Abstract
Background:
Fluid management is important in critically patients. The aim of this study was to determine the relationship between fluid balance and adverse outcomes of septic shock.
Methods:
A retrospective study was conducted in the medical Intensive Care Unit (ICU) of a tertiary university hospital in Thailand, over a 7-year period.
Results:
A total of 1048 patients with an ICU mortality rate of 47% were enrolled. The median cumulative fluid intake at 24, 48, and 72 h from septic shock onset were 4.2, 7.7, and 10.5 L, respectively. Nonsurvivors had a significantly higher median cumulative fluid intake at 24, 48, and 72 h (4.6 vs. 3.9 L, 8.2 vs. 7.1 L, and 11.4 vs. 9.9 L, respectively, P < 0.001 for all). Nonsurvivors also had a significantly higher cumulative and mean fluid balance within 72 h (5.4 vs. 4.4 L and 2.8 vs. 1.6 L, P < 0.001 for both). In multivariate logistic regression analysis, mean fluid balance quartile within 72 h, was independently associated with an increase in ICU and hospital mortality. Quartile 3 and 4 have statistically significant increases in mortality compared with quartile 1 (odds ratio [95% confidence interval] 3.04 [1.9–4.48] and 4.16 [2.49–6.95] for ICU mortality and 2.75 [1.74–4.36] and 3.16 [1.87–5.35] for hospital mortality, respectively, P < 0.001 for all). In addition, the higher amount of mean fluid balance was associated with prolonged ICU stays.
Conclusions:
Positive fluid balance over 3 days is associated with increased ICU and hospital mortality along with prolonged ICU stays in septic shock patients.
Keywords: Fluid, fluid balance, mortality, prognostic factor, septic shock
Introduction
Septic shock is a life-threatening condition with a high mortality rate.[1,2,3] It requires aggressive treatment and close monitoring in the Intensive Care Unit (ICU). Intravenous fluids, vasopressor administration, early and appropriate antibiotic therapy, source of infection control as well as ventilator support are essential for the treatment of these patients.[2,4]
Intravenous fluid administration is important for stabilizing hemodynamic status and improving tissue oxygenation. However, once there has been adequate fluid resuscitation, further fluid administration may increase intravascular pressure along with vascular permeability, causing fluid leakage which results in tissue edema, decreased oxygenation index, increase intra-abdominal pressure and increased mortality.[1,5,6,7,8]
Rivers et al. reported a prospective, randomized study of the early goal-directed therapy (EGDT) in severe sepsis and septic shock patients.[9] At the first 6 h mark, the EGDT group who received larger fluid volumes, compared to the standard group were associated with a significantly lower mortality. Consequently, aggressive fluid resuscitation, during the first 6 h, has been essential for the management of patients with septic shock.[2] The observational cohort studies of severe sepsis and septic shock patients have demonstrated the benefit of aggressive fluid management. Higher fluid volume resuscitation in the first 3 h, and during the first 3 days of these patients was associated with a significantly lower mortality.[10,11] However, several studies showed contrary results. In a large survey of patients with sepsis, a positive fluid balance was the strongest prognostic factor of ICU mortality.[1] An analysis of the septic shock patients from the Vasopressin and Septic Shock Trial (VASST) showed that a more positive fluid balance at 12 h and cumulatively over 4 days was associated with an increased risk of mortality.[6] A recent retrospective study showed that excessive positive fluid balance was an independent risk factor for mortality in severe sepsis patients.[12,13] Thus, the amount of intravenous fluid administration in septic shock management is still highly controversial.
We, therefore, conducted this study to determine the relationship between fluid balance within 72 h after the onset of septic shock, ICU, and hospital mortality along with the length of stay in the ICU.
Methods
This study was conducted at a tertiary referral university teaching hospital in Southern of Thailand. We studied retrospectively, consecutive patients admitted with septic shock to mixed medical-coronary ICU from January 2005 through until December 2011. Patients with a length of stay <24 h were excluded from this study. This study was approved by the Institutional Ethics Committee.
Definitions
Infection was identified based on clinical history, physical examination, laboratory findings as well as the administration of antibiotics. It was defined according to the International Sepsis Forum Consensus Conference.[14] Septic shock was defined as systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg for a duration of at least 1 h, despite adequate fluid resuscitation or the use of any vasopressors. The onset of septic shock was defined as; the time of vasopressor initiation. Severity was evaluated using the Acute Physiology and Chronic Health Evaluation II (APACHE) II[15] and the Sequential Organ Failure Assessment (SOFA) scores.[16] Organ failure was defined as; a SOFA score of >2 for each involved organ.[1] Community-acquired infection was defined as: Manifestation of infection before or within 48 h after admission, whereas hospital-acquired infection manifested later than 48 h after hospital admission. Acute respiratory distress syndrome (ARDS) was defined according to Berlin definition.[17]
Fluid intake was calculated as the sum of any intravenous fluid and oral feeding, which patients received from the onset of septic shock. Fluid output included urinary output and other outputs such as drainage, thoracentesis, paracentesis, and ultrafiltration. Fluid balance was calculated as the difference between total fluid intake and total output within the first 3 days of admission to the ICU. The mean fluid balance was the calculated for each day during a 3-day admission to the ICU.
Data collection
Our data were derived from a previous prospectively registered data of severity score and sepsis. Patients demographic, laboratory, and clinical data were gathered. This included: Age, gender, source of ICU admission, severity of illness (based on the APACHE II and SOFA scores), site of infection, positive cultures, ARDS, vasopressor use, ICU and hospital outcome, and ICU lengths of stay. The medical electronic database for fluid intake and output, during the first 3 days of an ICU stay was retrospectively reviewed.
Statistical analysis
The primary outcome was ICU mortality, while the secondary outcomes were hospital mortality and ICU lengths of stay. Categorical data were expressed as frequency distributions, using the Chi-squared test to determine if differences existed between groups. Continuous data were reported as; the median with interquartile range and compared by the Wilcoxon's rank sum test. Fluid intake and fluid balance were divided into quartiles for statistical analysis. Kaplan–Meier curves were used to evaluate 24-h fluid intake, and mean fluid balance quartiles then compared with the log-rank test. Variables found to be significant to P < 0.2 on univariate analysis were entered into the multivariate logistic regression analysis. We performed multivariate logistic regression, with backward elimination, to determine the variables independently associated with the ICU and hospital mortality. Cox proportional hazards model was used to select factors associated with ICU lengths of stay. The multicollinearity was assessed by using a variance inflation factor and any variable with variance factors >2.5 were rejected from the model.[18] The mean fluid balance was used in the models due to high multicollinearity with cumulative fluid balance. All statistics were two-tailed and the value P < 0.05 was considered to be statistically significant. The analyses were performed using R 3.1 software and Stata 7 software (Stata Corporation, College Station Tx, USA).
Results
During the 7-year period 1048 patients, diagnosed with septic shock, were enrolled in this study. The ICU and hospital mortalities were 47% and 57.8%. The baseline characteristics in addition to the fluid volumes are shown in Table 1. Nonsurvivors were statistically younger had greater severity of illness (measured by the APACHE II and SOFA scores), shorter lengths of ICU stay, fewer community-acquired infections, along with a higher frequency of ARDS [Table 2]. Nonsurvivors had a significantly higher median cumulative fluid intake within 24, 48, and 72 h, cumulative fluid balance and mean fluid balance within 72 h [Table 2]. Nonsurvivors also received daily fluid intakes at 48 and 72 h at a higher rate than survivors (3.69 [2.71–4.9] vs. 3.11 [2.27–4.13]) and (3.36 [2.44–4.27] vs. 2.61 [2–3.38]), P < 0.001 for both [Figure 1]. The fluid intake at 24 h was significantly higher in patients with nosocomial-acquired septic shock than those with community-acquired septic shock (4.38 [3.32–5.72] vs. 4.1 [3.08–5.37], P = 0.02). However, cumulative mean fluid balance within 72 h was not clinically significant between these patients (2.07 [1.4–3.38] vs. 1.99 [0.99–3], P = 0.02). Mean fluid intake at 24 h and cumulative mean fluid balance within 72 h were comparable between septic shock patients with or without ARDS (4.05 [3.24–5.28] vs. 4.2 [3.12–5.68] and 1.98 [1.23–3.09] vs. 2.04 [1.14–3.14], respectively).
Table 1.
Baseline characteristics and fluid volumes
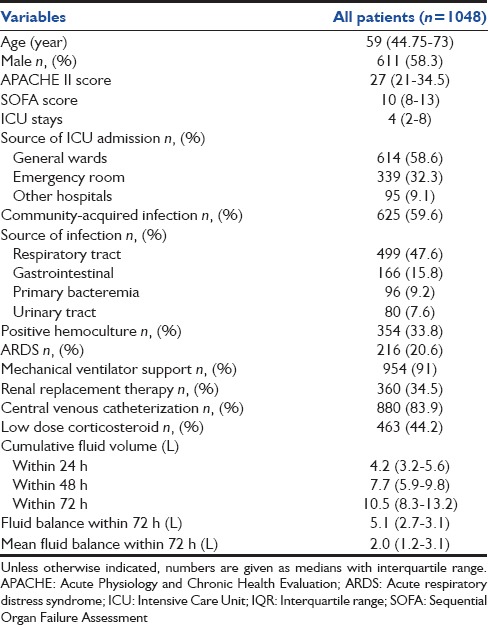
Table 2.
Clinical characteristics of ICU survivors and non-ICU survivors
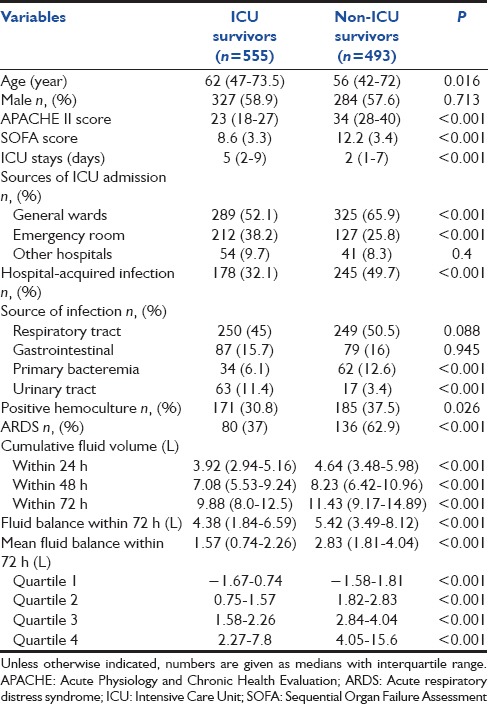
Figure 1.
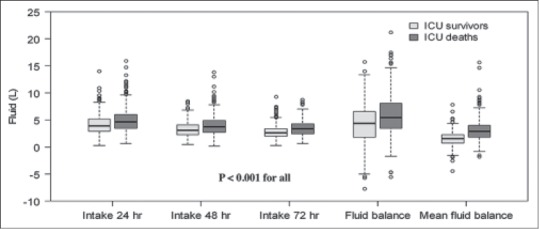
Box plots depicting daily fluid intake and fluid balance within 72 h
Kaplan–Meier survival curves, estimated by 24-h fluid intake and mean fluid balance within 72 h, are shown in Figures 2 and 3, respectively. Patients with excessive fluid balance showed a lower survival rate. At 24 h, the risk of survival in quartile 4 was significantly lower than in quartile 1, 2, and 3. (P < 0.001, Figure 2). The median survival time for 24 h fluid quartile 1, 2, 3, and 4 was 15, 10, 10, and 6 days, respectively. Similarly, cumulative fluid balance within 72 h, quartile 4 showed a significant increase in mortality than other quartiles (P < 0.001, Figure 3). The patients in quartile 4 of cumulative fluid balance within 72 h had a shorter median survival time than patients in quartile 3, 2, and 1 (2, 6, 14, and 16 days, respectively).
Figure 2.
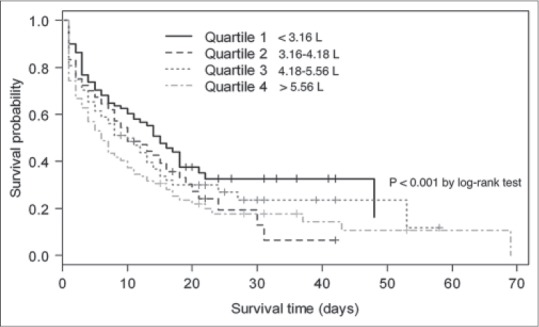
Kaplan–Meier survival curve for 24-h fluid intake quartiles
Figure 3.
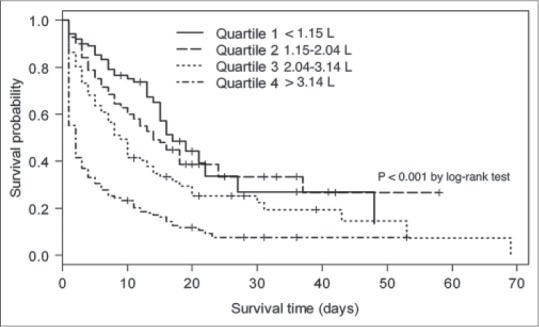
Kaplan–Meier survival curve for mean fluid balance quartiles within 72 h
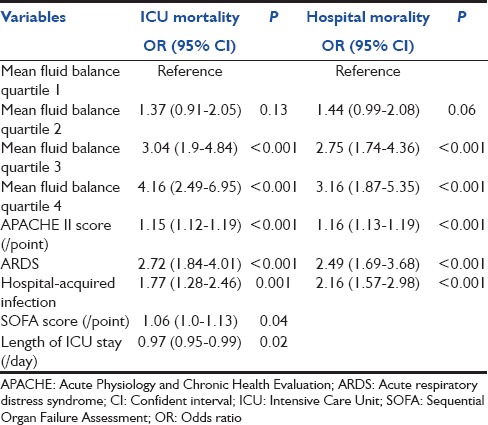
Univariate analysis showed that several factors were associated with a significantly higher ICU mortality in patients with septic shock these being: Age (P = 0.021), APACHE II score (P < 0.001), SOFA score (P < 0.001), ICU stays (P < 0.001), hospital-acquired infection (P < 0.001), urinary tract infection (P < 0.001), primary bacteremia (P < 0.001), positive hemoculture (P = 0.022), ARDS (P < 0.001), vasopressor use (P = 0.027), fluid intake within the first 24 h (P < 0.001), 48 h (P < 0.001), and 72 h (P < 0.001), and mean fluid balance within 72 h (P < 0.001). However, after multivariate logistic regression analysis parameters showed independent predictors of increased risk of ICU or hospital mortality are presented in Table 3. Mean cumulative fluid balance at day 3 shows a strong impact on the ICU and hospital mortality. Quartiles 3 and 4 have statistically significant increases in both the ICU and hospital mortality, compared with quartile 1 [Table 3]. However, quartile 2 showed a nonsignificant trend to increased mortality. In addition to this, the higher amount of mean fluid balance within 72 h was associated with an increase in ICU stays (quartile 3 hazard ratio 2.19, 95% confidence interval [CI]: 1.62–2.96, and quartile 4 h 2.54, 95% CI: 1.86–3.48, P < 0.001 for both) when adjusting for age, APACHE II, SOFA scores, and comorbidities.
Table 3.
Multivariate logistic regression analysis of independent risk factors for ICU and hospital mortality
Discussion
In this study, the association between fluid volume and unfavorable outcomes in patients with septic shock was explored. We found a dose-response between 24-h fluid intake and 72-h mean fluid balance quartiles and ICU mortality. Whereas multivariate logistic regression analysis indicated that only mean fluid balance within 72 h of the onset of septic shock was a significantly independent risk factor for ICU and hospital mortality coupled with the increase of ICU lengths of stay.
Early and aggressive fluid resuscitation is essential for the management of patients with severe sepsis and septic shock.[2,9] However, a more positive fluid balance was associated with the adverse outcome in these patients. Two retrospective studies showed that positive fluid balance was associated with an increased mortality in patients with septic shock.[13,19] The large European study, Sepsis Occurrence in Acutely Ill Patients, demonstrated that positive fluid balance within the first 72 h of onset of sepsis did correlate with ICU mortality.[1] In the VASST trial, positive fluid balance at both 12 h and over a period of 4 days correlated significantly with increased 28-day mortality.[6] Similar to our study, quartiles of fluid balance within 72 h were associated with an increased risk of mortality in septic shock patients.
Several studies have found relationships between positive fluid balance and the worse outcome in other groups of critically ill patients. Fluid accumulation in patients with acute lung injury was associated with increased mortality[19] and the length of stay.[5] In surgical critically ill patients, excessive positive fluid balance was related to mortality[20,21,22] and ICU complications.[22] de Almeida et al. reported that positive fluid balance was independently associated with mortality in critically ill cancer patients.[23] Teixeira et al. established that a higher fluid balance is correlated with 28-day mortality in critically ill patients with acute kidney injury.[24]
Early and aggressive fluid resuscitation have been recommended for management in septic shock patients.[2] Several studies have demonstrated that aggressive fluid resuscitation during 3 and 6 h of severe sepsis and septic shock are associated with a decrease in mortality. Data from EGDT showed that the EGDT group, who received larger fluid volumes within the first 6 h was associated with significantly lower 60-day mortality.[9] Recent retrospective study found that higher fluid resuscitation within the first 3 h is associated with a decrease in hospital mortality in severe sepsis and septic shock.[11] Cumulative fluid accumulation is common in critically ill patients due to aggressive hemodynamic resuscitation. However, after hemodynamic stabilization, further fluid administration depended on the individual basis of physical examination, blood chemistry, and clinical course.[7,25] Fluid removal is indicated when fluid accumulation contributes or is likely to contribute to patient morbidity. Intravenous diuretics or continuous ultrafiltration should be used to promote negative fluid balance.[25,26] Therefore, intensivists must find a balance among fluid resuscitation, hemodynamic stability, and organ perfusion while avoiding excessive fluid accumulation.
This study demonstrated that excessive fluid balance correlated with prolonged ICU stays when adjusted with severity of illness and comorbidities. Our results are consistent with previous studies conducted on other groups of critically ill patients. Stein et al. found a significant association between fluid overload and the length of the ICU stay in cardiac surgical patients.[27] Fluid and catheter treatment trial showed that conservative fluid management shorted duration of ICU stays in acute lung injury patients.[5]
There are several mechanisms for explaining the correlation of positive fluid balance and adverse outcomes in sepsis patients. Positive fluid balance could increase extravascular lung water,[28] prolong mechanical ventilator days,[5] and contribute to the occurrence of ventilator-associated pneumonia. In addition, the positive fluid balance could also result in intra-abdominal hypertension along with abdominal compartment syndrome contributing to the development of organ dysfunction.[7,28] Furthermore, positive fluid balance is associated with delayed renal recovery[29,30] and increased risk of acute kidney injury.[27]
Our study included a larger number of septic shock patients with higher disease severity than previous reports.[6,10,12,13] However, this study has some limitations. First, this being a retrospective study, we were unable to determine a causal relationship between fluid balance and outcomes. Severity of illness, hemodynamic monitoring techniques and endpoints, types of fluid, and fluid management protocol may be possible confounders and may not be fully accounted for in are retrospective analysis. Hence, we would suggest further randomized controlled trials, so as to best determine the fluid balance in the fluid management of patients with septic shock. Second, this study was performed in the mixed medical-coronary ICU of a tertiary university teaching hospital. Due to these factors the cases-mixed could be more severe, and the results may not be generalizable to other types of institutions or ICUs. Third, we did not estimate the amount of early fluid administration during first 3–6 h. Finally, the consideration of the fluid balance without considering the time of recovery from shock would change the mean cumulative fluid balance.
Conclusions
A more positive cumulative fluid balance over the period of 3 days from the onset of septic shock is associated with higher ICU and hospital mortality as well as ICU length of stay. Physicians should carefully assess the need for fluids in both early and late resuscitation periods. Restrictive fluid protocols need further study to determine the efficacy when compared to the standard fluid resuscitation protocols.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khwannimit B, Bhurayanontachai R. The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting. Epidemiol Infect. 2009;137:1333–41. doi: 10.1017/S0950268809002027. [DOI] [PubMed] [Google Scholar]
- 4.Raghavan M, Marik PE. Management of sepsis during the early “golden hours”. J Emerg Med. 2006;31:185–99. doi: 10.1016/j.jemermed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 7.Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361–80. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, de Louw E, Niemi M, Nelson R, Mark RG, Celi LA, et al. Association between fluid balance and survival in critically ill patients. J Intern Med. 2015;277:468–77. doi: 10.1111/joim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.Smith SH, Perner A. Higher vs.lower fluid volume for septic shock: Clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012;16:R76. doi: 10.1186/cc11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Ramar K, Park JG, Gajic O, Li G, Kashyap R. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: A retrospective cohort study. Chest. 2014;146:908–15. doi: 10.1378/chest.13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30:97–101. doi: 10.1016/j.jcrc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 18.Miller RR, 3rd, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–9. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 20.Shim HJ, Jang JY, Lee SH, Lee JG. The effect of positive balance on the outcomes of critically ill noncardiac postsurgical patients: A retrospective cohort study. J Crit Care. 2014;29:43–8. doi: 10.1016/j.jcrc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, et al. Impact of positive fluid balance on critically ill surgical patients: A prospective observational study. J Crit Care. 2014;29:936–41. doi: 10.1016/j.jcrc.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Silva JM, Jr, de Oliveira AM, Nogueira FA, Vianna PM, Pereira Filho MC, Dias LF, et al. The effect of excess fluid balance on the mortality rate of surgical patients: A multicenter prospective study. Crit Care. 2013;17:R288. doi: 10.1186/cc13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Almeida JP, Palomba H, Galas FR, Fukushima JT, Duarte FA, Nagaoka D, et al. Positive fluid balance is associated with reduced survival in critically ill patients with cancer. Acta Anaesthesiol Scand. 2012;56:712–7. doi: 10.1111/j.1399-6576.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein S, Bagshaw S, Cecconi M, Okusa M, Wang H, Kellum J, et al. Pharmacological management of fluid overload. Br J Anaesth. 2014;113:756–63. doi: 10.1093/bja/aeu299. [DOI] [PubMed] [Google Scholar]
- 26.Rosner MH, Ostermann M, Murugan R, Prowle JR, Ronco C, Kellum JA, et al. Indications and management of mechanical fluid removal in critical illness. Br J Anaesth. 2014;113:764–71. doi: 10.1093/bja/aeu297. [DOI] [PubMed] [Google Scholar]
- 27.Stein A, de Souza LV, Belettini CR, Menegazzo WR, Viégas JR, Costa Pereira EM, et al. Fluid overload and changes in serum creatinine after cardiac surgery: Predictors of mortality and longer intensive care stay. A prospective cohort study. Crit Care. 2012;16:R99. doi: 10.1186/cc11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, et al. Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;2:S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27:956–61. doi: 10.1093/ndt/gfr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]


