Abstract
Background
The introduction of neurological stroke units and of thrombolysis with the intravenous (IV) administration of recombinant tissue-type plasminogen activator (tPA) have markedly improved the treatment of stroke. Five randomized trials of catheter-based interventional treatment of stroke with special stents were published in 2015.
Method
Recently published randomized trials of mechanical thrombectomy are selectively reviewed.
Results
These trials documented the clinical efficacy of mechanical thrombectomy (MT) in the treatment of occlusion of a major cerebral artery in the distribution of the internal carotid artery (evidence level 1a, recommendation grade A). Roughly 4–10% of all stroke patients could benefit from such an intervention. In the trials, 85% of the patients were first treated with IV-tPA. A recanalization of the occluded vessel was achieved by MT in 59–88% of patients. The percentage of patients with no deficit or only a mild deficit was 33–71% among those who received the intervention, compared to 19–40% in the control groups. The trial data indicate that MT is effective for elderly patients as well (age over 80). Thrombectomy did not increase the rate of secondary, symptomatic intracranial hemorrhage.
Conclusion
MT can only be used to treat the occlusion of major cerebral arteries. In appropriate patients, it expands the spectrum of treatment options for stroke. Long-term data are not yet available.
Care for acute stroke has become an essential task for neurology centers and requires close, well-coordinated teamwork between the involved disciplines: in the first instance between neurology and neuroradiology, and also between angiology, cardiology, and neurosurgery. The two great milestones in stroke treatment were the introduction of neurological stroke units in 1994 and the National Institute of Neurological Disorders and Stroke (NINDS) study in 1996 that provided the evidence in favor of intravenous thrombolysis (IVT) using tissue-type plasminogen activator (tPA) (1, 2). This resulted in the concept of primary care for patients in stroke units, which reduces lasting impairment and mortality (odds ratio [OR]: 0.76; 95% confidence interval [CI]: 0.67 to 0.86; p = 0.0001; combined outcome versus care outside stroke units) (3). The first-line treatment for ischemic stroke is IVT within 4.5 hours of symptom onset (4). However, the current authors see many patients in clinical practice whose treatment outcomes are not satisfactory. An intensive search for better treatment and diagnostic methods therefore remains ongoing.
2015 saw the publication of five randomized trials on mechanical thrombectomy (MT), intervention for stroke. They examined the treatment of ischemic stroke caused by the occlusion of a large artery serving the brain, such as the distal internal carotid artery or the proximal middle cerebral artery (territorial infarctions). This review aims to explain these studies and their consequences for neuroradiological aspects of stroke treatment and cross-sectional imaging diagnostics. Overall, 85% of patients in the five trials were initially treated with IVT. It should therefore be made clear immediately that MT is an adjunct to IVT, not a replacement for it. MT can probably be considered for 4 to 10% of all stroke patients (5, 6). Even taking into account the new trial data, primary hospital care of patients is still to be provided in stroke units.
Intervention
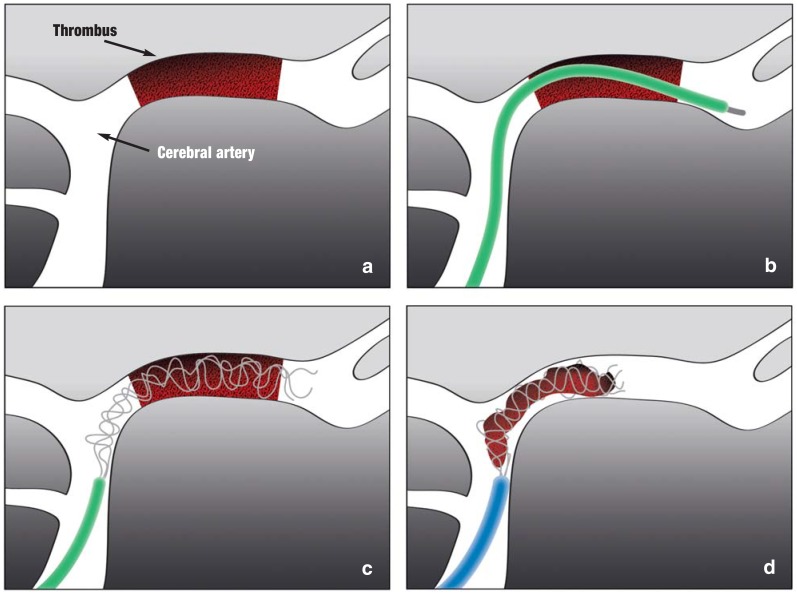
Since 2008, thrombectomy has been performed using fully expanded intracranial stents (stent retrievers). This procedure promised superior recanalization rates and seemed to lead to positive outcomes more frequently than average in patients with large proximal vessel occlusions who were seriously clinically affected. Older mechanical treatments were not very satisfactory; this was shown not least in three negative trials (IMS III, SYNTHESIS Expansion, and MR RESCUE [7,]) and led to a certain pessimism regarding MT. In early 2015, five independent randomized trials were published: ESCAPE (8), EXTEND-IA (9), MR CLEAN (10), REVASCAT (11), and SWIFT-PRIME (12). These showed endovascular stroke treatment to be clearly superior, mainly in combination with IVT versus IVT alone or, if intravenous (IV) tPA was contraindicated, also versus other noninvasive therapy. The MT procedure tested in the new studies (Figure 1) is very different from the intra-arterial fibrinolysis treatment that had been used to treat stroke since the early 1980s.
Figure 1.
If there is a proximal thromboembolic occlusion of the middle cerebral artery (a), the stent retriever is run past the intra-arterial thrombus in a microcatheter (b). When the microcatheter is retracted, the stent retriever is pushed out and released inside the thrombus. After a few minutes, the stent expands into the thrombus so that the mesh of the stent hooks into the thrombus (c). The expanded stent, together with the whole thrombus, is then removed into a larger catheter (d).
Recent trials
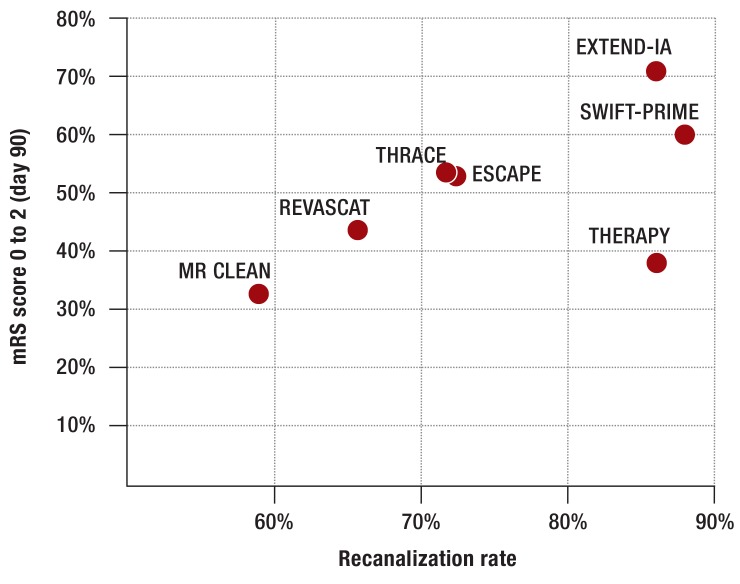
The MR CLEAN trial (10) was conducted at 16 sites in the Netherlands. The inclusion criteria stated no limitations regarding clinical symptoms or size of infarction on imaging. Patients initially received standard treatment (89% IV tPA, after a median of 1.5 hours) and were then randomized to a group either with or without additional endovascular treatment (after 3.4 hours). This may have led to the enrollment mainly of patients who showed no improvement after IV tPA. Endovascular treatment was administered significantly later (after 4.3 hours). The probability of a favorable clinical and functional outcome (modified Rankin Scale score 0 to 2 after 90 days) was 33% with MT and 19% without. The recanalization rate, strictly defined, was 59%. Despite these numerically rather modest outcomes in comparison with trials published later (Figure 2, Table), and although MT was used as the last option following failure of IV tPA, MR CLEAN was the first trial to yield positive outcomes and triggered early analyses of the other trials which were still ongoing.
Figure 2.
Relationship between clinical/functional outcome (rate of mRS score 0 to 2 after 90 days) and recanalization rate (TICI IIb/III rate) in thrombectomy arm of the five 2015 randomized trials on thrombectomy.
mRS: Modified Rankin Scale; TICI: Thrombolysis in cerebral infarction
Table. Characteristics of the five randomized trials on mechanical thrombectomy published in 2015.
| Trial | n patients: MT/control | IV tPA rate | Groin puncture after symptom onset | Median NIHSS score: MT/control | Vessel occlusion | Recanalization rate: MT | mRS score 0 to 2: control | mRS score 0 to 2: MT | Symptom onset to groin puncture/reperfusion (min) | SICB: MT | SICB: control | NNT (mRS score 0 to 2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR CLEAN | 233/267 | 89% | Intra-arterial therapy <6 hours possible | 17/18 | Distal ICA, M1, M2, A1, A2 | 59% | 19% | 33% | 260/- | 7.7% | 6.4% | 7 |
| ESCAPE | 165/150*1 | 76% | Patient recruitment <12 hours | 16/17 | ICA + M1, M1, M2 | 72% | 29% | 53% | -/241 | 3.6% | 2.7% | 4 |
| EXTEND-IA | 35/35*1 | 100% | IV <4.5 hours, intra-arterial therapy begun <6 hours, ended <8 hours | 17/13 | ICA, M1, M2 | 86% | 40% | 71% | -/210 | 0 | 5.7% | 3 |
| SWIFT-PRIME | 98/98*1 | 98% | IV <4.5 hours, ia | 17/17 | ICA, M1 | 88% | 35% | 60% | 224/252*2 | 0 | 3.1% | 4 |
| REVASCAT | 103/103*1 | 73% | Beginning of standard therapy or intra-arterial therapy <8 hours | 17/17 | ICA + M1, intracranial ICA, M1 | 66% | 28% | 44% | 269/355 | 1.9% | 1.9% | 6 |
n: Number; MT: Mechanical thrombectomy; tPA: Tissue-type plasminogen activator; I
VT: Intravenous thrombolysis; IA: Thrombectomy; NIHSS: National Institutes of Health Stroke Scale; ICA: Internal carotid artery; M1: Proximal, horizontal segment of middle cerebral artery; M2: Insular segment of middle cerebral artery following M1; mRS: Modified Rankin Scale; SICB: Symptomatic intracranial bleeding; NNT: Number needed to treat
*1Trial halted early due to lack of equipoise
*2Time to first deployment of stent retriever
EXTEND-IA is a research project in which CT perfusion data for the selected patients was automatically evaluated at 10 sites in Australia and New Zealand. Only patients receiving IV tPA were enrolled (after less than 4.5 hours). Endovascular treatment was administered using Solitaire stent retrievers and was required to have begun no later than 6 hours after symptom onset. In view of the scope of its sample, EXTEND-IA was interpreted only for the primary outcomes “reperfusion after 24 hours” and “early improvement in neurological symptoms”: cases in which National Institutes of Health Stroke Scale (NIHSS) score fell by at least 8 points or was between 0 and 1 on the third day after symptom onset. The absolute risk reduction (aRR) for impairment and death was 31%.
The ESCAPE trial recruited patients from Canada, the USA, South Korea, and Ireland. Unlike the two trials described above, this was a pragmatic trial. Within a wide time window, up to 12 hours after symptom onset, patients who had undergone standard treatment were enrolled into groups with and without additional endovascular treatment. The choice of standard therapy was guided in the first instance by the time window; 76% of patients were treated with IV tPA. The aRR for impairment and death was 24%.
SWIFT-PRIME was the only industry-sponsored trial (Covidien/Medtronic). Patients receiving IV tPA treatment less than 4.5 hours after symptom onset were enrolled at 39 sites in the USA and Europe. Those in the trial’s endovascular arm received additional Solitaire stent retriever treatment. Groin puncture had to be performed within 6 hours. Clinical and functional outcomes (aRR: 25%) and recanalization rates (88%) in this transatlantic trial were similar to those in EXTEND-IA. SWIFT-PRIME was the only trial involving German trial sites.
REVASCAT was fundamentally different from the trials described above, as it included only patients who did not respond to IV tPA treatment within 8 hours after symptom onset (73%) and those for whom IV tPA was contraindicated. Another distinguishing feature of REVASCAT is that patients were enrolled at four trial sites in Catalonia, and during the trial’s observation period only eight patients were treated outside the trial. This makes selection bias highly unlikely. The treatment arms were the best possible medical treatment in the stroke unit either alone or in combination with Solitaire stent retriever treatment. Here, too, the treatment outcomes for the group that also received endovascular treatment were superior (mRS score 0 to 2 in 44% of patients versus 28% for those receiving standard treatment alone; aRR: 16%).
Already presented but not yet published are the results of the French THRACE trial (mRS score 0 to 2 in 54% of patients versus 42% with standard treatment alone; aRR: 12%, p = 0.016; presented by S. Bracard at the European Stroke Organisation Conference on 18 April 2015) and those of the transatlantic THERAPY trial (mRS 0 to 2 in 38% of patients versus 30% of those receiving standard treatment alone; aRR: 8%; p = 0.521; presented by J. Mocco et al. at the European Stroke Organisation Conference on 17 April 2015).
Common to all these trials is the fact that the frequency of symptomatic intracranial hemorrhages was not increased by MT (Table). There are therefore no safety issues regarding the use of MT in combination with IV tPA. Because the trials were completed only a few months before the time of writing, no long-term outcomes are available so far.
Selection criteria
The technical nature of MT means that only patients with an occlusion in a large cerebral artery can benefit from it. This includes the distal carotid artery, including the carotid T, and the proximal middle cerebral artery (segment M1); these are occluded in 4 to 10% of all stroke patients (5, 6). If this is shown to be the case, the patient should be referred to a suitable center with a supraregional stroke unit. It is problematic if vascular diagnostics using computed tomography (CT) or magnetic resonance imaging (MRI) angiography are not available round the clock. This is currently the rule rather than the exception.
Advanced patient age alone is not a contraindication for MT. Taken strictly, overall there is a lack of evidence in favor of patient stratification by criteria such as age, time window, or NIHSS score. The mean NIHSS score in the five published trials on thrombectomy was 17; these were therefore seriously affected patients. There is a clear correlation between NIHSS score and size of an occluded vessel (13). The probability of occlusion of a large cerebral artery is 3.3 times higher if the NIHSS score is 11 or higher (14), while within a time window of 3 to 6 hours following stroke the NIHSS threshold score fell by 2 to 3 points. In other research an NIHSS score of 9 or higher was the best predictor of proximal vessel occlusion and also fell by 2 points after the end of a 3-hour time window (15).
The procedure suggested by this data is to refer patients with an NIHSS score of 9 or above to a facility for MT within a time window of 3 hours, and those with a score of 7 or above within 3 to 6 hours after symptom onset (16). This has also been suggested in a consensus paper. Such a procedure will only be feasible in Germany on the basis of consensus between supraregional stroke units and smaller and medium-sized hospitals.
Principles of neurological diagnostics
In ischemic stroke there is a critical decrease in perfusion pressure in the brain parenchyma caused by occlusion of a cerebral artery further upstream. The resulting territorial reduction in perfusion to the parenchyma causes an ischemic core of irreversible cell damage. This is surrounded by an area of critically diminished perfusion (penumbra). Around the penumbra is an area of noncritically diminished perfusion (oligemia). Although this macroscopic model of the pathophysiology simplifies the actual processes at the molecular level, it is helpful in interpreting imaging findings.
In terms of treatment, the most important aspect of cerebral imaging is still to rule out hemorrhage as the cause of symptoms. This can be done effectively and rapidly using native CT, immediately clearing the way for standard IV tPA therapy.
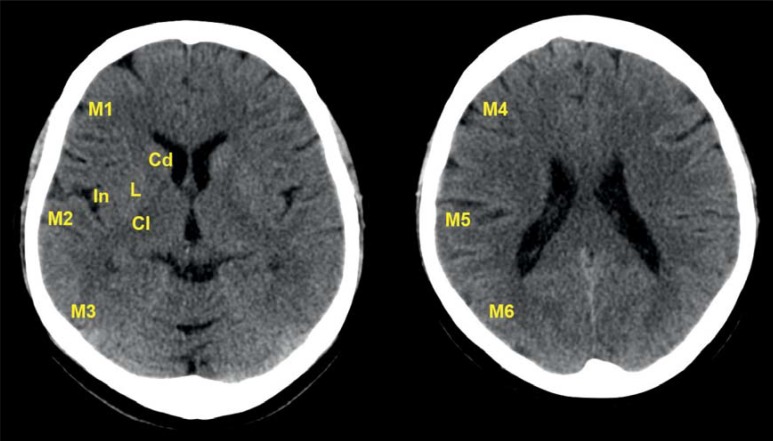
The next aim in stroke imaging should be to determine the extent of the ischemic core on the basis of the ischemic edema. This is done using either native CT or MRI diffusion-weighted imaging (DWI); the latter is substantially more sensitive. The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is used to provide a standardized description of the extent of edema (Figure 3). The ASPECTS divides the area supplied by the middle cerebral artery into 10 sections (17). A normal image is given an ASPECTS of 10, while ischemic edema throughout the area supplied by the middle cerebral artery scores 0.
Figure 3.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) describes the spatial extent of ischemic lesions in the cortical and subcortical areas supplied by the middle cerebral artery (MCA). It can take values between 10 and 0. Ten is the normal score for intact brain parenchyma supplied by the MCA, while 0 corresponds to diffuse ischemia throughout the area supplied by the middle cerebral artery (MCA).
Cd: Caudate nucleus; L: Lentiform nucleus; IC: Internal capsule; In: Insular cortex; M1: anterior cortex supplied by MCA; M2: Lateral insular cortex; M3: Posterior cortex supplied by MCA; M4, M5, M6: Anterior, lateral, and posterior MCA territories, directly above (rostrally) M1, M2, and M3 respectively and therefore also rostral to the basal ganglia. (M1 in the context of ASPECTS does not correspond to the horizontal, proximal segment M1 of the middle cerebral artery. The fact that the names are identical are coincidental.)
Multiparameter CT diagnostics (native CT, perfusion CT, CT angiogram) or multiparameter MRI (DWI, diffusion MRI, MR angiogram) provide important information on the at-risk tissue in the penumbra, i.e. on the sections of the brain parenchyma that threaten to cease functioning but which may possibly still be saved. Differentiation between the outer border of the penumbra and the oligemia currently remains unreliable, however, and can only be usefully interpreted in conjunction with clinical and neurological findings.
Consequences for imaging diagnostics
MT is only useful if a vessel that can be accessed using a catheter is occluded. This means that vessels must be imaged in the acute phase in all patients with significant neurological involvement. Only then can effective triage be performed between purely IV tPA-based therapy and combination IV tPA and thrombectomy therapy.
All five trials mentioned here required CT angiogram or MR angiogram detection of occlusion of a proximal vessel. However, their requirements for brain tissue imaging differed significantly. While the size of ischemic edema in the brain parenchyma played no role in MR CLEAN, EXTEND-IA required imaging of cerebral perfusion using perfusion CT which had to be evaluated using a specific software program. The other trials set an ASPECTS threshold value (Figure 3) of 6 to 10 or 7 to 10, with the recommendation of additional imaging of collaterals in ESCAPE.
It is thus likely that thrombectomy will lead to a therapeutic benefit in patients with an ASPECTS of 6 to 10 or 7 to 10 and confirmed proximal vessel occlusion. In subgroup analyses, treatment effects in patients with an ASPECTS of 8 to 10 were clear (ESCAPE, SWIFT-PRIME, REVASCAT, MR CLEAN), whereas no effect could be shown for scores ranging from 0 to 4 in MR CLEAN. The ASPECTS threshold value for treatment effect may therefore be between 4 and 6. More research is needed to narrow this down further.
It is neither useful nor the aim of this article to stratify patients on the basis of imaging diagnostics alone according to whether treatment can be expected to be effective or not. Such stratification must always include clinical and neurological evaluation. Even a patient with manifest ischemic edema can still benefit from recanalization provided the edema has not yet invaded the entire area supplied by the affected vessel.
Open questions
The borders delineating the indication of thrombectomy have not yet been defined in detail (18). The imaging criteria are not completely clear; this concerns particularly the maximum size of early ischemic edema (19), as well as how to deal with more distal occlusions of the middle cerebral artery, in segments M2 and M3. There are no results from randomized trials on MT for occlusions in the area supplied by the vertebral artery. The maximum time window for MT is as unclear as the procedure for handling patients who have suffered a stroke while asleep and in whom the time window since symptom onset is therefore unknown. In the latter group, the circumstances in which IVT is indicated have, as yet, never been shown. This is currently being investigated in a multicenter European trial (WAKE-UP) (20). Treatments involving MT for wake-up stroke that go beyond the scope of its authorization are therefore problematic.
Questions on the technical specifications of MT such as the role of aspiration procedures, choice of catheter, how to deal with proximal stenoses before the intracranial occlusion is reached—e.g. in the internal carotid artery—and method of anesthesia also remain open. Lastly, it must be mentioned that the trials of MT were conducted in centers that had substantial experience with the intervention. Their results cannot therefore simply be extrapolated to any location. Quality assurance, including supraregional registers, will be very important.
Summary
The trials discussed here demonstrate the effectiveness of MT for confirmed occlusion of a large cerebral artery in the area supplied by the internal carotid artery. This holds true for patients with considerable neurological impairment, a total of 4 to 10% of all stroke patients. There is no justification for setting a maximum age limit. IVT within 4.5 hours of symptom onset remains the first-line treatment for all ischemic stroke patients. MT must not hinder IVT. For diagnosis, the consequence of the new trials of MT is that multimodal imaging of stroke using MRI or CT plus CT angiogram and CT perfusion imaging is gaining value and is performed considerably more frequently than in the past. Implementing the new treatment options all over Germany requires supraregional stroke units and neurovascular networks to be coordinated with smaller and medium-sized hospitals, constructively and on the basis of consensus. It is very likely that remuneration structures will also need to be adapted. Modern care for stroke involves ever greater amounts of teamwork.
Key Messages.
The five trials published so far this year demonstrate that mechanical thrombectomy (MT) is clinically effective in stroke caused by occlusion of a proximal cerebral artery in the area supplied by the internal carotid artery.
Indication of MT requires evidence of a cerebral artery occlusion in the form of either MRI or CT plus CT angiogram.
Overall, 85% of the patients in the five trials were initially treated with intravenous tissue-type plasminogen activator (tPA). In most cases MT was thus an adjunct to tPA, not a replacement for it.
No upper age limit can be established for the indication of MT.
The maximum time window for intervention, the imaging criteria, and the boundaries for indication have not yet been definitively established.
Acknowledgments
Translated from the original German by Caroline Shimakawa-Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Fiehler has received consultancy fees from Boehringer Ingelheim, Codman, and Microvention. He has received reimbursement of travel expenses from Covidien and Penumbra. He has received lecture fees from Boehringer, Covidien, and Penumbra. He has received study funding (third-party funds) from Covidien (the SWIFT-PRIME trial) and Microvention. He is a member of the Management Committee of the Professional Association of German Neuroradiologists (BDNR, Berufsverband Deutscher Neuroradiologen), the German Neuroradiology Society (DGNR, Deutsche Gesellschaft für Neuroradiologie), the European Society of Minimally Invasive Neurological Therapy (ESMINT), and the Interventional Neuroradiology Committee of the European Society of Neuroradiology (ESNR).
Prof. Gerloff has received consultancy fees from Bayer Vitral, Boehringer Ingelheim, GlaxoSmithKline, Lundbeck, Pfizer, Silk Road Medical, and Sanofi Aventis. He has received reimbursement of travel expenses and lecture fees from Boehringer Ingelheim, Sanofi Aventis, and Bayer. Prof. Gerloff is coordinator of the WAKE-UP trial (EU FP7).
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne P, Williams BO, Gilchrist W, Howie K. Do stroke units save lives? Lancet. 1993;342:395–398. doi: 10.1016/0140-6736(93)92813-9. [DOI] [PubMed] [Google Scholar]
- 3.Stroke Unit Trialists Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD000197.pub3. CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD000213.pub3. CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogousslavsky J, van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14:846–854. doi: 10.1016/S1474-4422(15)00140-4. [DOI] [PubMed] [Google Scholar]
- 7.von Kummer R, Gerber J. MS-3, SYNTHESIS, and MR RESCUE: no disaster, but down to earth. Clin Neuroradiol. 2013;23:1–3. doi: 10.1007/s00062-013-0214-1. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 11.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Me d. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 13.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. 2007;38:2531–2535. doi: 10.1161/STROKEAHA.107.482554. [DOI] [PubMed] [Google Scholar]
- 14.Cooray C, Fekete K, Mikulik R, Lees KR, Wahlgren N, Ahmed N. Threshold for NIH stroke scale in predicting vessel occlusion and functional outcome after stroke thrombolysis. Int J Stroke. 2015;10:822–829. doi: 10.1111/ijs.12451. [DOI] [PubMed] [Google Scholar]
- 15.Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–1157. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke. 2015 doi: 10.1177/1747493015609778. in press. [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 18.Mordasini P, Zubler C, Wha-Vei Hsieh K, Chan PK, Gralla J. Stent-retriever thrombectomy: impact on the future of interventional stroke treatment. Clini Neuroradiol. 2014;24:17–22. doi: 10.1007/s00062-014-0299-1. [DOI] [PubMed] [Google Scholar]
- 19.Bendszus M. Interventional stroke treatment: challenges after MR CLEAN. Clin Neuroradiol. 2015;25 doi: 10.1007/s00062-015-0378-y. [DOI] [PubMed] [Google Scholar]
- 20.Thomalla G, Fiebach JB, Ostergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) Int J Stroke. 2014;9:829–836. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]