Abstract
Background:
Maintenance pemetrexed is a standard treatment option for selected non squamous nonsmall cell lung carcinoma patients having a response to platin based doublet. We conducted a clinical audit of such selected patients and report the outcome among the Indian population.
Aim:
To evaluate the outcomes with maintenance pemetrexed in the patients with locally advanced and metastatic adenocarcinoma lung.
Objectives:
To calculate the progression free survival (PFS), overall survival (OS), and factors affecting the outcome.
Materials and Methods:
Data of patients with locally advanced and metastatic adenocarcinoma lung were retrieved from prospectively maintained lung cancer database registered between June 2011 and March 2014. The patients who achieved partial response (n = 87) or stable disease (n = 101) after 6 cycles of pemetrexed platin based doublet and received the maintenance pemetrexed were selected for final analysis (n = 188). Kaplan–Meir survival analysis was used for PFS and OS. Log rank test was used to evaluate the factors affecting the outcome.
Results:
Median follow-up is 14 months. The median number of maintenance pemetrexed cycles received is 6 (1–38). Common reason for the discontinuation are disease progression (n = 127), renal toxicity (n = 4), and social/financial (n = 7). Median PFS and OS are 8 months and 20 months, respectively. The patients with baseline pleural effusion had better PFS (9 months vs. 7 months, P = 0.02) and OS (26 months vs. 18 months, P = 0.05). The patients receiving more than 6 cycles of maintenance had improved PFS (12 vs. 7 months, P = 0.002) and OS (26 vs. 16 months, P = 0.05).
Conclusion:
Maintenance pemetrexed is feasible and well tolerated by the majority of Indian patients who achieved the response after platin based doublet. The patients with baseline pleural effusion benefit more with maintenance pemetrexed.
Keywords: Effusion, maintenance, pemetrexed
INTRODUCTION
Lung cancer is the leading cause of death from cancer with an estimated 1.8 million new case per year. In India, approximately 63,000 new lung cancer cases are reported every year.[1] More than 85% of which are nonsmall cell lung carcinoma (NSCLC) and 40% of them present with either malignant effusion or metastatic disease.[1,2] Therapeutic options include cytotoxic chemotherapy and targeted therapy intended to prolong survival, control disease related symptoms and improve the quality of life (QOL).[3] Despite the significant advances in the treatment of advanced NSCLC, overall prognosis remains poor.[3,4] Recently, maintenance therapy has been evaluated and accepted as a standard treatment option for selected patients with disease control after the induction chemotherapy and good performance status. We conducted the clinical audit of our prospectively maintained lung cancer database to evaluate the outcome of the patients who received maintenance pemetrexed and, hence present first data from the Indian subcontinent.
MATERIALS AND METHODS
We retrieved the data of patients with locally advanced and metastatic NSCLC from our prospectively maintained lung cancer database in our institute registered between June 2011 and March 2014. For this study, we selected the patients with non squamous NSCLC histology who have received the induction pemetrexed platinum doublet and subsequently received maintenance pemetrexed after achieving disease control. All patients having cytological or histological diagnosis of locally advanced and metastatic non squamous NSCLC (stage IIIB and stage IV), no previous systemic therapy, age more than 18 years, one or more evaluable lesion as per Response Evaluation in Solid Tumor (RECIST, version 1.1) and an adequate organ function were included for analysis. This study was approved by the Institutional Ethics Committee.
During the induction phase, patients were treated with intravenous pemetrexed 500 mg/m2 and intravenous cisplatin 75 mg/m2 or carboplatin (area under the curve = 5) on day 1 of 21 days cycle for 6 cycles. This phase is followed by a maintenance phase of maintenance pemetrexed 500 mg/m2 every 3 weeks in the patients who have achieved the disease control (complete or partial response or stable disease) after the induction chemotherapy. Maintenance pemetrexed was delivered until the disease progression, unacceptable adverse effects or decision of patient/physician to stop further therapy. During both phases, all the patients received folic acid, Vitamin B12, and prophylactic dexamethasone as per the standard recommendation with pemetrexed based therapy. Response evaluation was done after every 3 cycles of chemotherapy with contrast enhanced computed tomography scan during the induction phase and continued during the maintenance phase. Follow-up was taken from the case records, electronic medical records, and telephonic conversation with a patient or their relatives.
The objective of our study was to calculate progression free survival (PFS), overall survival (OS), and factors affecting the outcome in the non squamous NSCLC patients receiving maintenance pemetrexed. PFS was defined as the interval between the date of first induction chemotherapy till the date of progression or death. OS was defined as the interval between the date of first induction chemotherapy till the date of death. Kaplan-Meier method was used to analyze PFS and OS. Log rank test was used to access the factors affecting the outcome.
RESULTS
Between June 2011 and March 2014, 384 patients were diagnosed with stage IIIB and stage IV non squamous NSCLC histology and received induction pemetrexed platin doublet as per our database. After the careful scrutiny, 52 patients were excluded due to the erroneous entry in a database such as wrong histology, previous chemotherapy, Gefitinib maintenance or upfront, no maintenance, wrong case file numbers, etc., [Figure 1]. Out of remaining 332 patients, 66 patients (20%) had disease progression after 3 cycles while 78 (23%) had disease progression after 3 cycles of induction chemotherapy. Remaining 188 (57%) who received maintenance pemetrexed were chosen for the final analysis [Table 1].
Figure 1.
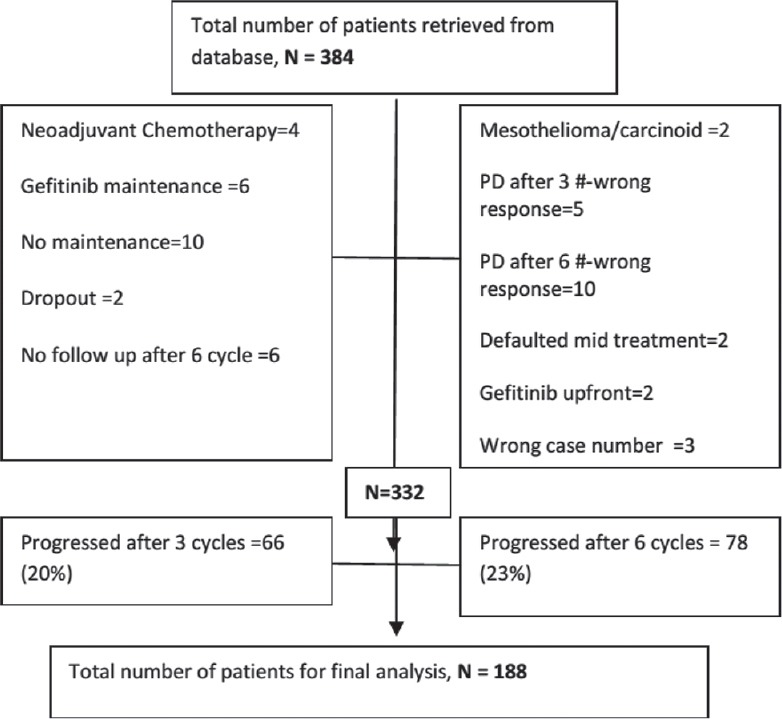
CONSORT diagram representing the schema for the patients selected for final analysis
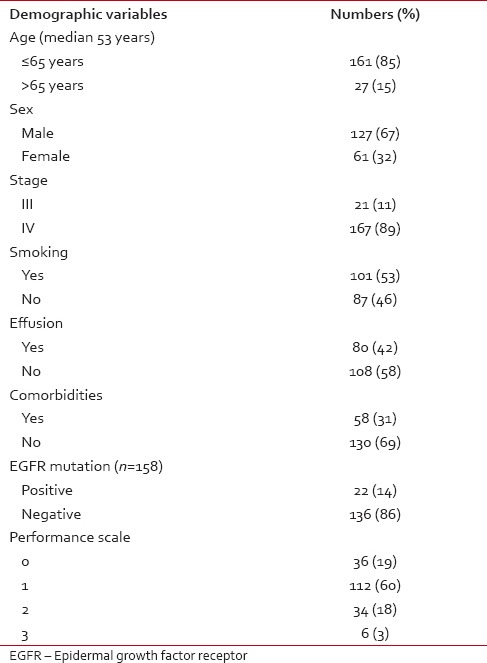
Table 1.
Demographic profile of patients who received maintenance pemetrexed
After 6 cycles of induction chemotherapy, 87 patients (26%) achieved complete or partial response while 188 (57%) achieved the disease control (complete or partial response or stable disease). The median number of maintenance pemetrexed cycles received is 6, with 96 patients (51%) receiving more than 6 cycles. Median follow-up is 14 months. For the patients receiving maintenance pemetrexed (n = 188), their median PFS and OS are 8 months and 20 months, respectively [Figures 2 and 3]. The benefit of maintenance pemetrexed was seen irrespective of age, baseline PS, smoking status, comorbidities, epidermal growth factor receptor (EGFR) mutation status, and response to induction chemotherapy [Table 2]. The patients with baseline pleural effusion had better PFS (9 months vs. 7 months, P = 0.02) and OS (26 months vs. 18 months, P = 0.05) [Figure 4]. Similarly, the patients receiving more than 6 cycles of maintenance pemetrexed had improved PFS (12 months vs. 7 months, P = 0.002) and OS (26 months vs. 16 months, P = 0.05).
Figure 2.
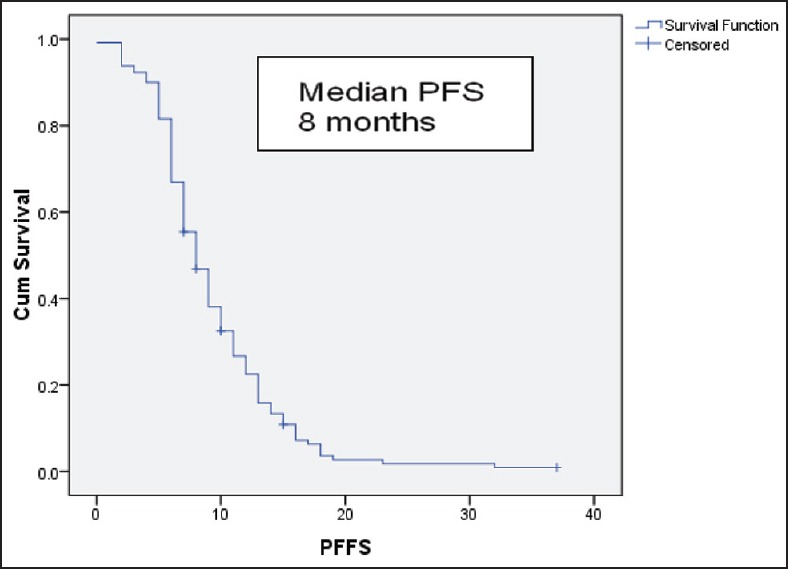
Progression free survival for the patients receiving maintenance pemetrexed
Figure 3.
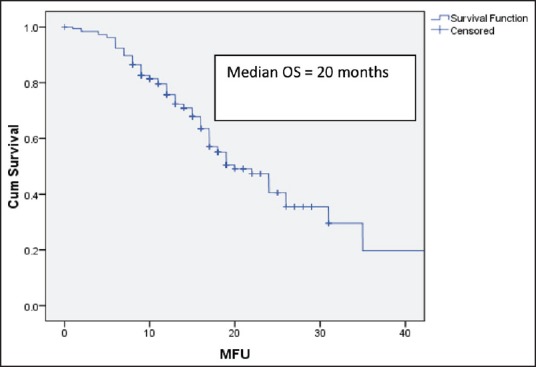
Overall survival for the patients receiving maintenance pemetrexed
Table 2.
Factors affecting outcome with maintenance pemetrexed
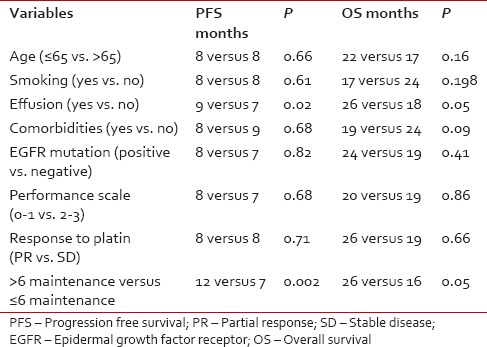
Figure 4.
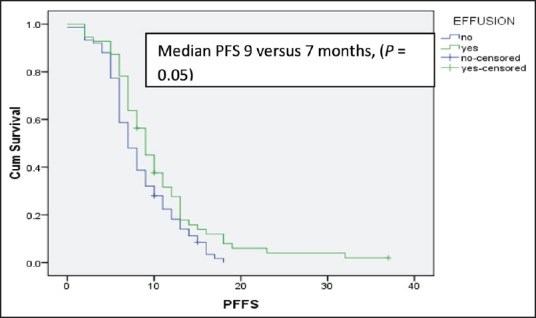
Median progression free survival for the patients receiving maintenance pemetrexed with or without the baseline pleural effusion
Most common reason for discontinuation of maintenance pemtrexed are disease progression (n = 127), renal toxicity (n = 4), and social financial constraints (n = 7). Total 102 out of 188 patients (54%) went on to receive second line therapy, with the majority of patients received single agent docetaxel (n = 49) followed by erlotinib (n = 22), gefitinib (n = 15), paclitaxel (10), crizotinib (n = 4), and gemcitabine (n = 2). EGFR mutation status was available in 158 patients (82%). Twenty-two patients (14%) had EGFR mutation positive. 18 out of 22 patients had disease progression after maintenance pemetrexed and went on to receive gefitinib (n = 10) and erlotinb (n = 8) while 4 patients are still on maintenance pemetrexed at the time of analysis. The benefit of maintenance pemetrexed was evident irrespective of EGFR mutation status.
DISCUSSION
Outcomes of locally advanced and metastatic lung carcinoma have reached a plateau with the treatment of induction chemotherapy. Maintenance therapy in the patients with disease control after the induction chemotherapy has recently shown to improve the outcomes.[5,6,7,8] Maintenance therapy when given in the patients with good performance status prevents the worsening of performance status and prolongs the time to progression such that more patients remain eligible for second line therapy at the disease progression. Use of maintenance therapy in selected patients has been shown to prolong the time to worsening of symptoms such as pain, hemoptysis, and reduce analgesic use, hence improving QOL.[9,10]
We report clinical audit of our prospectively maintained lung cancer database of the patients of locally advanced and metastatic non squamous NSCLC who received induction pemetrexed platinum doublet chemotherapy followed by the maintenance pemetrexed, the first among the Indian population. Disease control after the induction chemotherapy was achieved in 188 out of 332 patients (57%), who subsequently received the maintenance pemetrexed, which is similar to other reported major studies.[5,6] Improved PFS and OS achieved after the maintenance pemetrexed compared to that among the patient who progressed with induction chemotherapy shows the proof of principle that induction chemotherapy helps in selecting the patients with better biology and responsive tumors in such a way that the patient having aggressive disease fare poorly to induction and succumb to disease early. Benefit obtained in responding patients to induction chemotherapy is further improved upon by the use of maintenance pemetrexed with PFS and OS improving to 8 months and 20 months, respectively, similar to other maintenance studies.[5,6]
Median follow-up of our study is 14 months. More than 51% of patients went on to receive more than 6 cycles of maintenance pemetrexed which is superior to that reported by Paramount study (23%) and similar to that reported in JMEN trial (48%).[5,6] Patients who received more than 6 cycles of maintenance pemetrexed had significantly better outcomes. Similarly, patients having baseline pleural effusion had significantly better progression free and OS. None of the previously mentioned studies has evaluated or reported benefit in therapy in the patient with third space collection. The precise reason for such benefit could not be ascertained, however, the immune-modulator effect of maintenance pemetrexed in the patients with effusion cannot be ruled out.[11] Further studies are required to explore this hypothesis.
After progression from maintenance pemetrexed, 54% went on to receive the second line therapy which is higher compared to that reported by JMEN trial and similar to that in the paramount study. Single agent docetaxel (48%) was the most common regime used, followed by erlotinib and gefitinib. Only 14% of our patients had EGFR mutation positive which is much less compared to the prevalence of EGFR mutation reported in the Indian population.[12,13] This is probably because a considerable number of patients who initially waited for their EGFR mutation status before starting the chemotherapy, first chose to receive the first line tyrosine kinase inhibitor if they were diagnosed with EGFR mutation positive NSCLC. The benefit of pemetrexed maintenance was seen irrespective of EGFR mutation status.
Eleven out of 188 patients (6%) discontinued maintenance pemetrexed, out of which, 4 patients developed renal toxicity while remaining 7 patients had social and financial constraints. Detailed toxicity of maintenance pemetrexed could not be assessed due to the paucity of meticulous record keeping. Being retrospective study, we do not rule out any selection bias which might have inflated the overall outcomes as only patients entered in our database were analyzed which might not have included all the patients with locally advanced and metastatic NSCLC cases treated or referred to our institute.
Maintenance pemetrexed is a feasible option for the patients achieving disease control in locally advanced and metastatic non squamous NSCLC among the Indian population. Patients receiving maintenance pemetrexed enjoy longer progression free and OS with delay in symptomatic worsening. The benefit is consistent across subgroups including response to induction therapy; however, the patients with baseline pleural effusion and more than 6 cycles of maintenance pemetrexed have significantly better outcomes. The patient specific factors, wishes, and cost of therapy should be taken into consideration before advocating maintenance pemetrexed to such select cohort of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 2015 Apr 15]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Noronha V, Dikshit R, Raut N, Joshi A, Pramesh CS, George K, et al. Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian J Cancer. 2012;49:74–81. doi: 10.4103/0019-509X.98925. [DOI] [PubMed] [Google Scholar]
- 3.Felip E, Stahel RA, Pavlidis N. ESMO Guidelines Task Force. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of non-small-cell lung cancer (NSCLC) Ann Oncol. 2005;16(Suppl 1):i28–9. doi: 10.1093/annonc/mdi821. [DOI] [PubMed] [Google Scholar]
- 4.Fleming ID, Cooper JS, Henson DE. 5th ed. Philadelphia: Lippincott-Raven; 1997. Lung. AJCC Cancer Staging Manual; pp. 127–37. [Google Scholar]
- 5.Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placeboimmediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 6.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 7.Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089) J Clin Oncol. 2013;31:3004–11. doi: 10.1200/JCO.2012.42.3749. [DOI] [PubMed] [Google Scholar]
- 8.Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–57. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belani CP, Brodowicz T, Ciuleanu TE, Krzakowski M, Yang SH, Franke F. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): Results from a randomised, double-blind, phase 3 study. Lancet Oncol. 2012;13:292–9. doi: 10.1016/S1470-2045(11)70339-4. [DOI] [PubMed] [Google Scholar]
- 10.Juhász E, Kim JH, Klingelschmitt G, Walzer S. Effects of erlotinib first-line maintenance therapy versus placebo on the health-related quality of life of patients with metastatic non-small-cell lung cancer. Eur J Cancer. 2013;49:1205–15. doi: 10.1016/j.ejca.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Davis M, Conlon K, Bohac GC, Barcenas J, Leslie W, Watkins L, et al. Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother. 2012;35:629–40. doi: 10.1097/CJI.0b013e31826c8a4f. [DOI] [PubMed] [Google Scholar]
- 12.Chougule A, Prabhash K, Noronha V, Joshi A, Thavamani A, Chandrani P, et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One. 2013;8:e76164. doi: 10.1371/journal.pone.0076164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noronha V, Prabhash K, Thavamani A, Chougule A, Purandare N, Joshi A, et al. EGFR mutations in Indian lung cancer patients: Clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8:e61561. doi: 10.1371/journal.pone.0061561. [DOI] [PMC free article] [PubMed] [Google Scholar]


