SUMMARY
Chromoblastomycosis (CMB) is a chronic fungal infection of the skin and the subcutaneous tissue caused by a transcutaneous traumatic inoculation of a specific group of dematiaceous fungi occurring mainly in tropical and subtropical zones worldwide. If not diagnosed at early stages, patients with CBM require long term therapy with systemic antifungals, sometimes associated with physical methods. Unlike other neglected endemic mycoses, comparative clinical trials have not been performed for this disease. Nowadays, therapy is based on a few open trials and on expert opinion. Itraconazole either as monotherapy or associated with other drugs, or with physical methods, is widely used. Recently, photodynamic therapy has been successfully employed in combination with antifungals in patients presenting with CBM. In the present revision the most used therapeutic options against CBM are reviewed as well as the several factors that may have impact on the patient's outcome.
Keywords: Chromoblastomycosis, Dematiaceous fungi, Fungal infections, Antifungal treatment
RESUMO
Cromoblastomicose (CMB) é uma infecção fúngica crônica da pele e tecido subcutâneo causada pela inoculação transcutânea traumática de um grupo específico de fungos dermatiáceos que ocorrem principalmente em zonas tropicais e subtropicais do mundo. Quando não são diagnosticados nas fases iniciais, pacientes com CBM necessitam de tratamentos prolongados com antifúngicos sistêmicos, por vezes associados a métodos físicos. Diferentemente de outras micoses endêmicas negligenciadas, não foram realizados ensaios clínicos comparativos para esta doença. Atualmente a terapia é baseada em alguns poucos ensaios abertos e em opiniões de especialistas. Itraconazol é amplamente utilizado como monoterapia ou em associação com outras drogas, ou com métodos físicos. Recentemente, a terapia fotodinâmica foi empregada com sucesso combinada a antifúngicos em pacientes com CBM. Neste manuscrito as opções terapêuticas mais utilizadas contra CBM foram revistas, assim como os diversos fatores que podem influenciar a evolução dos pacientes
INTRODUCTION
Neglected diseases constitute a group of tropical and subtropical infections which are endemic in low-income populations in developing regions of Africa, Asia, and Latin America. The World Health Organization (WHO) acknowledges the neglected diseases as a symptom of poverty and disadvantage. The most affected by the neglected diseases are the poorest populations often living in remote, rural areas, urban slums or in conflict zones. With little political support, neglected tropical diseases are not on the priority list of public health systems37. A series of endemic diseases including helminths, protozoa, bacterial and viral infections, but not fungal diseases other than mycetoma are considered neglected diseases by WHO36. Its global burden should be even greater than mycetoma and CBM can lead to potential incapacity for labor. The aim of this review is to update the main clinical, epidemiological and therapeutic topics on CBM, a typical orphan disease.
CHROMOBLASTOMYCOSIS
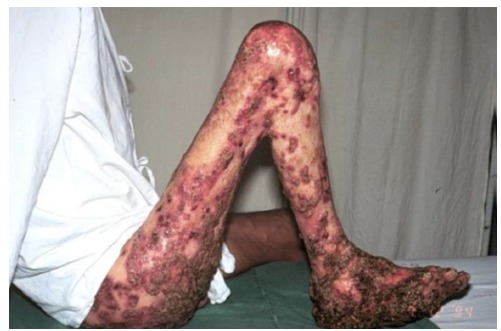
Chromoblastomycosis or chromomycosis is one of the most prevalent transcutaneous traumatic implantation or subcutaneous mycosis in individuals living in tropical and subtropical zones around the world. Although PEDROSO & GOMES observed some patients in 1910 in Sao Paulo, Brazil, the scientific report of these observations appeared only in 192020. This is the reason why the first description of CBM is actually attributed to Max RUDOLPH, a German doctor who published the first cases of CBM from the city of Estrela do Sul, Minas Gerais, Brazil, in 19149 , 32. This disease presents the following characteristics: primary lesion beginning at the site of inoculation; chronic involvement of cutaneous and subcutaneous tissues associated with a granulomatous, purulent, fibrotic tissue formation and a non-protective humoral immune response24. CBM lesions are usually recalcitrant and extremely difficult to eradicate. Due to its chronicity, CBM lesions may undergo neoplastic transformation leading to skin cancer24 , 27. Except for small initial lesions that can be cured by surgical removal, CBM lesions constitute a true therapeutic challenge for clinicians and patients (Fig. 1).
Fig. 1. - Severe and recalcitrant clinical form of chromoblastomycosis.
ECO-EPIDEMIOLOGY
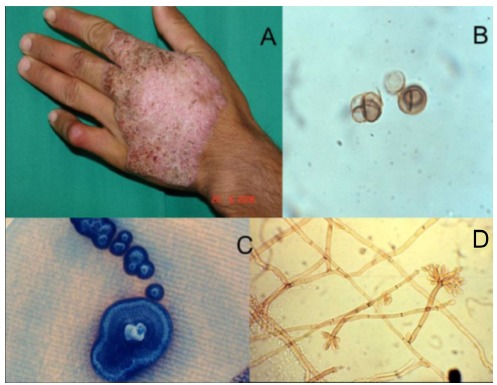
Chromoblastomycosis is the most common of several mycoses caused by melanized or black fungi. CBM agents are found on soil, plant thorns and debris15 , 35. These fungi belong mainly to Fonsecaea and Cladophialophora genus and, while scattered cases have been reported in Phialophora, Rhinocladiella and Exophiala genus. F. pedrosoi and C. carrionii are usually found in tropical and subtropical regions. F. pedrosoi is primarily found in humid areas, whereas C. carrionii is prevalent in semiarid climates11 , 23 , 24 , 34 , 38. As with other members of the Herpotrichiellaceae family, these agents have melanin in their cell wall, an important pathogenicity factor31. It is believed that CBM etiologic agents are soil and/or plant saprobes with typical mycelia in environmental samples, changing morphology to the muriform (sclerotic) form in tissue (Fig. 2)13 , 19.
Fig. 2. - Clinical and microbiological aspects of chromoblastomycosis: The etiologic agent is easily found in the "black dot" lesion covered area (circled) A. Muriform cells are pathognomonic for this disease. They are observed either on wet mount (B) or in histologic sections. Fonsecaea pedrosoi is one of the prevalent agents in humid areas. Figures C and D depicts its macro and micromorphology aspects.
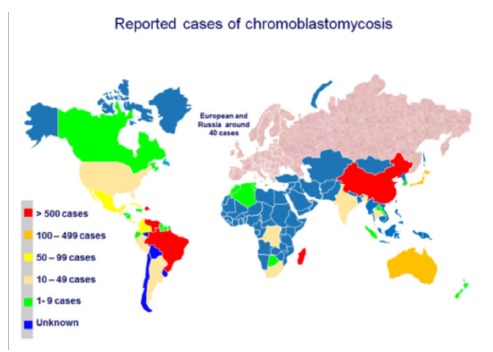
The highest prevalence of the disease is within a zone between 30° latitude North and 30° latitude South, coinciding with most of the tropical and subtropical zones. CBM is not a compulsory reportable disease so that all epidemiology data is derived from published case reports and surveys. Incidence rates range from 1: 6,800 (14/100,000) in Madagascar to 1: 8,625,000 (0.012/100,000) in USA. In Brazil the estimate incidence rate of CBM is 3/100,00025. Most of the reported cases occur in Latin America, the Caribbean, Asia, Africa and Australia. Madagascar, Brazil, Mexico, Dominican Republic, Venezuela, India and Southern China contribute with the majority of cases (Fig. 3)3 , 11 , 16 , 17 , 23 , 25 , 29 , 34 , 38.
Fig. 3. - Geographic distribution of chromoblastomycosis according to reported cases. (Courtesy of Dr Daniel Wagner dos Santos, University of Sao Paulo, Brazil).
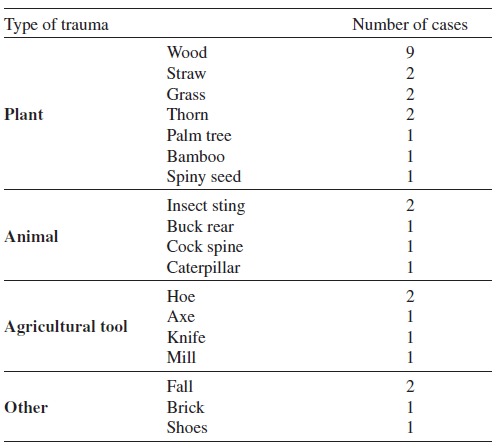
Chromoblastomycosis-causing fungi are found worldwide in soil and decaying plant debris, including wood. Because CBM is an implantation mycosis, occupation seems to play an important role1 , 24 , 25. This disease rarely occurs before adolescence with most patients in the age group between 40 to 50-years old, with a male-to-female ratio of 5:1 and 9:1.1 , 24 , 25. The majority of lesions are observed on the extremities of outdoor rural workers. The main risk factors associated with CBM infection are: lack of protective shoes, gloves or garments, poor nutrition and hygienic habits1. CBM is considered an occupational disease, occurring in farm workers, lumberjacks, or vendors of farm products. A potentially important source of infection was reported in an endemic area located in the Maranhao State, on the fringes of the Amazon rainforest in Brazil, where thousands of families are involved in babassu (Orbignya phalerata) a wild palm tree, harvesting. The local population collects babassu nuts to extract the babassu oil, an important component for local and international beauty product manufacturers. Because melanized fungi have been isolated from babassu shield fragments, this may be a risk factor for hundreds of people developing CBM after trauma that occurred at work (Fig. 4)18 , 33. Other occupational hazards are likely in other environments (Table 1).
Fig. 4. Babassu (Orbignya phalerata) nutcracker woman in the State of Maranhao, Brazil. (Courtesy of Professor Conceição Pedroso, Federal University of Maranhao, Brazil).

Table 1. Details of subcutaneous traumas leading to chromoblastomycosis in 32 Brazilian patients adapted from reference 18 .
CLINICAL MANIFESTATIONS
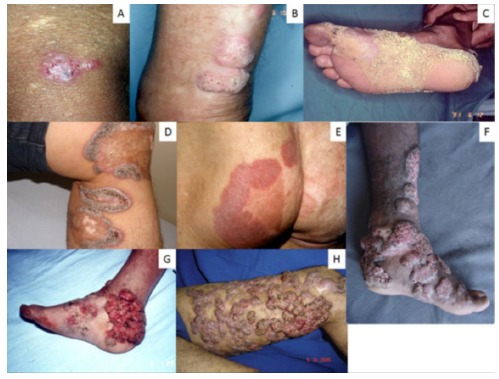
Following a transcutaneous traumatic implantation, and after an uncertain incubation period, the initial CBM lesion appears at the inoculation place. It may start as a solitary macular lesion, later progressing to a raised papule with a pink smooth surface that gradually increases over a few weeks before becoming scaly6 , 24 , 28. The initial skin lesion may progress and evolve with diverse clinical types including nodular, tumoral (cauliflower-like), verrucous or a scar-like appearance (Fig. 5)6 , 8 , 20 , 24 , 28. In advanced and severe cases, more than one type of lesion can be observed in the same patient. The clinical polymorphism of CBM lesions elicits multiple differential diagnoses including infectious and non-infectious possibilities. This may cause diagnosis delay, lack of therapeutic response and loss of mobility6 , 24 , 28. At the beginning, the initial lesions are asymptomatic, and usually do not interfere with the patient's activities. Over time, itching becomes the predominant symptom of the disease, which in the moderate forms is intense and may be accompanied by local pain. Because CBM lesions are very pruritic, it is accepted that the disease dissemination to other skin sites usually occurs by autoinoculation and/or contiguous lymphatic spread. As severity increases, edema and bacterial secondary infections affect the health of the patient as a whole, modifying the appearance of the skin and causing scars. In the most severe cases, chronic lymphedema and ankylosis develop and non-invasive squamous cell carcinomas may arise. All these complications can lead to definite disability (Fig. 1)6 , 24 , 28.
Fig. 5. - Lesions of chromoblastomycosis may depict clinical polymorphism end elicit several differential diagnoses. The initial lesion of chromoblastomycosis (1A), may evolve to five main clinical types: nodular lesions on the lower leg (1B), verrucous lesion of the foot (1C), scar lesions on the knee and lower leg (1D), plaque lesion on the buttocks (1E), tumoral (cauliflower) lesions on the foot (1G) and mixed lesions composed by plaque, nodular and verrucous lesions involving the lower limb (1H).
DIAGNOSIS
Diagnosis of CBM is mainly based on clinical and epidemiological suspicion in endemic areas but it must be confirmed by microbiological demonstration of the etiologic agents in clinical samples. Skin biopsies or scrapings should be taken from the surface of the lesion where "black dots" may be visible. When examined under light microscopy the pathognomonic "muriform cells" are depicted. These chestnut, rounded brown pigmented and cross chambered structures are distinctive and have been referred to as "sclerotic bodies, fumagoid cells" cooper pennies"1 , 24 , 25. Muriform cells are considered as a biological adaptation allowing the etiologic agent to survive in the hostile host tissue environment19 Histologically, CBM typically reveals pseudo epitheliomatous epidermal hyperplasia, hyperkeratosis, irregular acanthosis, alternating with areas of atrophy and collection of inflammatory cells forming epidermic abscesses. Granulomatous reaction with different grades of fibrosis can be found at the dermal level. Muriform cells may be observed among these structures or inside Langerhans giant cells4. When cultivated, all CBM agents grow slowly in culture. Initially, colonies are deep green, depicting a velvet dark aspect with time. Presumptive species identification may be achieved by mycological morphologic methods, but molecular techniques are suggested for definitive identification10.
THERAPY
Chromoblastomycosis lesions are recalcitrant and very difficult to treat. If not discovered early when the initial CBM lesions may be surgically removed, long periods of systemic antifungal therapy alone or in combination with several physical methods is the rule for many patients. The efficacy of therapy may be related to the severity and duration of the disease, to the etiologic agent and to the patient's compliance. As comparative trials on this disease are lacking, evidence that helps to select optimal therapy is based on a few open clinical studies and expert opinion. No ''gold standard'' therapy for CBM is available, but treatment options include systemic antifungals, as monotherapy or combined, physical methods and immune adjuvants (Table 2)6 , 27 , 28.
Table 2. Treatment options for chromoblastomycosis.
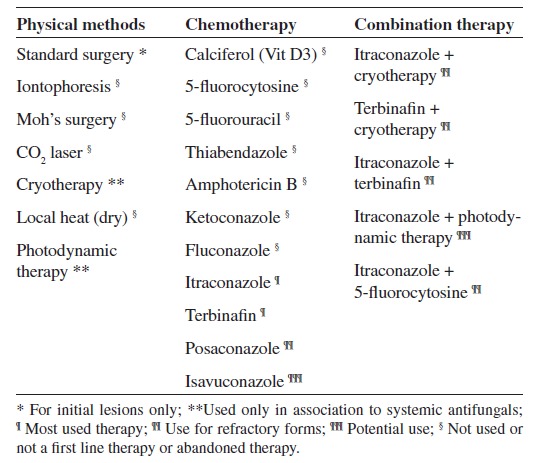
Over time, several therapeutic regimens have been tried, including physical methods such as surgery, thermo, laser and photodynamic therapies6 , 24 , 27 , 28. These therapeutic modalities are only indicated at the early stages of the disease. They can also be associated with systemic antifungal therapy. Initially, mild CBM lesions can be treated by surgical excision but unfortunately most of the patients present with moderate to severe forms, leading to long-term courses of systemic antifungal drugs. In addition, neither in vitro sensitivity tests are standard for the filamentous and the parasitic (muriform), nor experimental therapy models have been successfully developed31. Thus, it is accepted that the most used drugs are itraconazole and terbinafine at daily doses of 200-400 mg and 250-500 mg, respectively7 , 12 , 26 , 27 , 28 , 30. In refractory cases, the combination of these two drugs can be tempted27. Other effective treatments include posaconazole, 800 mg per day and the combination of itraconazole with 5-flucytosine2 , 5 , 14 , 21. The association of the latter with posaconazole may play an important role in the therapy of CBM. The duration of therapy must be based on clinical, mycological and histopathological criteria (Table 2). According to published data, cure rates with terbinafine or itraconazole vary from 15 to 80%, depending on the severity of the disease. As expected, in severe forms cure rates are lower and relapses are more common14 , 26 , 27.
REFERENCES
- 1.AL-DOORY Y. Di Salvo AF. Occupational mycoses. Philadelphia: Lea & Febiger; 1983. Chromomycosis; pp. 95–121. [Google Scholar]
- 2.Antonello VS, Appel da Silva MC, Cambruzzi E, Kliemann DA, Santos BR, Queiroz-Telles F. Treatment of severe chromoblastomycosis with itraconazole and 5-flucytosine association. Rev Inst Med Trop Sao Paulo. 2010;52:329–331. doi: 10.1590/s0036-46652010000600008. [DOI] [PubMed] [Google Scholar]
- 3.Attapattu MC. Chromoblastomycosis: a clinical and mycological study of 71 cases from Sri Lanka. Mycopathologia. 1997;137:145–151. doi: 10.1023/a:1006819530825. [DOI] [PubMed] [Google Scholar]
- 4.Avelar-Pires C, Simoes-Quaresma JA, Moraes de Macedo GM, Brasil-Xavier M, Cardoso de Brito A. Revisiting the clinical and histopathological aspects of patients with chromoblastomycosis from the Brazilian Amazon region. Arch Med Res. 2013;44:302–306. doi: 10.1016/j.arcmed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Bolzinger T, Pradinaud R, Sainte-Marie D, Dupont B, Chwetzoff E. Traitement de quatre cas de chromomycose àFonsecaea pedrosoipar l'association 5-fluorocytosine-itraconazole. Nouv Dermatol. 1991;10:462–466. [Google Scholar]
- 6.Bonifaz A, Carrasco-Gerard E, Saul A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1–7. doi: 10.1046/j.1439-0507.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonifaz A, Saul A, Paredes-Solis V, Araiza J, Fierro-Arias L. Treatment of chromoblastomycosis with terbinafine: experience with four cases. J Dermatolog Treat. 2005;16:47–51. doi: 10.1080/09546630410024538. [DOI] [PubMed] [Google Scholar]
- 8.Carrion AL. Chromoblastomycosis. Ann NY Acad Sci. 1950;50:1255–1282. doi: 10.1111/j.1749-6632.1950.tb39826.x. [DOI] [PubMed] [Google Scholar]
- 9.Castro RM, Castro LGM. On the priority of description chromomycosis. Mykosen. 1987;30:397–403. doi: 10.1111/j.1439-0507.1987.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 10.De Hoog GS, Attili-Angelis D, Vicente VA, Van Den Ende AH, Queiroz-Telles F. Molecular ecology and pathogenic potential ofFonsecaeaspecies. Med Mycol. 2004;42:405–416. doi: 10.1080/13693780410001661464. [DOI] [PubMed] [Google Scholar]
- 11.Esterre P, Andriantsimahavandy A, Ramarcel ER, Pecarrere JL. Forty years of chromoblastomycosis in Madagascar: a review. Am J Trop Med Hyg. 1996;55:45–47. doi: 10.4269/ajtmh.1996.55.45. [DOI] [PubMed] [Google Scholar]
- 12.Esterre P, Inzan CK, Rtasioharana M, Andriantsimahavandy A, Raharisolo C, Randrianiaina E. A multicenter trial of terbinafine in patients with chromoblastomycosis: effects on clinical and biological criteria. J Dermatolog Treat. 1998;9(Suppl 1):S29–S34. [Google Scholar]
- 13.Esterre P, Queiroz-Telles F. Management of chromoblastomycosis: novel perspectives. Curr Opin Infect Dis. 2006;19:148–152. doi: 10.1097/01.qco.0000216625.28692.67. [DOI] [PubMed] [Google Scholar]
- 14.Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis. 2009;22:559–563. doi: 10.1097/QCO.0b013e328332bbc5. [DOI] [PubMed] [Google Scholar]
- 15.Gezuele E, Mackinnon JE, Conti-Díaz IA. The frequent isolation ofPhialophora verrucosaandPhialophora pedrosoifrom natural sources. Sabouraudia. 1972;10:266–273. [PubMed] [Google Scholar]
- 16.Leslie DF, Beardmore GL. Chromoblastomycosis in Queensland: a retrospective study of 13 cases at the Royal Brisbane Hospital. Australas J Dermatol. 1979;20:23–30. doi: 10.1111/j.1440-0960.1979.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Lu C, Zhang J, Hu Y, Li X, Xi L. Chromoblastomycosis in Mainland China: a systematic review on clinical characteristics. Mycopathologia. 2013;175:489–495. doi: 10.1007/s11046-012-9586-z. [DOI] [PubMed] [Google Scholar]
- 18.Marques SG, Silva C de M, Saldanha PC, Resende MA, Vicente VA, Queiroz-Telles F. Isolation ofFonsecaea pedrosoifrom the shell of babassu coconut (Orbignya phalerataMartius) in the Amazon region of Maranhão, Brazil. Nihon Ishinkin Gakkai Zasshi. 2006;47:305–311. doi: 10.3314/jjmm.47.305. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Matsuda T, McGinnis MR, Ajello L. Clinical and mycological spectra ofWangiella dermatitidisinfections. Mycoses. 1993;36:145–155. doi: 10.1111/j.1439-0507.1993.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 21.Negroni R, Tobon A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47:339–346. doi: 10.1590/s0036-46652005000600006. [DOI] [PubMed] [Google Scholar]
- 22.Pedroso A, Gomes JM. Sôbre quatro casos de dermatite verrucosa produzida pelaPhialophora verrucosa . Ann Paul Med Cirur. 1920;11:53–61. [Google Scholar]
- 23.Perez-Blanco M, Hernández Valles R, Garcia-Humbria L, Yegres F. Chromoblastomycosis in children and adolescents in the endemic area of the Falcon State, Venezuela. Med Mycol. 2006;44:467–471. doi: 10.1080/13693780500543238. [DOI] [PubMed] [Google Scholar]
- 24.Queiroz-Telles F, Esterre P, Perez-Blanco M, Vitale RG, Salgado CG, Bonifaz A. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47:3–15. doi: 10.1080/13693780802538001. [DOI] [PubMed] [Google Scholar]
- 25.Queiroz-Telles F, Nucci M, Colombo AL, Tobón A, Restrepo A. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225–236. doi: 10.3109/13693786.2010.539631. [DOI] [PubMed] [Google Scholar]
- 26.Queiroz-Telles F, Purim KS, Fillus JN, Bordignon GF, Lameira RP, Van Cutsem J. Itraconazole in the treatment of chromoblastomycosis due toFonsecaea pedrosoi . Int J Dermatol. 1992;31:805–812. doi: 10.1111/j.1365-4362.1992.tb04252.x. [DOI] [PubMed] [Google Scholar]
- 27.Queiroz-Telles F, Santos DWCL. Chromoblastomycosis in the clinical practice. Curr Fungal Infect Rep. 2012;6:312–319. doi: 10.1007/s12281-012-0116-8. [DOI] [Google Scholar]
- 28.Queiroz-Telles F, Santos DW. Challenges in the therapy of chromoblastomycosis. Mycopathologia. 2013;175:477–488. doi: 10.1007/s11046-013-9648-x. [DOI] [PubMed] [Google Scholar]
- 29.Rajendran C, Ramesh V, Misra RS, Kandhari S, Upreti HB, Datta KK. Chromoblastomycosis in India. Int J Dermatol. 1997;36:29–33. doi: 10.1046/j.1365-4362.1997.00008.x. [DOI] [PubMed] [Google Scholar]
- 30.Restrepo A, Gonzalez A, Gomez I, Arango M, de Bedout C. Treatment of chromoblastomycosis with itraconazole. Ann N Y Acad Sci. 1988;544:504–516. doi: 10.1111/j.1749-6632.1988.tb40448.x. [DOI] [PubMed] [Google Scholar]
- 31.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph M. Über die brasilianische "Figueira" (Vorläufige Mitteilung) Arch Schiffs Tropen-Hyg. 1914;18:498–499. [Google Scholar]
- 33.Silva ACCM, Serra A, Neto, Galvão CES, Marques SG, Saldanha ACR, Silva CMP. Chromoblastomicose produzida porFonsecaea pedrosoino estado do Maranhão. I. Aspectos clínicos, epidemiológicos e evolutivos. Rev Soc Bras Med Trop. 1992;25:37–34. [PubMed] [Google Scholar]
- 34.Silva JP, de Souza W, Rozental S. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil) Mycopathologia. 1998;143:171–175. doi: 10.1023/a:1006957415346. [DOI] [PubMed] [Google Scholar]
- 35.Tschen JA, Knox JM, McGavran MH, Duncan WC. Chromomycosis. The association of fungal elements and wood splinters. Arch Dermatol. 1984;120:107–108. doi: 10.1001/archderm.120.1.107. [DOI] [PubMed] [Google Scholar]
- 36.Van de Sande W, Maghoub el S, Fahal AH, Goodfellow M, Welsh O, Zijlstra E. The mycetoma knowledge gap: identification of research priorities. PLOS Negl Trop Dis. 2014;8: doi: 10.1371/journal.pntd.0002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. Geneva: World Health Organization/Department of Control of Neglected Tropical Diseases; 2013. [Google Scholar]
- 38.Yegres F. Chromoblastomycosis in children and adolescents in the endemic area of the Falcon State, Venezuela. Med Mycol. 2006;44:467–471. doi: 10.1080/13693780500543238. [DOI] [PubMed] [Google Scholar]


