Abstract
Pyogenic liver abscesses are caused by various microorganisms and usually present with fever, abdominal pain, leukocytosis and liver enzyme abnormalities. This case presents the insidious manifestation of a pyogenic liver abscess in a 34-year-old immunocompetent male, where classical manifestations of a liver abscess were absent. The microorganisms cultured from the abscess belonged to oral cavity's and gastrointestinal tract's normal flora.
INTRODUCTION
Liver abscesses are the most common type of visceral abscess, accounting for 48% of them, in reports of intraabdominal abscesses. Various pathogens may lead to their formation. They are usually polymicrobial, consisting of a mixture of enteric facultative and anaerobic pathogens, with Streptococcus anginosus group (SAG) bacteria, Candida species and Klebsiella pneumoniae being among the most common pathogens [1–3].
Diagnosis is made from the patient's history, clinical examination and imaging followed by aspiration and culture of the abscess. Typical clinical manifestations of liver abscess include fever (90%) and abdominal pain (50–75%). In addition, white blood cells (WBCs) and liver function tests are usually increased [4].
An interesting case of an afebrile liver abscess is hereby presented, caused by Micrococcus luteus and Streptococcus intermedius, without liver function abnormalities, fever or leukocytosis.
CASE REPORT
A 34-year-old Cypriot male, with unremarkable past medical history, was admitted for investigation after complaining for blunt diffuse abdominal pain, starting a month ago, without any other constitutional symptoms. He denied the use of any recreational drugs or alcohol, recent traveling abroad or contact with any pets.
The physical examination was unremarkable. However, a right upper quadrant abdominal tenderness during deep palpation and a positive Murphy sign were present. The patient had also poor oral hygiene.
Laboratory examination was normal, besides a mild hypochromic microcytic anemia and a mild C-reaction protein (CRP) elevation (WBCs: 9.570/μl, neutrophils %: 67%, hemoglobin: 10.0 g/dl, mean corpuscular volume 75.9 fl, mean corpuscular hemoglobin: 23.4 fl and CRP 54.47 mg/l).
Abdominal ultrasonography showed a sizable liver lesion (cyst containing) confirmed by abdominal computed tomography (CT) scan (Fig. 1a). Patient was initiated intravenous metronidazole 500 mg three times daily and piperacillin/tazobactam 4.5 g three times daily. Blood and stool cultures were negative. Further investigation with procalcitonin, tuberculin skin test, serology for Echinococcus granulosus and hepatitis/HIV screening was negative. The abscess was drained under CT guidance and the fluid's biochemistry examination (Fig. 1c) was compatible with exudative etiology (pus; WBCs: 83.600/μl, neutrophils (#): 48 400, glucose 0 mg/dl, protein: 5.49 g/dl and LDH fluid/serum ratio: 8.16). Fluid's cytologic examination was negative and the gram stain showed necrotic WBCs. Streptococcus intermedius and M. luteus/lylae sensitive to oral β-lactams were isolated from the fluid culture.
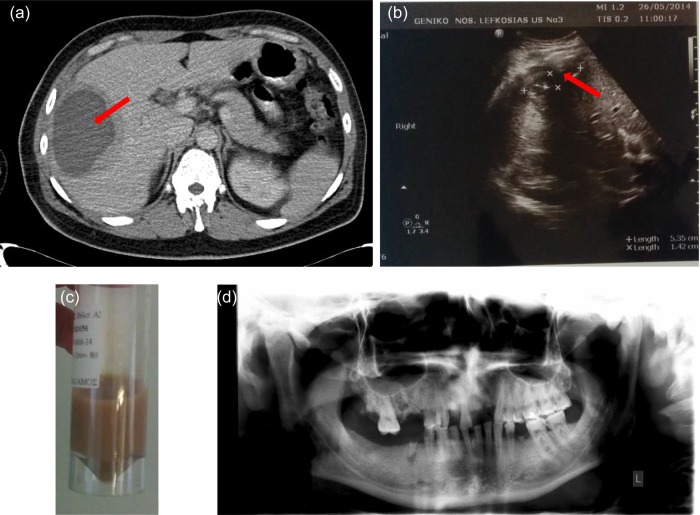
Figure 1:
(a) Abdominal CT scan with intravenous contrast showing a sizable (cyst containing) hypodense lesion with thickened wall (15 mm) in the right liver lobe (liver section 5 and 6), peripherally to the liver's capsule (red arrow). Lesions maximum dimensions were 10 × 10 × 8 cm. It displayed also minimum fluid collection around the liver. The imaging findings were primarily suggestive of an abscess. Another possibility would be a tumor with central necrosis. Additionally, there were small para-aortic lymph nodes with diameter <1 cm as well as mild splenomegaly (spleen diameter 13.5 cm). (b) Follow-up ultrasonography imaging a week after having the drainage catheter in situ: residual abscess (red arrow) cavity with dimensions of 5.35 × 1.42 cm with minimal effusion and air. (c) Light-brown abscess fluid sample, after CT-guided drainage. (d) Patient's panoramic radiograph with the absence of abscesses.
Transthoracic echocardiograph excluded infectious endocarditis. Endoscopic evaluation of the gastrointestinal tract revealed only internal hemorrhoids. Dentist referral concluded to a poor oral hygiene and an increased plaque deposition (Fig. 1d).
Follow-up with an ultrasound a day before removing the drainage catheter showed a residual abscess cavity (dimensions of 5.3 × 1.4 cm) with minimal effusion and air (Fig. 1b). The drainage catheter was removed after a week. After 23 days, the patient was discharged after switching to oral antimicrobial therapy, deescalating to amoxicillin/clavulanic acid 625 mg and metronidazole 400 mg three times daily, respectively, to complete 6 weeks of antibiotic therapy. Furthermore, the patient was scheduled for complete dental care, to ensure that poor oral hygiene will not be an infection source once again.
However, a week after his discharge, the patient was admitted to the General Surgery Department with high fever, shivering, abdominal pain, generalized maculopapular rash and diarrhea. From laboratory evaluation, the patient did not present leukocytosis (WBCs: 6.090/μl); however, other inflammation markers were elevated (CRP: 147.8 mg/l). Ultrasonography and abdominal CT imaging revealed a relapse of the abscess and the patient undergone a surgical abscess drainage, in combination with broad-spectrum intravenous antibiotic therapy with successful outcome. Cultures of pus after surgical drainage were positive for S. intermedius.
DISCUSSION
Liver abscesses are the cause of major complications (sepsis or multiorgan dysfunction/failure) and it can lead to death, if they are left untreated. Antibiotics and appropriate drainage are the standard of practice [4]. They are associated with underlying hepatobiliary or pancreatic disease, diabetes, liver transplantation and colorectal neoplasia (4-fold incidence of liver abscesses with colorectal carcinoma) [5].
According to the current literature review, fever is the most common clinical manifestation of liver abscesses. However, a minority present without fever (13.9–31%). In addition, a small subset have a normal WBC count (23–24.6%) and liver function tests (13–29.5%) at the time of diagnosis [6, 7]. In this interesting case, our patient was admitted afebrile with normal liver enzymes and WBCs.
Microorganisms associated with liver abscesses include most commonly gram-negative enteric bacteria (Escherichia coli, K. pneumoniae, Pseudomonas, and Proteus), gram-positive aerobes (Streptococcus milleri, Enterococcus, Staphylococcus aureus, Staphylococcus epidermidis and Streptococci sp.), anaerobic organisms (Bacteroides sp. and Fusobacterium), actinomyces, Candida albicans, Salmonella typhi, Brucella melitensis or other protozoa (Entamoeba histolytica and Echinococcus granulosus). The microbe type isolated from the liver abscess is correlated with its origin. Since liver receives blood from both systemic and portal circulation, liver is more prone to infections from biliary and gastrointestinal tract bacteria (especially the right liver lobe due to anatomic reasons) [2, 8].
In our case, the abscess culture isolated bacteria from oral cavity and gastrointestinal tract normal flora. Streptococcus intermedius is an anaerobic gram-positive, non-motile and catalase-negative coccus, which is a member of the SAG. It is considered the most common microorganism found in dental plaques and the one causing liver abscesses. This is due to its liver tropism and the hydrolytic enzymes (such as intermedilysin). We speculate that the infection originated from the oral cavity, as the patient exhibited poor oral hygiene with the presence of dental plaque [9].
Micrococcus luteus, the second organism cultured, is an aerobic gram-positive, gram-variable, non-motile, saprotrophic, urease and catalase-positive bacterium that belongs to the family of Micrococcaceae. It can be found in several materials and in the oral cavity as normal flora. Moreover, it colonizes the upper pharynx and the respiratory tract. It is a non-pathogenic organism; nevertheless, it can be infectious in immunocompromised patients. According to the literature review, M. luteus is not included to the pathogens causing liver abscesses. The fact that this microorganism was isolated from our patient's abscess makes the case very noteworthy. Furthermore, it is not fully understood how this organism caused the liver abscess to our immunocompetent patient. Thus, a further evaluation of M. luteus is needed in order to understand its pathogenicity [10].
In conclusion, what was interesting regarding our case was the absence of the classic manifestations of liver abscess, in an immunocompetent patient, and then the secondary admission of the patient with fever and abdominal pain, in the absence of leukocytosis. Our opinion is that during the first admission, the thick abscess wall entrenched the abscess successfully. However the abscess CT-guided drainage abolished that “protective” effect. In addition, a possible inadequate abscess drainage leaded to a more systemic inflammation, causing the patient's re-admission and further surgical intervention. Clinicians should be vigilant in order to establish the right diagnosis and give the correct treatment. The absence of fever, leukocytosis or liver function abnormalities can mascarate the presence of pyogenic liver abscess. Further evaluation with blood and abscess culture are crucial. Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal. A.I. is a guarantor.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
We thank our patient for his consent to publish the case and the related pictures.
REFERENCES
- 1.Huang CJ, Pitt HA, Lipsett PA, Osterman JR, Lillemoe KD, Cameron JL et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg 1996;223:600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004;39:1654–9. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032–8. [DOI] [PubMed] [Google Scholar]
- 4.Lok KH, Li KF, Li KK, Szeto ML. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect 2008;41:483–90. [PubMed] [Google Scholar]
- 5.Qu K, Liu C, Wang ZX, Tian F, Wei JC, Tai MH et al. Pyogenic liver abscesses associated with nonmetastatic colorectal cancers: an increasing problem in Eastern Asia. World J Gastroenterol 2012;18:2948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y-J, Lai Y-C, Lin Y-C, Yeh Y-H. Treatment and prognosis of pyogenic liver abscess. Int J Emerg Med 2010;3:381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: ten years experience in a UK centre. QJM 2002;95:797–802. [DOI] [PubMed] [Google Scholar]
- 8.Chemaly RF, Hall GS, Keys TF, Procop GW. Microbiology of liver abscesses and the predictive value of abscess gram stain and associated blood cultures. Diagn Microbiol Infect Dis 2003;46:245–8. [DOI] [PubMed] [Google Scholar]
- 9.Claridge JE, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (‘Streptococcus milleri group’) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis 2001;32:1511–5. [DOI] [PubMed] [Google Scholar]
- 10.Young M, Artsatbanov V, Beller HR, Chandra G, Chater KF, Dover LG et al. Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living Actinobacterium. J Bacteriol 2010;192:841–60. [DOI] [PMC free article] [PubMed] [Google Scholar]