant.
Results
Low levels of vitamins A and D in children and adults in Memphis, TN
RBP levels (surrogates for serum retinol 15) and vitamin D levels for 46 individuals in Memphis, TN were measured and results are shown in Table 1, with entries for samples from an Influenza Virus Study and from the TBS. Entries are listed in order of increasing RBP levels. In Fig. 1, measurements for RBP and vitamin D are plotted on an x–y axis. Vertical and horizontal lines indicate cut‐offs for vitamin deficiencies and insufficiencies. Among the 46 samples, one vitamin D level was not acquired. For the remaining samples, all but two exhibited deficient or insufficient levels for RBP, vitamin D or both. We found that RBP and vitamin D levels were correlated with each other (R S = 0·31, P = 0·04). We also observed a direct correlation between RBP and age (R S = 0·62, P < 0·0001).
Figure 1.
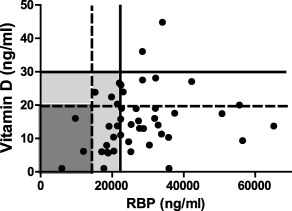
Retinol binding protein (RBP) comparisons with vitamin D and age. Comparisons were made between RBP (ng/ml) and vitamin D (ng/ml). Levels are shown for 45 samples and each symbol represents a different blood sample. One vitamin D sample was not evaluable among 46 tested samples. Cut‐offs for vitamin A (RBP) and D deficiencies (solid lines) and insufficiencies (dotted lines) are shown. Dark grey areas identify individuals with double deficiencies for RBP and vitamin D, and light grey areas identify individuals with insufficiencies for RBP, vitamin D or both.
High RBP concentrations associate with high levels of IgA and IgG4
Previous studies in vitamin A‐deficient (VAD) mice demonstrated a significant deficit in virus‐specific IgA antibody secreting cells and IgA antibodies compared to controls 7, 8, 9. To determine if vitamin A in humans (as measured by RBP) associated similarly with IgA production, we tested the 46 blood samples for IgM, IgG1, IgG2, IgG3, IgG4 and IgA. Numerical values for isotypes and isotype ratios were determined. Values for total IgG1 and IgA are shown for each individual in Table 1. We compared RBP levels with serum antibody isotype distributions and evaluated data using Spearman's rank correlation tests. Samples were examined within the whole population and also after stratification of young and old age groups (defined by the median of 31 years). As shown in Fig. 2, correlations were not significant in the age‐separated subsets, but when the population was evaluated as a whole, RBP correlated significantly with IgA (a) (P = 0·0009), IgA/IgM (b) (P = 0·03), IgA/IgG1 (c) (P = 0·0003) and IgG4 (d) (P = 0·0082). IgA also correlated with age, as expected based on previous reports 16, 17, 18 and based on our finding that RBP and age were correlated. As shown in Fig. 3, vitamin D correlated with IgM, IgG3, IgG3/IgG1 ratios and IgG3/IgA ratios.
Figure 2.
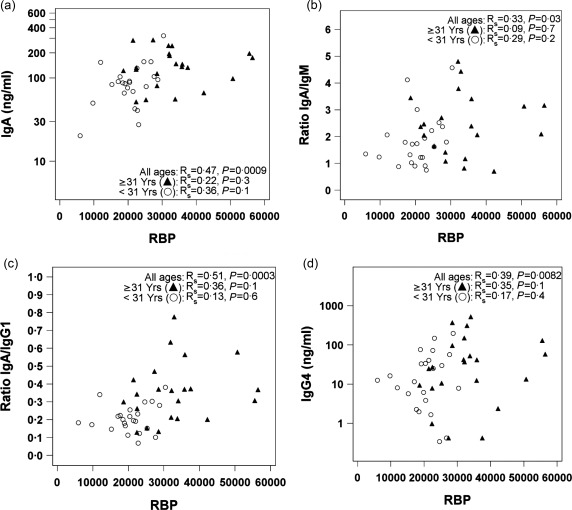
Retinol binding protein (RBP) comparisons with isotypes and isotype ratios. RBP levels (ng/ml) were plotted against isotypes or isotype ratios in individual panels as follows: (a) immunoglobulin (Ig)A, (b) IgA/IgM, (c) IgA/IgG1 and (d) IgG4. Blood samples were diluted 1 : 16 000 prior to isotype assays. Antibody concentrations are reported for the diluted samples in ng/ml. Statistical evaluations were with Spearman's rank correlation coefficients, estimated using combined data or with data from groups of younger or older individuals, subdivided by the median age (31 years).
Figure 3.
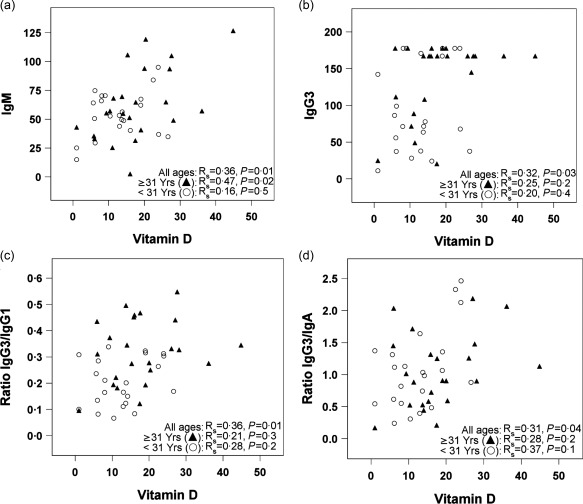
Vitamin D comparisons with isotypes and isotype ratios. Vitamin D levels (ng/ml) were plotted against isotypes or istoype ratios in individual panels as follows: (a) immunoglobulin (Ig)M, (b) IgG3, (c) IgG3/IgG1 and (d) IgG3/IgA. Blood samples were diluted 1 : 16 000 prior to isotype assays. Antibody concentrations are reported for the diluted samples in ng/ml. Statistical evaluations were with Spearman's rank correlation coefficients, estimated using combined data or with data from groups of younger or older individuals, subdivided by the median age (31 years).
To examine functional antibodies, influenza virus‐specific neutralization assays were performed on samples from the influenza virus study. Neutralization correlated significantly with RBP levels (Fig. 4, R S = 0·52, P = 0·015), but not with total IgG1 (R S = −0·09, P = 0·7) or total IgA (R S = 0·34, P = 0·13). Neutralization activities were probably a reflection of acute as well as historical exposures to influenza viruses. As shown in Table 1, not all individuals in this study had evidence of a recent infection, although they may have been exposed to influenza virus by contact with an infected household member.
Figure 4.
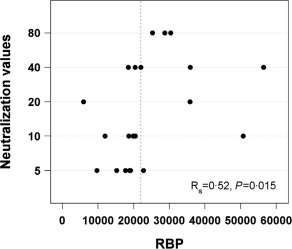
Retinol binding protein (RBP) association with neutralizing antibodies. Neutralizing antibody titres are shown relative to RBP (ng/ml). A score of 5 was used when the response was below detection.
Sufficient RBP associates with reduced cytokines in nasal passages
Because vitamin A‐deficient individuals are vulnerable to respiratory pathogens 7, 8, 9, and because respiratory tract disease is often indicated by increased cytokine and growth factor levels in the upper respiratory tract 14, 19, 20, 21, we asked if RBP levels correlated inversely with cytokine levels in NW samples. To address this question, we examined historical cytokine records for the 21 blood and NW samples from the Influenza Virus Study 14. Again, samples were grouped by age based on the median age for the 21 participants (median age = 25 years). When cytokine and growth factor levels were evaluated from blood, vitamin D, but not RBP, associated with increases in two cytokine levels, eotaxin (P = 0·01) and IL‐8 (P = 0·03). These correlations were each significant in younger, but not older study participants. When NW cytokines and growth factors were evaluated, negative correlations were observed with RBP, but not vitamin D. Cytokines and growth factors that were correlated negatively with RBP included PDGF‐AA (Fig. 5a), granulocyte–colony‐stimulating factor (G‐CSF) (Fig. 5b), IL‐1α (Fig. 5c), IP‐10 (Fig. 5d), EGF (Fig. 5e), MCP‐1 (Fig. 5f) and IL‐8 (not shown). These associations were also significant in older, but not younger individuals. Each sample from the Influenza Virus Study was scored for the number of NW cytokines or growth factors exceeding twice the lowest detectable value in the assay. As shown in Table 1, eight of the 13 individuals with insufficient/deficient levels of RBP exhibited 10 or more elevated cytokines or growth factors in NW samples, whereas such high numbers were not observed in any of the eight individuals with sufficient levels of RBP. Even when individuals with sufficient RBP were experiencing influenza virus infections at the time of sampling (F5014 and F5040C39 in Table 1), cytokine levels were relatively low in NW, perhaps indicative of healthier nasal tissues.
Figure 5.
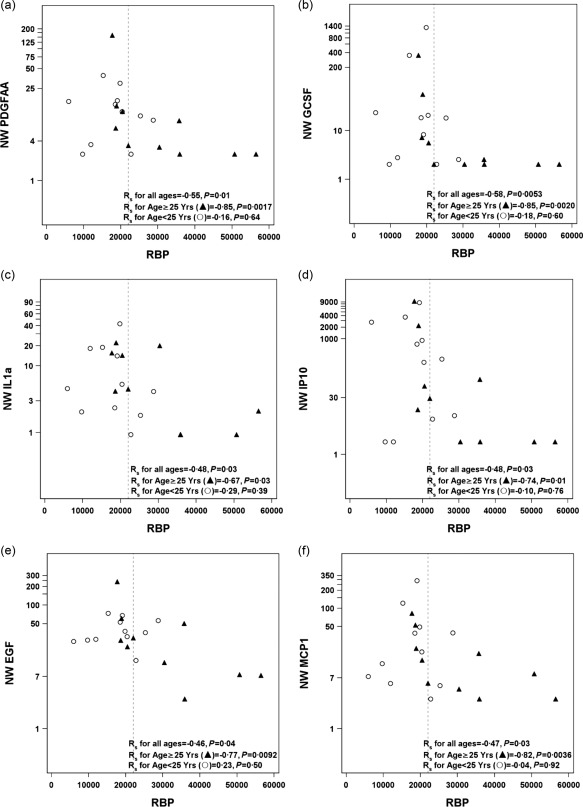
Retinol binding protein (RBP) association with nasal wash (NW) cytokines. RBP (ng/ml) associations with six NW cytokines/growth factors are shown. These were (a) platelet‐derived growth factor (PDGF)‐AA, (b) granulocyte‐colony stimulating factor (G‐CSF), (c) interleukin (IL)‐1α, (d) interferon gamma‐induced protein 10 (IP‐10), (e) epidermal growth factor (EGF) and (f) Monocyte chemoattractant protein‐1 (MCP‐1). Experiments were performed as per the manufacturer's instructions and then scored as pg/ml. When scores were below or above the standard curve, they were plotted with the assay's lowest or highest detectable values, respectively.
Discussion
Results in this report demonstrate relationships between RBP (a surrogate of vitamin A), vitamin D, antibody isotype patterns, neutralizing antibodies and NW cytokine and growth factor patterns in humans. Specifically, individuals with higher RBP levels exhibited higher levels of IgA, IgA/IgM, IgA/IgG1, IgG4 and virus‐specific neutralizing antibodies, and lower levels of NW cytokines and growth factors (indicative of healthy mucosal tissue). RBP also associated with age which, in turn, associated with increased IgA concentrations 16, 17, 18, 22. RBP correlated with vitamin D, and vitamin D correlated with IgM, IgG3, IgG3/IgG1 and IgG3/IgA. While cause–effect relationships could not be discerned in this human study, results from small‐animal studies support the hypothesis that vitamins directly influence isotype expression 7, 8, 9. Perhaps in humans, vitamins also instruct B cell development and the antibody isotype switch. Vitamin sufficiency may then support effective antibody function, effective virus control and mucosal tissue integrity.
What might be the mechanism for vitamin A and vitamin D association with antibody isotype expression patterns? There are multiple influences of vitamins on the immune system. Vitamins may support dendritic cell development necessary for antigen presentation, T cell activation and homing, B cell activation and division, B cell heavy chain switch rearrangement, maturation of B cells and/or the stabilization of end‐differentiated antibody‐producing cells. When focusing on the heavy chain class switch rearrangement (CSR) event, we note that functional CH genes are arranged in the order 5′‐Cµ, Cδ, Cγ3, Cγ1, Cα1, Cγ2, Cγ4, Cɛ, Cα2‐3′ along the locus. Because intervening DNA sequences are usually deleted during CSR, isotype switching will generally progress irreversibly in the 5′→3′ direction. B cells that express IgM and IgG3 at early stages of development (as may be prompted by vitamin D) have the potential to continue switching. However, once B cells express IgA or IgG4 (as may be prompted by vitamin A), continued CSR is limited due to deletion of upstream genes Cµ, Cδ, Cγ3 and Cγ1. Preferential expression of IgA and IgG4 relative to others over time may be a simple consequence of successive CSR. However, there are also environmental and developmental conditions that differentially influence the rearrangement apparatus 23, 24, 25, 26. Our previous in‐vitro studies showed that IgA production by stimulated B cells was enhanced by retinol in the presence of epithelial cells that expressed retinal dehydrogenase (RALDH) (necessary for metabolism of retinal to retinoic acid 6). This IgA expression was associated with up‐regulation of IL‐6 and GM‐CSF within the culture system, and was inhibited when cytokines were blocked. IL‐6, like vitamin A, is multi‐faceted in that it has a direct influence on IL‐6R‐bearing B cells, but will also influence B cells indirectly via T cell help and innate cell partners 27. Clearly, vitamin influences on CSR and stabilization of switched cells are multi‐faceted and warrant continued investigation 28.
An additional striking feature of the data described here is the frequency of insufficient vitamins A or D observed in the study populations. Whereas low levels of vitamins in residents of developing countries have been reported routinely 29, 30, the extent of these insufficiencies and deficiencies in inner cities of the United States may not be fully appreciated. In our study, there were only two of 45 samples that scored in the ‘sufficient’ range for vitamin D (>30 ng/ml) 2, and only about half of the RBP samples were in the sufficient range (> 22 000 ng/ml). A few individuals were suffering from influenza virus infections (Table 1), a possible explanation for low levels, but the majority of individuals were not infected. Multiple factors may lend to nutritional deficits. For individuals of low socio‐economic status there is ready access to processed foods, but limited access to fresh produce or fortified foods 31. Skin exposure to UVB light is no longer encouraged 32. Genetics will also influence vitamin status. RBP values differ among races, and polymorphisms in RBP will alter serum concentrations 33, 34. A close examination of nutritional habits and genetic backgrounds (e.g. RBP single nucleotide polymorphisms, SNPs) is now warranted to provide explanations for the low vitamin levels that we have observed.
In conclusion, our results show that Memphian samples in this study exhibited low levels of RBP and vitamin D. Vitamin D correlated with IgM and IgG3, while RBP correlated with IgA and IgG4. Individuals with the higher RBP levels exhibited higher influenza virus neutralization titres in blood and lower levels of cytokines in NW indicative of healthy mucosal tissues. Given that the surveillance of respiratory tract mucosa is often mediated by IgA, a reduction in this antibody isotype as a consequence of insufficient or deficient vitamin A may explain an individual's increased vulnerability to respiratory viral disease. Results encourage correction of vitamin deficiencies/insufficiencies among individuals in both developed and developing countries, particularly at the time of respiratory virus vaccination 8, 9 to improve vaccine efficacy and confer protection against respiratory tract disease.
Disclosure
There were no competing interests associated with this work.
Author contributions
B. G. J., C. M. O. and S. S. W. conducted the experiments. J. L. H., B. G. J., C. M. O., S. S. W., R. W. and P. G. T. designed the experiments. J. L. H., L. T., Y. S., R. W., R. B., S. S. W., C. M. O. and P. G. T. evaluated the data. J. L. H., R. W. and P. G. T. provided financial support. J. L. H., L. T., Y. S., R. W., R. B., S. S. W., C. M. O. and P. G. T. contributed to manuscript preparation.
Acknowledgements
We thank Dr Jay for conduct of vitamin D assays in the Department of Pathology at St Jude Children's Research Hospital. The research was funded in part by NIH NIAID R01 AI088729, NCI P30 CA21765, the St Jude Center of Excellence for Influenza Research and Surveillance, HHSN272201400006C and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1. Butler JC, Havens PL, Sowell AL et al Measles severity and serum retinol (vitamin A) concentration among children in the United States. Pediatrics 1993; 91:1176–81. [PubMed] [Google Scholar]
- 2. Chesney RW. Vitamin D and the Magic Mountain: the anti‐infectious role of the vitamin. J Pediatr 2010; 156:698–703. [DOI] [PubMed] [Google Scholar]
- 3. Querfeld U. Vitamin D and inflammation. Pediatr Nephrol 2013; 28:605–10. [DOI] [PubMed] [Google Scholar]
- 4. Sarma PC, Goswami BC, Gogoi K, Bhattacharjee H, Barua AB. A new approach to the assessment of marginal vitamin A deficiency in children in suburban Guwahati, India: hydrolysis of retinoyl glucuronide to retinoic acid. Br J Nutr 2009; 101:794–7. [DOI] [PubMed] [Google Scholar]
- 5. Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008; 8:685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rudraraju R, Jones BG, Surman SL, Sealy RE, Thomas PG, Hurwitz JL. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLOS ONE 2014; 9:e86554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Surman SL, Rudraraju R, Sealy R, Jones B, Hurwitz JL. Vitamin A deficiency disrupts vaccine‐induced antibody‐forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol 2012; 25:341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Surman SL, Jones BG, Sealy RE, Rudraraju R, Hurwitz JL. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 2014; 32:2521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Surman SL, Jones BG, Rudraraju R, Sealy RE, Hurwitz JL. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A‐deficient host. Clin Vaccine Immunol 2014; 21:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Renegar KB, Small PA Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol 1991; 146:1972–8. [PubMed] [Google Scholar]
- 12. Renegar KB, Small PA Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978–86. [DOI] [PubMed] [Google Scholar]
- 13. Seibert CW, Rahmat S, Krause JC et al Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol 2013; 87:7793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oshansky CM, Gartland AJ, Wong SS et al Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med 2014; 189:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almekinder J, Manda W, Soko D, Lan Y, Hoover DR, Semba RD. Evaluation of plasma retinol‐binding protein as a surrogate measure for plasma retinol concentrations. Scand J Clin Lab Invest 2000; 60:199–203. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez‐Quintela A, Alende R, Gude F et al Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008; 151:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oyeyinka GO, Salimonu LS, Williams AI, Johnson AO, Ladipo OA, Osunkoya BO. Range of normal serum immunoglobulin (IgG, IgA and IgM) values in Nigerians. Afr J Med Med Sci 1984; 13:169–76. [PubMed] [Google Scholar]
- 18. Weber‐Mzell D, Kotanko P, Hauer AC et al Gender, age and seasonal effects on IgA deficiency: a study of 7293 Caucasians. Eur J Clin Invest 2004; 34:224–8. [DOI] [PubMed] [Google Scholar]
- 19. Wood LG, Simpson JL, Wark PA, Powell H, Gibson PG. Characterization of innate immune signalling receptors in virus‐induced acute asthma. Clin Exp Allergy 2011; 41:640–8. [DOI] [PubMed] [Google Scholar]
- 20. Gandhi VD, Vliagoftis H. Airway epithelium interactions with aeroallergens: role of secreted cytokines and chemokines in innate immunity. Front Immunol 2015; 6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung HL, Lee EJ, Park HJ, Lee KH. Increased epidermal growth factor in nasopharyngeal aspirates from infants with recurrent wheeze. Pediatr Pulmonol 2015; 50:841–7. [DOI] [PubMed] [Google Scholar]
- 22. El‐Madhun AS, Cox RJ, Haaheim LR. The effect of age and natural priming on the IgG and IgA subclass responses after parenteral influenza vaccination. J Infect Dis 1999; 180:1356–60. [DOI] [PubMed] [Google Scholar]
- 23. Beagley KW, Eldridge JH, Lee F et al Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA‐committed B cells. J Exp Med 1989; 169:2133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrhardt RO, Strober W, Harriman GR. Effect of transforming growth factor (TGF)‐beta 1 on IgA isotype expression. TGF‐beta 1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J Immunol 1992; 148:3830–6. [PubMed] [Google Scholar]
- 25. Sonoda E, Hitoshi Y, Yamaguchi N et al Differential regulation of IgA production by TGF‐beta and IL‐5: TGF‐beta induces surface IgA‐positive cells bearing IL‐5 receptor, whereas IL‐5 promotes their survival and maturation into IgA‐secreting cells. Cell Immunol 1992; 140:158–72. [DOI] [PubMed] [Google Scholar]
- 26. Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today 1993; 14:15–17. [DOI] [PubMed] [Google Scholar]
- 27. Fujihashi K, McGhee JR, Lue C et al Human appendix B cells naturally express receptors for and respond to interleukin 6 with selective IgA1 and IgA2 synthesis. J Clin Invest 1991; 88:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stavnezer J, Schrader CE. IgH chain class switch recombination: mechanism and regulation. J Immunol 2014; 193:5370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D, DEVTA Team . Vitamin A supplementation every 6 months with retinol in 1 million pre‐school children in north India: DEVTA, a cluster‐randomised trial. Lancet 2013; 381:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sommer A. Vitamin A, infectious disease, and childhood mortality: a 2¢ solution? J Infect Dis 1993; 167:1003–7. [DOI] [PubMed] [Google Scholar]
- 31. Rose D, Richards R. Food store access and household fruit and vegetable use among participants in the US Food Stamp Program. Public Health Nutr 2004; 7:1081–8. [DOI] [PubMed] [Google Scholar]
- 32. Edlich RF, Winters KL, Cox MJ et al National health strategies to reduce sun exposure in Australia and the United States. J Long Term Eff Med Implants 2004; 14:215–24. [DOI] [PubMed] [Google Scholar]
- 33. Munkhtulga L, Nakayama K, Utsumi N et al Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia,. Hum Genet 2007; 120:879–88. [DOI] [PubMed] [Google Scholar]
- 34. Dessein PH, Tsang L, Norton GR, Woodiwiss AJ, Solomon A. Retinol binding protein 4 concentrations relate to enhanced atherosclerosis in obese patients with rheumatoid arthritis. PLOS ONE 2014; 9:e92739. [DOI] [PMC free article] [PubMed] [Google Scholar]


