Abstract
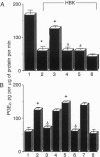
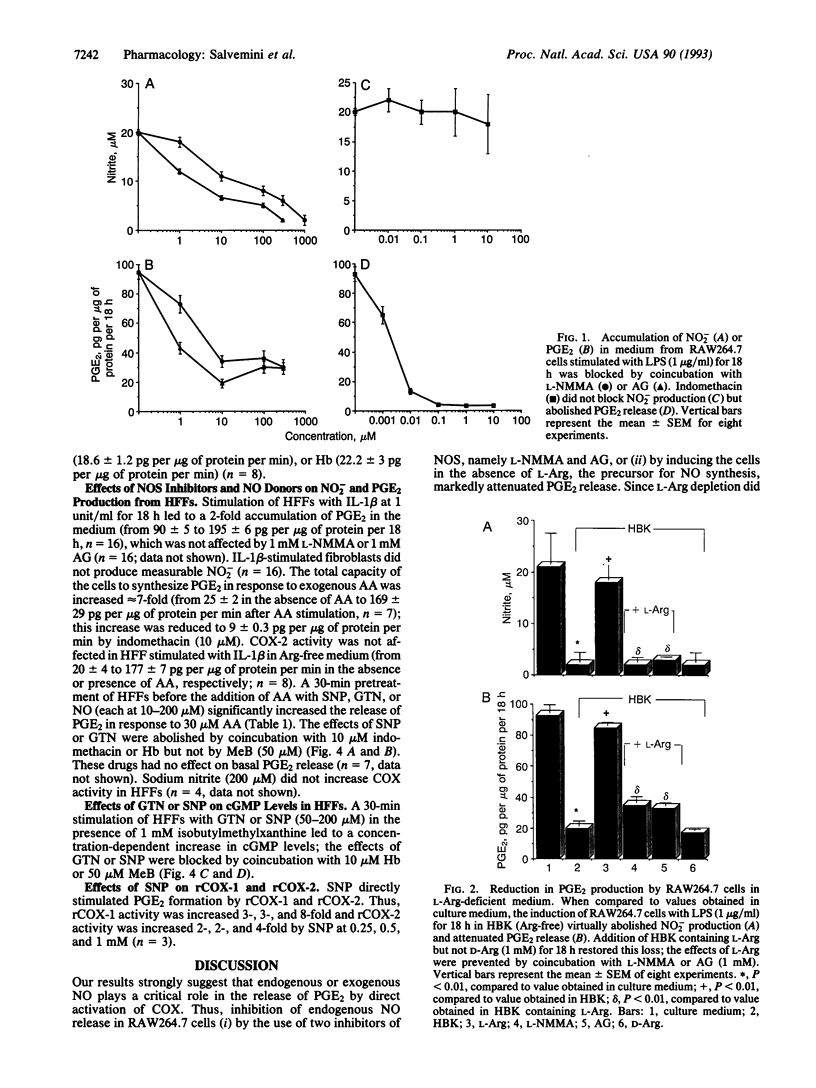
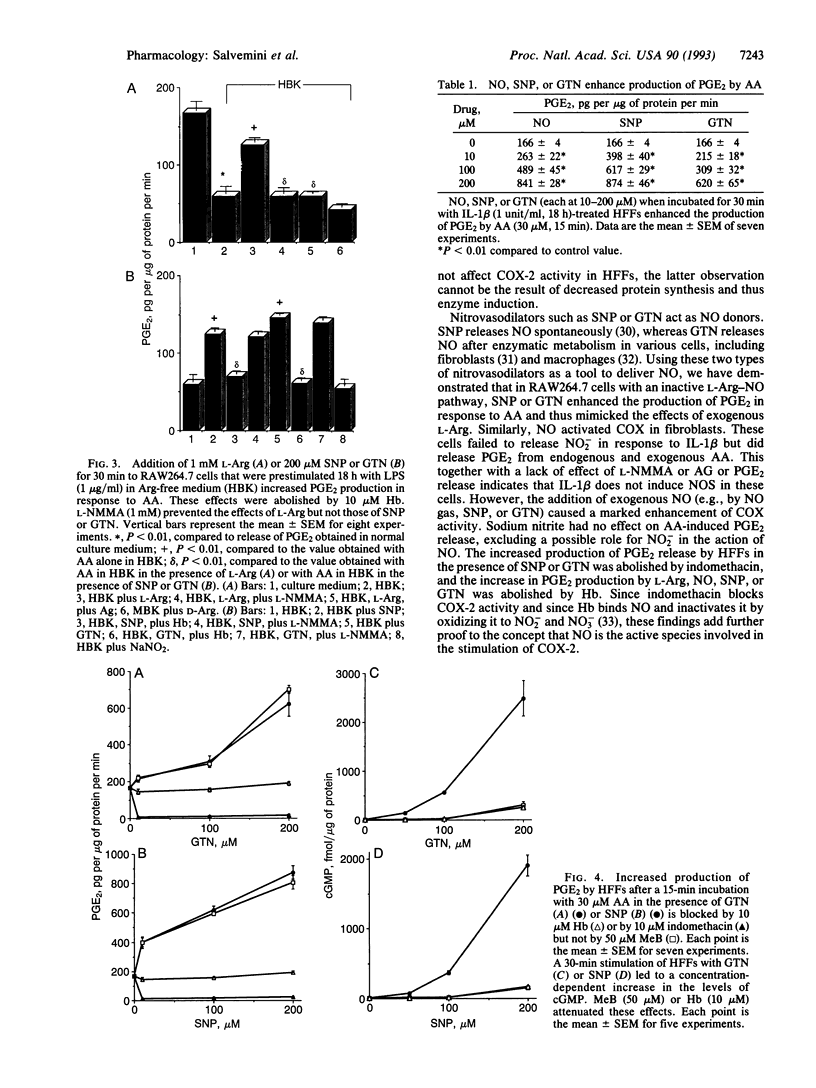
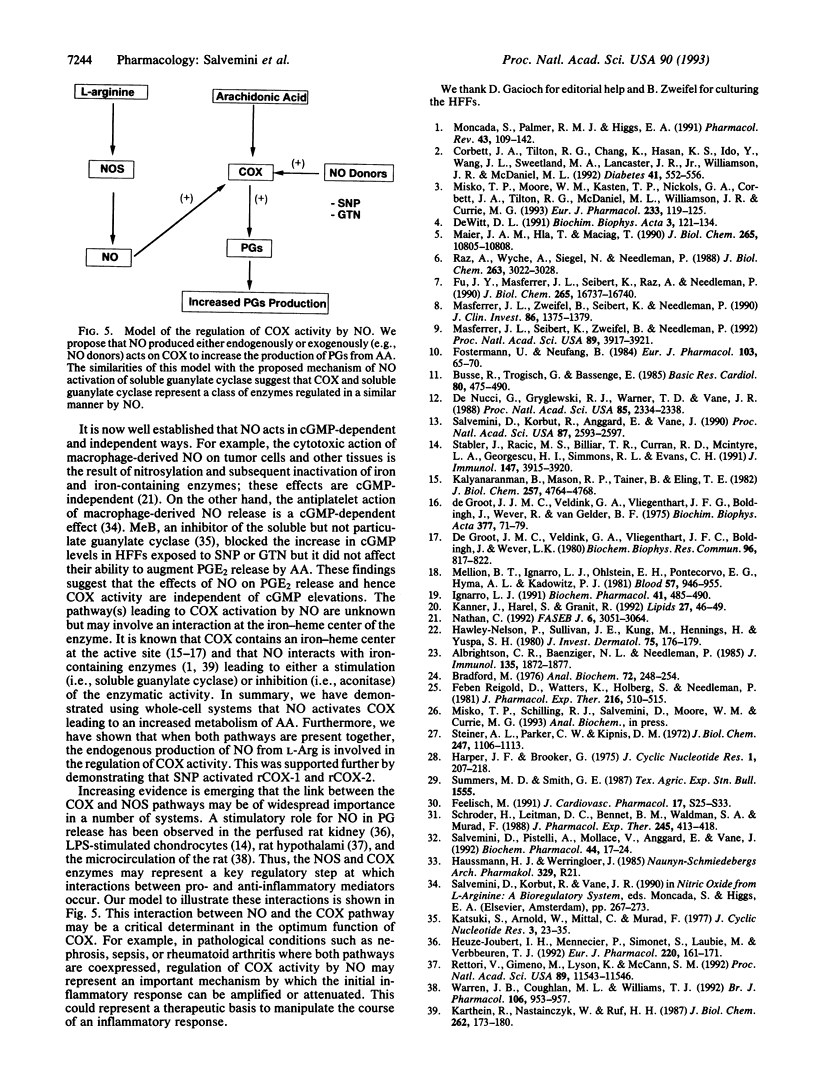
We have evaluated the role of nitric oxide (NO) on the activity of the constitutive and induced forms of cyclooxygenase (COX; COX-1 and COX-2, respectively). Induction of NO synthase (NOS) and COX (COX-2) in the mouse macrophage cell line RAW264.7 by Escherichia coli lipopolysaccharide (1 microgram/ml, 18 h) caused an increase in the release of nitrite (NO2-) and prostaglandin E2 (PGE2), products of NOS and COX, respectively. Production of both NO2- and PGE2 was blocked by the NOS inhibitors NG-monomethyl-L-arginine or aminoguanidine. The effects of NG-monomethyl-L-arginine or aminoguanidine were reversed by coincubation with L-Arg, the precursor for NO synthesis, but not by D-Arg. RAW264.7 cells stimulated for 18 h with lipopolysaccharide in L-Arg-free medium (to reduce NO generation by the endogenous NOS pathway) failed to release NO2- and accumulated at least 4-fold less PGE2 when compared to cells in the presence of L-Arg. PGE2 production elicited by a 15-min arachidonic acid treatment of lipopolysaccharide-induced RAW264.7 cells in L-Arg-deficient medium was decreased 3-fold when compared to the release obtained with cells induced in medium containing L-Arg. To examine the NO activation of the induced form of COX in the absence of an endogenous L-Arg, human fetal fibroblasts were first stimulated for 18 h with interleukin 1 beta. These cells released PGE2 but not NO2-, consistent with the induction of COX but not NOS in the fibroblast. Exogenous NO either as a gaseous solution or released by a NO donor, sodium nitroprusside or glyceryl trinitrate, increased COX activity in the interleukin 1 beta-stimulated fibroblasts by 5-fold; these effects were abolished by coincubation with hemoglobin (10 microM), which binds and inactivates NO, but not by methylene blue, an inhibitor of the soluble guanylate cyclase. Furthermore, sodium nitroprusside (0.25-1 mM) increased arachidonic acid-stimulated PGE2 production by murine recombinant COX-1 and COX-2. These results demonstrate that NO enhances COX activity through a mechanism independent of cGMP and suggest that, in conditions in which both the NOS and COX systems are present, there is an NO-mediated increase in the production of proinflammatory prostaglandins that may result in an exacerbated inflammatory response. The data suggest that NO directly interacts with COX to cause an increase in the enzymatic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991 May 8;1083(2):121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Förstermann U., Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol. 1984 Aug 3;103(1-2):65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- Greenwald J. E., Alexander M. S., Fertel R. H., Beach C. A., Wong L. K., Bianchine J. R. Role of ferric iron in platelet lipoxygenase activity. Biochem Biophys Res Commun. 1980 Sep 30;96(2):817–822. doi: 10.1016/0006-291x(80)91428-x. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hawley-Nelson P., Sullivan J. E., Kung M., Hennings H., Yuspa S. H. Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol. 1980 Aug;75(2):176–182. doi: 10.1111/1523-1747.ep12522602. [DOI] [PubMed] [Google Scholar]
- Heuzé-Joubert I., Mennecier P., Simonet S., Laubie M., Verbeuren T. J. Effect of vasodilators, including nitric oxide, on the release of cGMP and cAMP in the isolated perfused rat kidney. Eur J Pharmacol. 1992 Sep 22;220(2-3):161–171. doi: 10.1016/0014-2999(92)90744-o. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Mason R. P., Tainer B., Eling T. E. The free radical formed during the hydroperoxide-mediated deactivation of ram seminal vesicles is hemoprotein-derived. J Biol Chem. 1982 May 10;257(9):4764–4768. [PubMed] [Google Scholar]
- Kanner J., Harel S., Granit R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids. 1992 Jan;27(1):46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- Karthein R., Nastainczyk W., Ruf H. H. EPR study of ferric native prostaglandin H synthase and its ferrous NO derivative. Eur J Biochem. 1987 Jul 1;166(1):173–180. doi: 10.1111/j.1432-1033.1987.tb13499.x. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Masferrer J. L., Seibert K., Zweifel B., Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Reingold D. F., Watters K., Holmberg S., Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [PubMed] [Google Scholar]
- Rettori V., Gimeno M., Lyson K., McCann S. M. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Korbut R., Anggård E., Vane J. Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2593–2597. doi: 10.1073/pnas.87.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., Pistelli A., Mollace V., Anggård E., Vane J. The metabolism of glyceryl trinitrate to nitric oxide in the macrophage cell line J774 and its induction by Escherichia coli lipopolysaccharide. Biochem Pharmacol. 1992 Jul 7;44(1):17–24. doi: 10.1016/0006-2952(92)90032-e. [DOI] [PubMed] [Google Scholar]
- Schröder H., Leitman D. C., Bennett B. M., Waldman S. A., Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther. 1988 May;245(2):413–418. [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Warren J. B., Coughlan M. L., Williams T. J. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. doi: 10.1111/j.1476-5381.1992.tb14441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J. J., Veldink G. A., Vliegenthart J. F., Boldingh J., Wever R., van Gelder B. F. Demonstration by EPR spectroscopy of the functional role of iron in soybean lipoxygenase-1. Biochim Biophys Acta. 1975 Jan 23;377(1):71–79. doi: 10.1016/0005-2744(75)90287-9. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]