Abstract
Hepatosplenic T-cell lymphoma (HSTCL), a rare type of γδ T-cell lymphoma, is characterized by hepatosplenomegaly and cytopenias. It is associated with immunodeficiency and its age of onset is reportedly between the 20s and 30s. We herein report 4 Japanese HSTCL cases. Three of them, including an elderly case that was 74 years of age, were not at adolescence. No cases had a history of immunodeficiency. All other disease phenotypes were similar to the typical HSTCL cases. These findings suggest that there are a certain proportion of HSTCL patients who presented after middle age.
Keywords: Hepatosplenic T-cell lymphoma, Immunodeficiency, Elderly patients
Highlights
-
•
Three out of four HSTCL cases in a Japanese institute were over 59 years of age.
-
•
None of the patients had previous illness that was related to immunodeficiency.
-
•
There may be a certain proportion of HSTCL patients who presented after middle age.
1. Introduction
Hepatosplenic T-cell lymphoma (HSTCL) is a rare subtype of peripheral T-cell lymphoma, which is derived from cytotoxic T-cells typically expressing γδ T-cell receptor (TCR). It usually occurs in young men and is characterized by B symptoms such as a fever, weight loss and night sweats, hepatosplenomegaly, thrombocytopenia, and anemia. The patient's prognosis is typically poor [1].
Approximately 20% of all HSTCL cases are associated with immunodeficiency which include chronic immunosuppressive therapies after solid-organ transplantation and collagen diseases such as SLE, HIV, malaria infection, AML, Hodgkin's lymphoma, and inflammatory bowel diseases [2], suggesting that the immunodeficient status may contribute to the development of HSTCL.
Histologically, the lymphoma cells have medium-sized nuclei and a rim of pale cytoplasm, and homogeneous medium-sized CD3-positive lymphoma cells infiltrate the cords and sinuses in the red pulp of the spleen, sinusoids of the liver and the bone marrow [1]. Immunohistochemistry analyses generally show CD3(+), CD4(−), CD5(−), CD8(−/+), CD56(+/−), TCRδ1(+), TIA-1(+), and granzyme B(−).
HSTCL accounts for only 3% of all T-cell lymphomas in the United States and 2.3% in Europe. Notably, it occurs less frequently in Eastern and South-eastern Asian countries (0.2%) [3]. Because HSTCL is especially rare in Asia, its actual characteristics in Asia have not been well-documented [4]. We experienced 4 cases of HSTCL out of 292 cases (1.3%) of T cell lymphoma according to the clinical and pathological records between 1998 and 2014 in our institution. Surprisingly, 3 out of the 4 cases were middle-aged to elderly patients (cases 1–3), and only one case was an adolescent male [5].
We herein report these 4 HSTCL cases of Japanese patients.
2. Case presentation
The clinical and laboratory features of these 4 cases are summarized in Table 1 (cases 1–4). None of the patients had previous illness that was related to immunodeficiency, immunosuppression or an abnormal immunological status. All of the patients had B symptoms, hepatosplenomegaly and cytopenias at diagnosis, resulting in a high or high-intermediate International Prognostic Index (IPI).
Table 1.
Summary of the 4 cases of HSTCL.
| Case no. | Age (y)/Sex | Country | Immuno-deficiency | Clinical stage (cs) | B symptoms | Hepato-megaly | Spleno-megaly | IPI |
|---|---|---|---|---|---|---|---|---|
| 1 | 74/F | Japan | − | ⅣB | + | + | + | High |
| 2 | 64/M | Japan | − | ⅣB | + | + | + | High |
| 3 | 59/M | Japan | − | ⅣB | + | + | + | High |
| 4 | 23/M | Japan | − | ⅣB | + | + | + | High-int |
| Peripheral blood |
Histological detection of tumor cells | ||||
|---|---|---|---|---|---|
| WBC (109/L) | Hgb (g/dL) | Platelet (109/L) | Elevated LD (U/L) | anti-HTLV1 | |
| 5.9 | 7.2 | 109 | 449 | − | Liver, BM |
| 1.8 | 12.2 | 54 | 390 | − | Liver, BM |
| 0.3 | 5.2 | 25 | 577 | − | BM |
| 7.1 | 9.1 | 95 | 522 | − | BM |
| Immunohistochemical staining of tumor cells |
Outcome/follow-up (months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD2 | CD3 | CD4 | CD5 | CD7 | CD8 | CD56 | TIA-1 | Granzyme B | EBER | TCR | |
| + | + | − | − | + | − | + | + | − | − | γδ | Alive/12M |
| + | + | − | − | + | + | + | + | + | − | γδ | Dead/37M |
| NS | + | − | − | NS | − | NS | NS | NS | − | NS | Dead/11M |
| + | + | − | − | + | − | − | + | − | − | γδ | Dead/19M |
PS, performance status; IPI, International Prognostic Index; WBC, leukocyte count; Hgb, hemoglobin level; LD, lactate dehydrogenase level; BM, bone marrow; EBER, EB virus-encoded small RNAs; TCR, T-cell receptor; PSL, Prednisolone; NS, not specified
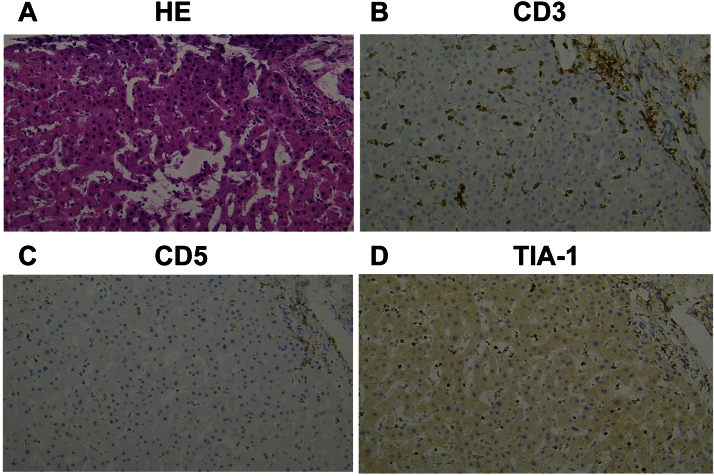
Case 1 was a 74-year-old female. A liver biopsy demonstrated the infiltration of small to medium-sized lymphoma cells in the sinusoids and portal area, and these cells were CD3(+), CD4(−), CD5(−), CD8(−), CD56(+), TIA-1(+), granzyme B(−) and Epstein–Barr virus-encoded RNA (EBER) in situ hybridization(−) (Fig. 1). The patient had a CR by treatment with 6 cycles of CHOP, and has remained alive for more than 12 months after achieving a CR.
Fig. 1.
Histologic appearance of the liver in Case 1. (A) Lymphoma cells infiltrated the sinusoids of the liver (hematoxylin and eosin stain, 200×). (B), (C), (D) An immunohistochemical analysis showed that the malignant cells were positive for CD3 and TIA-1, and negative for CD5.
Case 2 was a 64-year-old male. A liver biopsy demonstrated the infiltration of small to medium-sized lymphoma cells in the sinusoids and portal area, and these cells were CD3(+), CD4(−), CD5(−), CD8(+), CD56(+), TIA-1(+), granzyme B(+) and EBER(−). He was treated with 6 cycles of THP-COP (pirarubicin, cyclophosphamide, vincristine and prednisolone) to achieve a PR for 10 months. Combination chemotherapy with 2 cycles of CHASE (cyclophosphamide, cytarabine, etoposide and dexamethasone) was administered after the disease progression, however, the tumor cells infiltrated the central nervous system. He ultimately died 37 months after the diagnosis.
Case 3 was a 59-year-old male. A bone marrow clot demonstrated hypercellular bone marrow with medium- to large-sized lymphoma cells that were CD3(+), CD4(−), CD5(−), CD8(−) and EBER(−). He was initially treated with 2 cycles of CHOP followed by 2 cycles of ESHAP (etoposide, methylprednisolone and cytarabine), resulting in a PR. An autologous stem cell transplantation preceded by a preparative regimen MCEC (ranimustine, carboplatin, etoposide and cytarabine) provided a CR. He relapsed 3 months post-transplant and died 11 months after the diagnosis.
Case 4 was a 23-year-old male. A bone marrow biopsy demonstrated hypercellular bone marrow with medium- to large-sized lymphoma cells that were CD3(+), CD4(−), CD5(−), CD8(−), CD56(−), TIA-1(+), granzyme B(−) and EBER(−), as previously reported [5]. He was treated with 1 cycle of CHOP followed by 3 cycles of IVAC (ifosfamide, etoposide and cytarabine), resulting in a PR. Allogeneic bone marrow transplantation from an unrelated donor preceded by a preparative regimen composed of etoposide, cyclophosphamide and total body irradiation provided a CR. He relapsed 3 months post-transplant and died 19 months after the diagnosis.
3. Discussion
HSTCL predominantly occurs in young men, with a median age of 34 years. There have been few reports showing its frequency separated by age, but only 4 out of 90 cases (4.4%) undergoing stem cell transplantation have been reported as patients over 60 years of age [1]. In addition, HSTCL is believed to be related to immunodeficiency. In our institution, 3 out of the 4 HSTCL cases developed HSTCL after middle age, including an aged case at 74 years of age. All of these 4 cases had no previous illness. However, other disease phenotypes and their inferior prognoses were similar to the typical HSTCL cases.
Six HSTCL over 65 years of age have been reported in detail to date. The clinical and histopathological characteristics of the patients are summarized in Table 2 (cases 5–10) [[6], [7], [8], [9], [10]]. These characteristics, as well as our aged cases (Case 1) (Table 1), were also compatible with the typical HSTCL cases. Notably, three of these 7 aged HSTCL cases including our Case 1 were from Japan.
Table 2.
Summary of aged HSTCL cases in the literature.
| Case no. | Age (y)/Sex | Country | Immuno-deficiency | Clinical stage (CS) | B symptoms | Hepato-megaly | Spleno-megaly | IPI |
|---|---|---|---|---|---|---|---|---|
| 5 | 71/M | Japan | PSL for BOOP | ⅣB | + | + | + | High |
| 6 | 67/M | Japan | − | ⅣB | + | + | + | High |
| 7 | 80/F | U.S | − | ⅣB | + | + | + | High |
| 8 | 69/M | U.S | − | ⅣB | + | − | + | High |
| 9 | 65/M | U.S | − | Ⅳ | NS | + | + | ≧High-int |
| 10 | 65/M | U.S | − | ⅣB | + | + | + | High |
| Summary (N=6) | Range: 65–80 /M 5; F 1 | U.S: 4 Japan: 2 | 1/6 | CS IV 6/6 | 5/5 | 5/6 | 6/6 | High 5/≧High-int 1 |
| Peripheral blood |
Histological detection of tumor cells | ||||
|---|---|---|---|---|---|
| WBC (109/L)/Leukocytopenia | Hgb (g/dL)/Anemia | Platelet (109/L)/Thrombocytopenia | Elevated LD (U/L) | anti-HTLV1 | |
| 3.4 | 10.8 | 71 | 1622 | NS | Liver, BM |
| 4.9 | NS | 85 | 2240 | − | Liver |
| −3.7 | 14.1 | 55 | NS | NS | NT |
| −12.2 | NS | + | NS | NS | NT |
| NS | NS | NS | NS | NS | BM |
| NS | + | + | NS | NS | BM |
| 1/4 | 2/3 | 5/5 | 2/2 | 0/1 | BM 3; Liver 2 |
| Immunohistochemical staining of tumor cells |
Outcome/follow-up (months) | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD2 | CD3 | CD4 | CD5 | CD7 | CD8 | CD56 | TIA-1 | Granzyme B | EBER | TCR | ||
| NS | + | − | − | NS | − | − | + | + | + | γδ | Dead/3 days | [6] |
| + | + | − | − | + | + | − | + | + | + | γδ | Alive/1M | [7] |
| NS | + | − | NS | NS | + | − | + | + | − | αβ | Dead/4 days | [8] |
| + | + | − | − | + | − | + | + | − | − | αβ | Dead/18M | [8] |
| + | + | − | − | + | + | + | NS | NS | − | γδ | Alive/3M | [9] |
| + | + | − | − | + | + | − | + | NS | − | γδ | Alive/NS | [10] |
| 4/4 | 6/6 | 0/6 | 0/5 | 4/4 | 4/6 | 2/6 | 5/5 | 3/4 | 2/6 | γδ 4; αβ 2 | Alive 3 (NS-3M); Dead 3 (3 days-18M) | |
PS, performance status; IPI, International Prognostic Index; WBC, leukocyte count; Hgb, hemoglobin level; LD, lactate dehydrogenase level; BM, bone marrow; EBER, EB virus-encoded small RNAs; TCR, T-cell receptor; PSL, Prednisolone; BOOP, bronchiolitis obliterans organizing pneumonia; NS, not specified
In WBC counts, Hgb levels and platelet levels, available data are listed. In cases lacking detailed data, + or − is shown. In the summary column at the bottom, leukocytopenia, anemia and thrombocytopenia include a WBC less than 4.0×109/L, Hgb level less than 12.0 g/dL and platelet less than 100×109/L, respectively.
A possible mechanism of the initiation of HSTCL is a prolonged immunosuppressive status which generates the expansion of γδ T-cells recognizing multiple pathogens through excessive antigenic stimulation. Our cases did not have any previous illness related to immunosuppression, recurrent infectious episodes or laboratory evidence of HTLV-1 infection which has the potential of causing immunosuppression as well as a T-cell malignancy. The cause of their immunocompromised status was therefore unknown; an age-related decline in the immune function might be responsible for the lymphomagenesis of HSTCL in these cases. In addition, both of the two previous aged Japanese cases (cases 5 and 6) were positive for EBER (Table 2). In Eastern Asia, EBV infection and reactivation are thought to contribute to the tumorigenesis of T-cell or natural killer (NK)-cell posttransplant lymphoproliferative disorder (T-PTLD) and non-hepatosplenic/non-cutaneous γδ peripheral T-cell lymphomas [7]. Although EBER was undetectable in our cases, there might be a potential contribution of EBV infection and reactivation to the development of HSTCL in aged patients as well as other lymphoid malignancies [6], [7].
Regarding therapy management, the cases in this study were treated with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) or CHOP-like regimens as first-line therapy. The 5-year overall survival (OS) rate has been reported to be 7% [3]. Cases 2–4 in fact had faint responses, and these cases were not rescued by subsequent salvage therapies, autologous HSCT (auto-HSCT) and allogeneic HSCT (allo-HSCT). The survival benefit of HSTCL cases using allo-HSCT has been reported in a limited number of cases [11]; Considerable issues in allo-HSCT include the treatment-related mortality [12]. However, the evaluable sample size in the study was too small to estimate the risk of cytotoxic effects, GVHD and relapse after allo-HSCT. Therefore, large-scale studies are required to determine the indication for and appropriate strategy of allo-HSCT to improve the treatment outcome of HSTCL. Interestingly, Case 1, an exclusive female patient, achieved a CR that was maintained after 6 cycles of CHOP. This result was compatible with a previous retrospective analysis of 15 patients that showed that the overall survival (OS) was significantly prolonged in females than in males [13].
In conclusion, HSTCL occurred not only in young adulthood, but also in the middle-aged to elderly population. Further analyses with a greater accumulation of HSTCL cases are required to elucidate the potential differences. Substantial experience of treatment for elderly HSTCL patients and the development of new treatment modalities with lower adverse events are necessary to improve the prognosis of HSTCL.
Author contributions
H.K., collection and assembly of data, data analysis and interpretation, and manuscript writing; H.M., conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; K.O., collection and assembly of data, data analysis and interpretation; M.K., S.M., Y.O. and H.K, collection and assembly of the data; N.N., data analysis and interpretation, and manuscript writing, K.A., conception and design, administrative support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Contributor Information
Hiromichi Matsushita, Email: hmatsu@is.icc.u-tokai.ac.jp.
Kiyoshi Ando, Email: andok@keyaki.cc.u-tokai.ac.jp.
References
- 1.Visnyei K., Grossbard M.L., Shapira I. Hepatosplenic γδ T-cell lymphoma: an overview. Clin. Lymphoma Myeloma Leuk. 2013;13:360–369. doi: 10.1016/j.clml.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Francois A., Lesesve J.F., Stamatoullas A., Comos F., Lenormand B., Etienne I. Hepatosplenic gamma/delta T-cell lymphoma: a report of two cases in immunocompromised patients, associated with isochromosome 7q. Am. J. Surg. Pathol. 1997;21:781–790. doi: 10.1097/00000478-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Vose J., Armitage J., Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 4.Lu C.L., Tang Y., Yang Q.P., Wang M., Zhao S., Bi C.F. Hepatosplenic T-cell lymphoma: clinicopathologic, immunophenotypic, and molecular characterization of 17 Chinese cases. Hum. Pathol. 2011;42:1965–1978. doi: 10.1016/j.humpath.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M., Matsushita H. Hepatosplenic T-cell lymphoma appearing in the peripheral blood. Blood. 2013;122:1103. doi: 10.1182/blood-2013-01-478404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsunematsu S., Natsuizaka M., Fujita H., Otsuka N., Terashita K., Sato F. Hepatosplenic gamma-delta T-cell lymphoma associated with Epstein–Barr virus. Intern. Med. 2014;53:2079–2082. doi: 10.2169/internalmedicine.53.2236. [DOI] [PubMed] [Google Scholar]
- 7.Ohshima K., Haraoka S., Harada N., Kamimura T., Suzumiya J., Kanda M. Hepatosplenic gammadelta T-cell lymphoma: relation to Epstein–Barr virus and activated cytotoxic molecules. Histopathology. 2000;36:127–135. doi: 10.1046/j.1365-2559.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 8.Macon W.R., Levy N.B., Kurtin P.J., Salhany K.E., Elkhalife M.Y., Casey T.T. Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am. J. Surg. Pathol. 2001;25:285–296. doi: 10.1097/00000478-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad E., Kingma D.W., Jaffe E.S., Schrager J.A., Janik J., Wilson W. Flow cytometric immunophenotypic profiles of mature gamma delta T-cell malignancies involving peripheral blood and bone marrow. Cytometry B Clin. Cytom. 2005;67:6–12. doi: 10.1002/cyto.b.20063. [DOI] [PubMed] [Google Scholar]
- 10.Cooke C.B., Krenacs L., Stetler-Stevenson M., Greiner T.C., Raffeld M., Kingma D.W. Hepatosplenic T-cell lymphomas: a distinct clinicopathologic entity of cytotoxic gammadelta T-cell origin. Blood. 1996;88:4265–4274. [PubMed] [Google Scholar]
- 11.Konuma T., Ooi J., Takahashi S., Tomonari A., Tsukada N., Kobayashi T. Allogenic stem cell transplantation for hepatosplenic gammadelta T-cell lymphoma. Leuk. Lymphoma. 2007;48:630–632. doi: 10.1080/10428190601126941. [DOI] [PubMed] [Google Scholar]
- 12.Tanase A., Schmitz N., Stein H., Boumendil A., Finel H., Castagna L. Allogenic and Autologous stem cell transplantation for hepatosplenic T-cell lymphoma: a retrospective study of the EBMT Lymphoma Working Party. Leukemia. 2015;29:686–688. doi: 10.1038/leu.2014.280. [DOI] [PubMed] [Google Scholar]
- 13.Falchook G.S., Vega F., Dang N.H., Samaniego F., Rodriguez M.A., Champlin R.E. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann. Oncol. 2009;20:1080–1085. doi: 10.1093/annonc/mdn751. [DOI] [PMC free article] [PubMed] [Google Scholar]