Abstract
Background:
Nephrotoxicity is one of the known side effects of methotrexate (MTX) therapy despite the use of conventional protective measures. Our objectives were to evaluate the effects of N-acetylcysteine (NAC) on MTX-induced toxicity in renal tubular cells and to evaluate whether adjunctive use of NAC interferes with MTX antitumor activity in the B-cell lymphoma.
Methods:
Kidney Epithelial (Madin-Darby canine kidney [MDCK]) cells were exposed to MTX (10 μM or 100 μM) alone and with NAC (0.2 mM or 0.4 mM). Reactive oxygen species (ROS) generation at 1, 2, 4, and 24 h was measured by flow cytometer. Quantification of total glutathione (GSH) was performed by using GSH assay kit. To measure the impact of NAC on the antitumor activity of MTX, B lymphoma cells were exposed to MTX alone and with NAC. A percentage of apoptosis was measured using fluorescein isothiocyanate in both cell lines. Quantitative data was presented as a means ± standard deviation, and P values were analyzed using the Student's t-test.
Results:
Apoptosis in MDCK cells were observed after 24 h of incubation with both 10 μM and 100 μM MTX. Maximum ROS generation was observed at 4 h and corresponded to GSH production. Treatment with 0.2 and 0.4 mM of NAC led to decrease percentages of apoptotic MDCK cells. NAC did not change either proliferation or apoptosis of B-cell lymphoma.
Conclusion:
Using NAC for kidney protection may not interfere with the antitumor activity of MTX. Further in vivo studies are warranted to confirm noninterference between MTX and NAC and assess synergistic antitumor effects.
Keywords: B-cell lymphoma, Madin-Darby canine kidney epithelial cells, methotrexate, N-acetylcysteine, nephrotoxicity
INTRODUCTION
Nephrotoxicity is one of the serious dose-limiting complications of methotrexate (MTX) when used in the treatment of various malignancies including leukemias and lymphomas, and nononcologic diseases such as rheumatoid arthritis, psoriasis, and refractory inflammatory bowel disease.[1,2,3] Children appear to be at a higher risk of nephrotoxicity than adults because of metabolic differences associated with maturation.[4] MTX treatment significantly decreased glomerular filtration rate (GFR) and may cause albuminuria in pediatric cancer patients several years after treatment.[3] There are different clinically available methods to measure in biological fluids.[5] To quantify MTX metabolites, high performance liquid chromatography is the preferred assay technique.[6]
High-dose administration of MTX in oncology can lead to direct tubular toxicity and subsequent renal failure.[7] A recent study showed that poly (adenosine diphosphate-ribose) polymerase (PARP) activity plays a substantial role in MTX-induced nephrotoxicity.[2] It is interesting to note that nicotinamide adenine dinucleotide (NAD+) is required as a substrate for generating adenosine diphospate-ribose monomers. The overactivation of PARP may deplete the stores of cellular NAD+ and induce a progressive adenosine 5’-triphosphate (ATP) depletion. Glucose oxidation is inhibited, and necrotic cell death is observed.[8] Reactive oxygen species (ROS) can result in activation of mitochondrial membrane permeability and loss of its potential (ΔΨm), leading to cell death.[7,9] Thus, oxidative stress, through different pathways, plays a significant role in renal damage caused by MTX.
N-acetylcysteine (NAC) is a sulfhydryl source for glutathione (GSH) synthesis and acts as a thiol antioxidant by scavenging ROS such as O2•-, H2O2, and •OH. NAC also acts as a nucleophile for the direct nonenzymatic detoxification of reactive, electrophilic metabolites. NAC provides protection as a GSH precursor, increasing its synthesis, and replenishing its stores. It also increases GSH-S-transferase activity, as well as neutralizes free radicals through its nucleophilic activity.[9,10,11,12,13] It is currently used as an antidote for acetaminophen overdose and as a topical antimucolytic agent.[12,13] NAC has demonstrated protective effects on the kidneys in ischemia/reperfusion injury and CP- and cyclosporine-induced nephrotoxicity in experimental systems.[14,15,16] In some studies, NAC is used in patients on high-dose chemotherapy with ifosfamide and cis-platinum.[17] NAC is a promising agent against MTX-induced renal damage,[1] but the question remains whether NAC also protects tumor cells. MTX is widely used for the treatment of B-cell lymphoma.
We investigated the hypothesis that NAC provides renoprotection by decreased oxidative stress damage in renal proximal tubular epithelial cells (Madin-Darby canine kidney [MDCK]-cells). Evaluate whether the use of NAC does not compromise the anticancer activity of MTX, while providing renoprotection. In addition, we evaluated whether the use of NAC does not compromise the anticancer activity of MTX, while providing renoprotection.
METHODS
Cell culture
The cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA): SuDHL-4, B-cell lymphoma, (ATCC® CRL-2957™) and MDCK (NBL-2), kidney cell with characteristics of distal epithelial cells (ATCC® CCL-34™). All cells were employed with the media and conditions provided by ATCC manufacture instruction. The cells were subcultured every 3 days by trypsinization using 0.25%, 0.03% EDTA. The cells were grown to 70-80% confluence with monolayer appearance.
Treatment conditions
In the experiments, SuDHL-4 and MDCK cells were exposed to MTX (10 μM or 100 μM) and/or NAC (0.2 and 0.4 mM) for 1, 4, 8, 12, and 24 h after culture achieved 70% confluence. The cell growth media was changed daily. Normal saline exposure served as a control.
Cell proliferation assay
Cellular viability for SuDHL-4 cells were assessed by using BURdu cell proliferation ELISA kit (Abcam®, Cambridge, MA, USA). For SuDHL-4 cells, two types of controls were used to ensure the validity of the experiment. For control 1, (blank) only tissue culture media without cells was used. For control 2, culture media with cells was used, but BrdU reagent was not added. Cells were seeded in different treatment conditions in sterile 96 well tissue culture plates at 2 × 105 cells/ml in 100 μl/well in respective culture media along with both controls. Proliferation assay was performed in triplicates as per manufacturer instruction. Plates were read using a spectrophotometric microtiter plate reader set at a dual wavelength of 450/550 nm.
Apoptosis
Before exposure to the treatment conditions, cells were grown in 12-well plates until 70% confluence. Detection of apoptosis was done by Annexin V/PI Assay (BD Pharmingen, Bedford, MA, USA), according to the manufacturer's protocol. Briefly, cells were collected by trypsinization, washed twice with PBS and stained with 5 μl of Annexin V-fluorescein isothiocyanate and 5 μl of PI (5 μg/ml) in binding buffer for 15 min at room temperature in dark. The apoptotic cells were determined by FACSVerse (Model RUO 3L8C) flow cytometer (BD Biosciences).
Detection of reactive oxygen species using 2’,7’-dichlorofluorescin diacetate
ROS was detected by staining the cells with a general oxidative stress indicator: 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Molecular Probes, Grand Island, NY, USA). Cells were incubated with 10 μM CM-H2DCFDA for 30 min. The fluorescent intensity was recorded by excitation at 485 nm and emission at 535 nm using FACSVerse flow cytometer (Model RUO 3L8C, BD Biosciences).
Intracellular glutathione assays
The intracellular GSH cell contents was quantified using a GSH/GSSG assay kit (Biovision, Mountain View, CA, USA) after 1, 2, 4, and 6 h exposure to the treatment under investigation. Cells were homogenized in an ice-cold GSH buffer, and proteins were removed by precipitation using perchloric acid and centrifugation. After neutralization of the supernatants using KOH, the GSH contents were determined using an O-phthalaldehyde (OPA) probe. In the assay, OPA reacts with GSH (not GSSG), generating fluorescence proportional to the GSH content. The fluorescence were measured at 420 nm (excitation 340 nm) using a Synergy HT Automated Microplate Reader.
Statistical analysis
Quantitative data is presented as a mean ± standard deviation from three independent experiments, and P value was obtained by Student's t-test by using prism GraphPad software (GraphPad software Inc. La Jolla, CA, USA).
RESULTS
N-acetylcysteine prevented apoptosis in Madin-Darby canine kidney cells
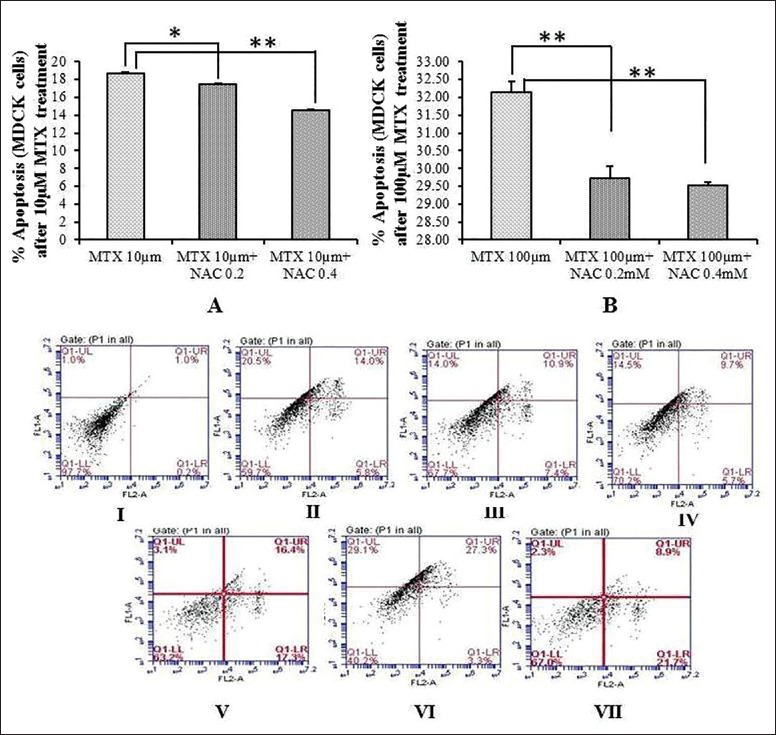
Percentage of apoptosis in MDCK cells was high after 24 h of incubation with 10 μM and 100 μM MTX [Figure 1]. When MDCK cells were co-treated with 0.2 and 0.4 mM NAC% of apoptotic cells decreased significantly as compared to apoptosis in cells which were treated with MTX alone. This was true for both MTX concentrations [Figure 1a and b].
Figure 1.
Apoptosis in Madin-Darby canine kidney cell line; (a) Percent of apoptotic cells at 24 h in Madin-Darby canine kidney cells, after the treatment with 10 μM methotrexate alone and in combination of 10 μM methotrexate and 0.2 and 0.4 mM N-acetylcysteine; (b) Apoptosis in Madin-Darby canine kidney cells at 4 and 24 h after the treatment with 100 μM methotrexate alone and in combination of 100 μM methotrexate and 0.2 and 0.4 mM N-acetylcysteine; *P > 0.05, **P > 0.00. I: Control (PI and Annexin); II: 10 μM methotrexate; III: 10 μM methotrexate + 0.2 mM N-acetylcysteine; IV: 10 μM methotrexate + 0.4 mM N-acetylcysteine; V: 100 μM methotrexate; VI: 100 μM methotrexate + 0.2 mM N-acetylcysteine; VII: 100 μM methotrexate + 0.4 mM N-acetylcysteine
Reactive oxygen species generation in experimental models
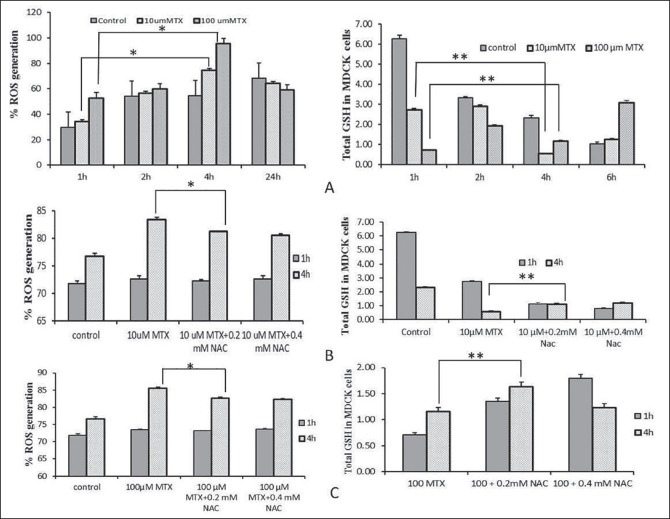
Maximum ROS generation was observed 4 h after the treatment in MDCK cells after the treatment either with 10 μM or 100 μM MTX [Figure 2a]. Co-treatment with 0.2 and 0.4 mM NAC led to significant decrease of ROS production [Figure 2b and c].
Figure 2.
Percent changed of reactive oxygen species generation in Madin-Darby canine kidney cell line; (a) Percent of reactive oxygen species generation and total glutathione generation (nmoles) in Madin-Darby canine kidney cells after treatment with 10 or 100 μM methotrexate; (b) Percent of reactive oxygen species generation and total glutathione generation (nmoles) in Madin-Darby canine kidney cells at 1 and 4 h after co-treatment with 10 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine; (c) Percent of reactive oxygen species and total glutathione generation (nmol) in Madin-Darby canine kidney cells at 1 and 4 after co-treatment with 100 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine. *P > 0.05, **P > 0.001
ROS generation corresponded with GSH production in all experimental conditions. When cells were treated with low (10 μM) or high (100 μM) concentrations of MTX, GSH production was depleted in a time-dependent manner [Figure 2a]. Again, minimum GSH production was observed at 4 h [Figure 2b and c].
Does N-acetylcysteine compromise antitumor effect of methotrexate in B-cell lymphoma?
There was no change observed in percent of apoptotic cells after 4 or 24 h of incubation [Figure 3a and b]. Suppressive effect of MTX on the proliferation of B-cell lymphoma cells also was not compromised by NAC in the studied concentration confirmed by a cell proliferation assay [Figure 4a and b].
Figure 3.
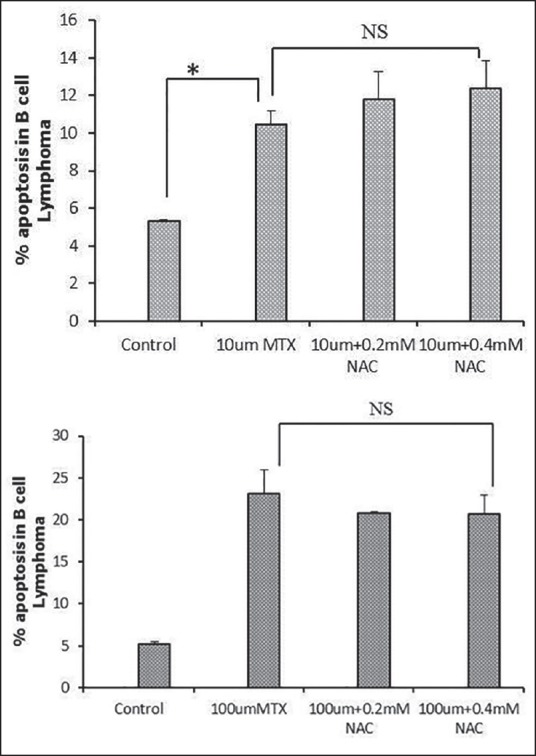
Percent of apoptosis in B-cell lymphoma cell line; (a) Comparison of percent of apoptosis in B-cell lymphoma cells at 24 h after treatment with 10 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine; (b) Comparison of apoptosis in B-cell lymphoma cells at 24 h treatment with 100 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine; *P > 0.05; NS: Not significant
Figure 4.
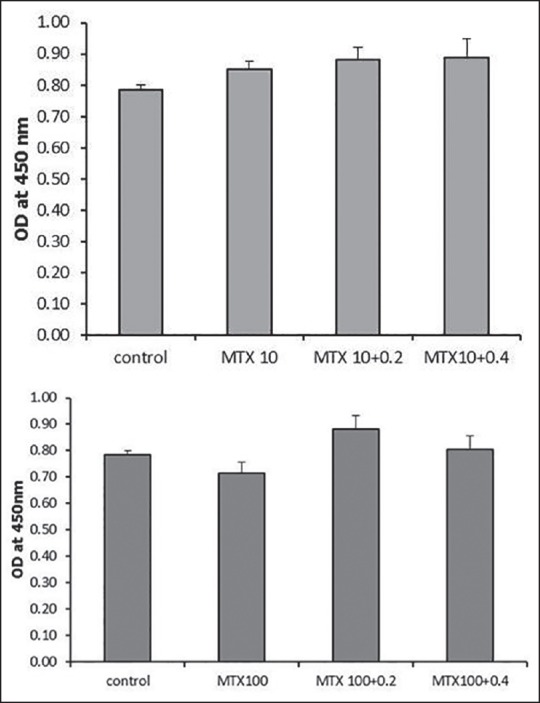
Cell proliferation assay in B-cell lymphoma cell line; (a) Comparison of cell proliferation in B-cell lymphoma cells at 24 h after the treatment with 10 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine; (b) Comparison of cell proliferation in B-cell lymphoma cells at 24 h after the treatment with 100 μM methotrexate alone and in combination with 0.2 and 0.4 mM N-acetylcysteine
DISCUSSION
High-dose of MTX is related with toxicities. MTX toxicity mainly affects liver, kidneys, skin, lungs, bone marrow, and gastrointestinal system, particularly the oral mucosa.[18] The effectiveness of MTX, as an anticancer agent, is frequently limited by severe renal toxicity in cancer patients.[18] MTX mainly cleared by kidney, MTX-induced renal toxicity can be life threatening as it may affect MTX excretion resulting in a high concentration of MTX in plasma and related other adverse events.[19] Some clinical studies results demonstrated that temporary decline in GFR is comparatively common during MTX treatment.[20,21,22] MTX have a direct toxic effect on renal cells which may leads to permanent tubular damage.[23] Genestier in 2000 has been proposed the possible mechanism of MTX action in leukemia patients he suggested that MTX leads to decrease in purine synthesis and subsequently decrease in DNA synthesis which leads to apoptosis.[23] However, how does MTX affects the nonmalignant cell is unclear. Our results also indicated that percentage of apoptosis increased in tubular cells when incubated with MTX in a dose-dependent manner and was observed after 24 h.
In normal cells, including renal MDCK cells, the metabolic process and production of ROS might be very different from the tumor cells. The data showed that as in the tumor cells, in renal tubular cells, apoptosis increased with increasing MTX concentrations.
Preincubation of MDCK with the antioxidant NAC decreased MTX-induced ROS production. This shows an interaction between the MTX apoptotic and pro-oxidant effects and the susceptibility of proximal tubular cells, which causes nephrotoxicity. A suppression of the antioxidant defense system by MTX may play a role in the heightened ROS generation in MDCK cell line. In this study, when MDCK cells were incubated with NAC, ROS production significantly decreased at 4 h as compared to when treated with MTX alone. This is because NAC inhibit ROS production by GSH. NAC, a thiol antioxidant, when added has blocked oxidative stress induced MDCK cell apoptosis. This is consistent with the concept that an apoptotic death signal was triggered by an early loss of GSH-to-GSSG balance.[24] To put it briefly, in proximal tubular cells, MTX-induced apoptosis by ROS production while NAC, an antioxidant, prevented apoptosis. The inhibition MTX-induced apoptosis by NAC was found to be of a lesser extent in B-cell lymphoma than in MDCK cells.
CONCLUSION
NAC may provide renoprotection in MTX-induced nephrotoxicity without affecting B-cell lymphoma cell apoptosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Çaglar Y, Özgür H, Matur I, Yenilmez ED, Tuli A, Gönlüsen G, et al. Ultrastructural evaluation of the effect of N-acetylcysteine on methotrexate nephrotoxicity in rats. Histol Histopathol. 2013;28:865–74. doi: 10.14670/HH-28.865. [DOI] [PubMed] [Google Scholar]
- 2.Dalaklioglu S, Sahin P, Ordueri EG, Celik-Ozenci C, Tasatargil A. Potential role of poly(ADP-ribose) polymerase (PARP) activation in methotrexate-induced nephrotoxicity and tubular apoptosis. Int J Toxicol. 2012;31:430–40. doi: 10.1177/1091581812457430. [DOI] [PubMed] [Google Scholar]
- 3.Grönroos MH, Jahnukainen T, Möttönen M, Perkkiö M, Irjala K, Salmi TT. Long-term follow-up of renal function after high-dose methotrexate treatment in children. Pediatr Blood Cancer. 2008;51:535–9. doi: 10.1002/pbc.21650. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Aleksa K, Woodland C, Rieder M, Koren G. N-Acetylcysteine prevents ifosfamide-induced nephrotoxicity in rats. Br J Pharmacol. 2008;153:1364–72. doi: 10.1038/bjp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crom WR, Evans WE. Methotrexate. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring. Fourth edition. LWW; (May 19, 2005); 1992. pp. 29-1–29-42. [Google Scholar]
- 6.Aboleneen H, Simpson J, Backes D. Determination of methotrexate in serum by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;681:317–22. doi: 10.1016/0378-4347(95)00580-3. [DOI] [PubMed] [Google Scholar]
- 7.Chan WH, Wu CC, Yu JS. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J Cell Biochem. 2003;90:327–38. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- 8.Piskunova TS, Yurova MN, Ovsyannikov AI, Semenchenko AV, Zabezhinski MA, Popovich IG, et al. Deficiency in poly (ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Curr Gerontol Geriatr Res 2008. 2008:754190. doi: 10.1155/2008/754190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–27. [PubMed] [Google Scholar]
- 10.De Vries N, De Flora S. N-acetyl-l-cysteine. J Cell Biochem Suppl. 1993;17F:270–7. doi: 10.1002/jcb.240531040. [DOI] [PubMed] [Google Scholar]
- 11.De Flora S, Bennicelli C, Camoirano A, Serra D, Romano M, Rossi GA, et al. In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and/or mutagenic compounds. Carcinogenesis. 1985;6:1735–45. doi: 10.1093/carcin/6.12.1735. [DOI] [PubMed] [Google Scholar]
- 12.Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcystine: The treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097–100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–4. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 14.Sehirli AO, Sener G, Satiroglu H, Ayanoglu-Dülger G. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol. 2003;16:75–80. [PubMed] [Google Scholar]
- 15.Mishima K, Baba A, Matsuo M, Itoh Y, Oishi R. Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic Biol Med. 2006;40:1564–77. doi: 10.1016/j.freeradbiomed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14:923–9. doi: 10.1093/ndt/14.4.923. [DOI] [PubMed] [Google Scholar]
- 17.Momeni A, Hajigholami A, Geshnizjani S, Kheiri S. Effect of silymarin in the prevention of Cisplatin nephrotoxicity, a clinical trial study. J Clin Diagn Res. 2015;9:OC11–3. doi: 10.7860/JCDR/2015/12776.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 19.Chan AJ, Rajakumar I. High-dose methotrexate in adult oncology patients: A case-control study assessing the risk association between drug interactions and methotrexate toxicity. J Oncol Pharm Pract. 2014;20:93–9. doi: 10.1177/1078155213482602. [DOI] [PubMed] [Google Scholar]
- 20.Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, et al. High-dose methotrexate: On the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL) Cancer Chemother Pharmacol. 2003;51:311–20. doi: 10.1007/s00280-002-0552-1. [DOI] [PubMed] [Google Scholar]
- 21.Deray G, Khayat D, Cacoub P, Bourbouze R, Musset L, Baumelou A, et al. The effects of diltiazem on methotrexate-induced nephrotoxicity. Eur J Clin Pharmacol. 1989;37:337–40. doi: 10.1007/BF00558496. [DOI] [PubMed] [Google Scholar]
- 22.Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106:459–65. doi: 10.1016/s0002-9343(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 23.Genestier L, Paillot R, Quemeneur L, Izeradjene K, Revillard JP. Mechanisms of action of methotrexate. Immunopharmacology. 2000;47:247–57. doi: 10.1016/s0162-3109(00)00189-2. [DOI] [PubMed] [Google Scholar]
- 24.Pias EK, Aw TY. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ. 2002;9:1007–16. doi: 10.1038/sj.cdd.4401064. [DOI] [PubMed] [Google Scholar]