Abstract
We describe a case of Takotsubo cardiomyopathy in a case of pituitary macroadenoma in acute adrenal crisis. A 48-year-old man presented with acute onset altered sensorium, vomiting, and gasping. On admission, he was unresponsive and hemodynamically unstable. He was intubated and ventilated and resuscitated with fluids and inotropes. The biochemical evaluation revealed hyponatremia, hyperkalemia, and hypocortisolism. Hyponatremia was corrected with 3% hypertonic saline. Contrast enhanced computed tomography (CT) scan of the brain revealed a sellar-suprasellar mass with hypothalamic extension with no evidence of pituitary apoplexy. A diagnosis of invasive pituitary adenoma with the Addisonian crisis was made and steroid replacement was initiated. Despite volume resuscitation, he had persistent refractory hypotension, recurrent ventricular tachycardia, and metabolic acidosis. Electrocardiogram (ECG) showed ST elevation and T-wave inversion in lateral leads; cardiac-enzymes were increased suggestive of acute coronary syndrome. Transthoracic echocardiography showed severe regional wall motion abnormalities (RWMAs) involving left anterior descending territory and low ejection fraction (EF). Coronary angiogram revealed normal coronaries, apical ballooning, and severe left ventricular dysfunction, consistent with a diagnosis of Takotsubo's cardiomyopathy. Patient was managed with angiotensin-converting enzyme inhibitors and B-blockers. He improved over few days and recovered completely. At discharge, ECG changes and RWMA resolved and EF normalized to 56%. In patients with Addisonian Crisis with persistent hypotension refractory to optimal resuscitation, possibility of Takotsubo's cardiomyopathy should be considered. Early recognition of association of Takotsubos cardiomyopathy in neurological conditions, prompt resuscitation, and supportive care are essential to ensure favorable outcomes in this potentially lethal condition.
Keywords: Pituitary adenoma, secondary adrenal insufficiency, takotsubos cardiomyopathy
Introduction
Reversible left ventricular (LV) dysfunction and Takotsubo's cardiomyopathy has been described in endocrine conditions such as Addison's disease,[1] isolated adrenocorticotropic hormone deficiency,[2] primary adrenal insufficiency in adrenal crisis,[3] pheochromocytoma,[4] thyrotoxicosis,[5] and multiple endocrine neoplasia.[6] It has also been described in various neurological conditions such as stroke,[7] Guillian–Barre syndrome,[8] seizures,[9] subarachnoid bleed[10,11] empty sella syndrome,[12] and reversible posterior leukoencephalopathy syndrome.[13] The most common cause of secondary adrenal insufficiency is a tumor of the hypothalamic-pituitary region, usually associated with panhypopituitarism caused by tumor growth or treatment with surgery or irradiation. However, Takotsubo's cardiomyopathy in a case of the pituitary tumor with secondary adrenal insufficiency has not been described to the best of our knowledge.
Case Report
A 48-year-old man with no known comorbid illness, presented to the emergency department with a progressive decrease in vision and headache for 2 months, vomiting for 1 day, and acute onset altered sensorium for 4 h. At admission, the patient was unresponsive with Glasgow Coma Scale score of 6/15 (E1M4V1). Endotracheal intubation was done to secure the airway, and mechanical ventilation was initiated. He was resuscitated with intravenous (IV) fluids (2.0 L of crystalloid and 1.0 L of colloid within 1 h) and vasopressors (noradrenaline infusion at 0.2 mcg/kg/h). Chest X-ray and ECG were normal. Laboratory evaluation revealed hyponatremia (108 mEq/L) and hyperkalemia (5.5 mEq/L). IV hydrocortisone 100 mg was administered. The temperature was 36°C.
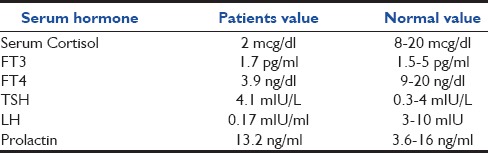
In view of the progressive loss of vision and altered sensorium, contrast enhanced CT brain was performed. It revealed a dense sellar-suprasellar mass lesion extending into the hypothalamus, right cavernous sinus, and anteriorly under the frontal lobe of size 40 mm × 23 mm. suggestive of pituitary adenoma without the evidence of pituitary apoplexy or hydrocephalus. Hormonal profile revealed hypocortisolism [Table 1]. A provisional diagnosis of hypopituitarism with secondary adrenal insufficiency in adrenal crisis was considered.
Table 1.
Hormonal profile of the patient
Resuscitation was continued, and central venous pressure (CVP) was maintained between 8 and 10 cm of H2O. Hyponatremia was corrected with 3% hypertonic saline. After adequate fluid replacement (CVP-12), noradrenaline infusion (0.5 mcg/kg/h), and dobutamine (3 mcg/kg/h) infusions were started. Despite continuing active resuscitation and hormonal replacement, there was persistent hypotension, tachycardia, and worsening acidosis. A cardiac cause for hypotension was suspected.
A 12 lead ECG revealed sinus tachycardia with T wave inversion and ST-elevation in lead 1, 2, aVF, avL, V1-V6, Poor R wave progression in V1-V4 [Figure 1]. Serum biomarkers showed borderline elevation (troponin I - 0.55 ng/ml and CPK-MB -264). Bedside transthoracic echocardiography revealed regional wall motion abnormality involving the left anterior descending artery territory with ejection fraction (EF) <40% [Figure 2]. An acute coronary event was suspected, and the patient was started on antiplatelets and anticoagulant (aspirin 300 mg, clopidogrel 600 mg, and IV Heparin 600 IU/h).
Figure 1.
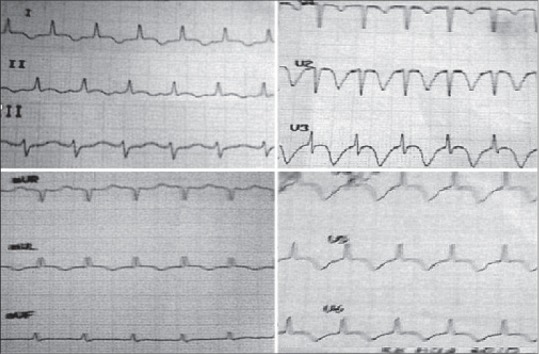
Electrocardiogram changes at the time of hypotension
Figure 2.
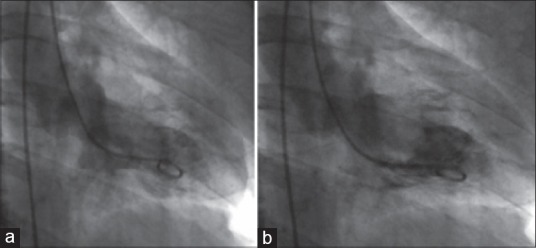
Left ventricular angiogram (a) hyperkinetic base (b) akinetic apex
The patient developed two episodes of ventricular tachycardia, which reverted to sinus rhythm following defibrillation. In view of the refractory hemodynamic instability, emergency coronary angioplasty was done. Coronary angiogram showed normal coronaries and ventriculogram revealed apical ballooning of left ventricle and severe LV dysfunction with an EF of <30% [Figure 3]. A diagnosis of stress cardiomyopathy or Takutsubo's syndrome was considered. He was managed conservatively with dobutamine and noradrenaline. Inotropic supports were slowly tapered over 24 h and stopped.
Figure 3.
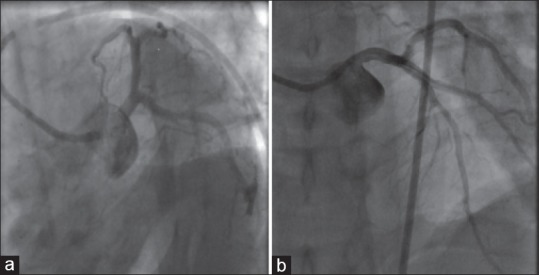
(a and b) Angiogram demonstrating normal coronaries
Beta-blocker therapy was initiated with atenolol 25 mg BD. His clinical condition improved rapidly over the next few days. The patient was weaned off mechanical ventilation and was continued on replacement doses of hydrocortisone and fludrocortisones. An echocardiogram was done after 7 days showed no wall motion abnormalities and complete recovery of LV function (EF 54%).
Discussion
Stress cardiomyopathy, also referred to as Takotsubo's cardiomyopathy or LV apical ballooning syndrome, is a clinical syndrome which manifests as chest pain, ST segment elevation, mild elevation of cardiac enzyme, biomarker levels, and transient apical systolic LV dysfunction.[14] As seen in our patient, the clinical presentation markedly resembles myocardial ischemia. However, there is an absence of obstructive epicardial coronary disease.[15,16] It is more common in postmenopausal women.[17,18] Approximately, 1.2–2.2% of admissions for acute myocardial infarction have been ultimately diagnosed to be apical ballooning syndrome due to various etiologies.[16,19] The pathophysiology of stress cardiomyopathy remains unclear. Emotional stress and the corresponding increase in catecholamines have been reported in many patients following Takotsubo's cardiomyopathy.[20] Proposed mechanisms for catecholamine-mediated stunning in stress cardiomyopathy include epicardial spasm, microvascular dysfunction, hyperdynamic contractility with midventricular or outflow tract obstruction, and direct effects of catecholamines on cardiomyocytes.[21] Recent reviews have proved that the apical ballooning that characterizes Takotsubo's cardiomyopathy reflects toxic high local concentrations of catecholamines and not coronary artery or microvascular disease. The elevated catecholamine levels have been shown to cause direct myocyte injury via an increase in intracellular calcium and oxygen-free radicals. The pattern of LV dysfunction may result from both myocardial cellular rupture and withdrawal of beta-adrenoceptors. The LV apex contains a higher concentration of adrenoceptors. Therefore, myocardial responsiveness to adrenergic stimulation is pronounced in the apex.[22] Another proposed mechanism is that the cardiac functional and ECG abnormalities in Takotsubo cardiomyopathy might reflect activation of central neurogenic mechanisms analogous to those evoked by subarachnoid hemorrhage.[23] Myocardial histopathological findings seen in acute myocardial dysfunction associated with brain injury are similar to that of Takotsubo's cardiomyopathy.[24] Therefore, Takotsubo cardiomyopathy may reflect stunned myocardium from a neurogenic source. Although a reversible syndrome, it can lead to complications such as left heart failure, LV intracavitary obstruction, and arrhythmias. In extreme cases, it can lead to sudden and unexpected death.[7] However, aggressive management is associated with good prognosis. Patients with hypopituitarism presenting with hemodynamic instability present a diagnostic dilemma as to whether it is primarily an Addisonian crisis or a myocardial event. In this case, there was an acute hemodynamic worsening, with inverted T waves and elevated cardiac biomarkers indicative of a cardiac event, soon after the initial stabilization of the Addisonian crisis. Although the clinical picture resembled myocardial ischemia, coronary angiography did not reveal any obstructive lesions and prompt resuscitation and supportive measures helped in the management of a potentially lethal condition. Therefore, in patients with pituitary adenoma with panhypopituitarism, cardiac dysfunction due to hyper catecholaminergic states may present as potentially reversible conditions such as Takotsubo's cardiomyopathy. With more reports of neurological conditions either precipitating or presenting as Takotsubo's cardiomyopathy being reported, it is to be an important differential diagnosis in patients with neurological conditions presenting with hemodynamic instability. Although the exact pathogenesis is as yet unclear, the relationship between cardiac dysfunction secondary to neurological conditions is being firmly established.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Punnam SR, Gourineni N, Gupta V. Takotsubo cardiomyopathy in a patient with Addison disease. Int J Cardiol. 2010;144:e34–6. doi: 10.1016/j.ijcard.2008.12.191. [DOI] [PubMed] [Google Scholar]
- 2.Ukita C, Miyazaki H, Toyoda N, Kosaki A, Nishikawa M, Iwasaka T. Takotsubo cardiomyopathy during acute adrenal crisis due to isolated adrenocorticotropin deficiency. Intern Med. 2009;48:347–52. doi: 10.2169/internalmedicine.48.1662. [DOI] [PubMed] [Google Scholar]
- 3.Wolff B, Machill K, Schulzki I, Schumacher D, Werner D. Acute reversible cardiomyopathy with cardiogenic shock in a patient with Addisonian crisis: A case report. Int J Cardiol. 2007;116:e71–3. doi: 10.1016/j.ijcard.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, Gutterman DD. Pheochromocytoma with reversible focal cardiac dysfunction. Am Heart J. 1995;130:909–11. doi: 10.1016/0002-8703(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 5.Goldman LE, Sahlas DJ, Sami M. A case of thyrotoxicosis and reversible systolic cardiac dysfunction. Can J Cardiol. 1999;15:811–4. [PubMed] [Google Scholar]
- 6.Frustaci A, Loperfido F, Gentiloni N, Caldarulo M, Morgante E, Russo MA. Catecholamine-induced cardiomyopathy in multiple endocrine neoplasia. A histologic, ultrastructural, and biochemical study. Chest. 1991;99:382–5. doi: 10.1378/chest.99.2.382. [DOI] [PubMed] [Google Scholar]
- 7.Cho HJ, Kim HY, Han SH, Kim HJ, Moon YS, Oh J. Takotsubo cardiomyopathy following cerebral infarction involving the insular cortex. J Clin Neurol. 2010;6:152–5. doi: 10.3988/jcn.2010.6.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iga K, Himura Y, Izumi C, Miyamoto T, Kijima K, Gen H, et al. Reversible left ventricular dysfunction associated with Guillain-Barré syndrome – An expression of catecholamine cardiotoxicity? Jpn Circ J. 1995;59:236–40. doi: 10.1253/jcj.59.236. [DOI] [PubMed] [Google Scholar]
- 9.Stöllberger C, Wegner C, Finsterer J. Seizure-associated takotsubo cardiomyopathy. Epilepsia. 2011;52:e160–7. doi: 10.1111/j.1528-1167.2011.03185.x. [DOI] [PubMed] [Google Scholar]
- 10.Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112:3314–9. doi: 10.1161/CIRCULATIONAHA.105.558239. [DOI] [PubMed] [Google Scholar]
- 11.Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–6. doi: 10.1161/01.str.30.4.780. [DOI] [PubMed] [Google Scholar]
- 12.Sakihara S, Kageyama K, Nigawara T, Kidani Y, Suda T. Ampulla (Takotsubo) cardiomyopathy caused by secondary adrenal insufficiency in ACTH isolated deficiency. Endocr J. 2007;54:631–6. doi: 10.1507/endocrj.k07-012. [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou J, Tsirantonaki M, Koukoulitsios G, Papageorgiou D, Mandila C, Karakitsos D, et al. Reversible posterior leukoencephalopathy syndrome and takotsubo cardiomyopathy: The role of echocardiographic monitoring in the ICU. Hellenic J Cardiol. 2009;50:436–8. [PubMed] [Google Scholar]
- 14.Grawe H, Katoh M, Kühl HP. Stress cardiomyopathy mimicking acute coronary syndrome: Case presentation and review of the literature. Clin Res Cardiol. 2006;95:179–85. doi: 10.1007/s00392-006-0346-2. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001;38:11–8. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 16.Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): Frequency, mechanisms, and prognosis. Chest. 2007;132:809–16. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 17.Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: First series in white patients. Heart. 2003;89:1027–31. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–9. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 19.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27:1523–9. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 20.Sealove BA, Tiyyagura S, Fuster V. Takotsubo cardiomyopathy. J Gen Intern Med. 2008;23:1904–8. doi: 10.1007/s11606-008-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy – A novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–9. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, et al. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–8. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- 23.Benarroch EE. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 24.White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation. 1995;92:2183–9. doi: 10.1161/01.cir.92.8.2183. [DOI] [PubMed] [Google Scholar]


