Abstract
Objective:
Acute doses of alcohol impair memory when administered before encoding of emotionally neutral stimuli but enhance memory when administered immediately after encoding, potentially by affecting memory consolidation. Here, we examined whether alcohol produces similar biphasic effects on memory for positive or negative emotional stimuli.
Method:
The current study examined memory for emotional stimuli after alcohol (0.8 g/kg) was administered either before stimulus viewing (encoding group; n = 20) or immediately following stimulus viewing (consolidation group; n = 20). A third group received placebo both before and after stimulus viewing (control group; n = 19). Participants viewed the stimuli on one day, and their retrieval was assessed exactly 48 hours later, when they performed a surprise cued recollection and recognition test of the stimuli in a drug-free state.
Results:
As in previous studies, alcohol administered before encoding impaired memory accuracy, whereas alcohol administered after encoding enhanced memory accuracy. Critically, alcohol effects on cued recollection depended on the valence of the emotional stimuli: Its memory-impairing effects during encoding were greatest for emotional stimuli, whereas its memory-enhancing effects during consolidation were greatest for emotionally neutral stimuli. Effects of alcohol on recognition were not related to stimulus valence.
Conclusions:
This study extends previous findings with memory for neutral stimuli, showing that alcohol differentially affects the encoding and consolidation of memory for emotional stimuli. These effects of alcohol on memory for emotionally salient material may contribute to the development of alcohol-related problems, perhaps by dampening memory for adverse consequences of alcohol consumption.
Powerful drug-related memories play a crucial role in substance use and substance use disorders. Drugs of abuse act on reward systems that normally facilitate learning and memory for motivational behaviors necessary for survival (e.g., procuring food or sex; Everitt et al., 2001; Robbins et al., 2008). Drugs of abuse can “hijack” these systems, resulting in aberrant learning for emotionally salient and drug-associated stimuli (Hyman et al., 2006; Torregrossa et al., 2011). Once learned, these memories persist even after long periods of abstinence, and their activation often produces drug craving, followed by drug seeking and drug taking (Stacy, 1997). This is especially relevant to alcohol-dependent individuals, because alcohol-related memories play a pivotal role in relapse (Ludwig, 1986).
Surprisingly, alcohol can both enhance and impair memory, depending on when it is administered. Alcohol impairs learning when it is present during encoding (Zorumski et al., 2014), whereas it enhances learning and memory for salient environmental stimuli when it is present during consolidation. Parker et al. (1980, 1981) first demonstrated this in humans, showing that alcohol improved memory when it was administered immediately following stimulus presentation (i.e., during memory consolidation). Similar findings have since been reported from several different laboratories (Bruce & Pihl, 1997; Bruce et al., 1999a; Knowles & Duka, 2004; Mann et al., 1984). Although the mechanism underlying this post-encoding memory improvement is unknown, several possible explanations have been suggested. These include facilitation of trace consolidation via direct actions on brain regions implicated in memory, including the hippocampus, caudate, and amygdala (Bruce & Pihl, 1999a; White, 1996); activation of reward/incentive pathways (Bruce et al., 1999b; Esposito et al., 1984; Mann et al., 1984); and/or a reduction in post-learning interference (Mueller et al., 1983; Tyson & Schirmuly, 1994).
Most studies on the effects of alcohol on encoding and consolidation have used neutral stimuli rather than emotionally salient material. It is important to determine whether similar effects occur with emotional memories, considering that drugs are thought to affect emotional memory systems (Everitt et al., 2001; Hyman et al., 2006; Robbins et al., 2008). In two studies, alcohol (0.65–0.7 g/kg) did not differentially affect encoding of emotional stimuli (Knowles & Duka, 2004; Ray et al., 2012). However, these studies assessed retrieval on the same day as encoding, while blood alcohol levels were still elevated. Thus, it is not known if memory for emotional stimuli would be impaired when participants are tested in a sober state. Other studies have examined effects of alcohol on consolidation of emotional memory, with conflicting results. Knowles and Duka (2004) found that alcohol (0.65 g/kg) enhanced consolidation preferentially for emotional (positive and negative) relative to neutral images when retrieval was tested on the same day as encoding. Bruce et al. (1999b) found that alcohol (0.8 g/kg) increased memory consolidation for elating statements relative to negative statements when tested 24 hours later. However, in two other studies, alcohol (0.8 g/kg) had similar effects on memory consolidation for emotional and neutral stimuli when tested 24 hours later (Bruce & Pihl, 1997; Bruce et al., 1999a). It is possible that these inconsistent findings could be attributable in part to acute alcohol effects (when retrieval is tested on the same day as encoding) or lingering alcohol withdrawal or hangover effects (when retrieval is tested 24 hours after encoding). As such, it is important to test retrieval at least 48 hours after encoding to ensure that no lingering alcohol effects have persisted.
Alcohol effects on emotional memory could have important implications for memories of drinking episodes that occur in a nonlaboratory setting. Greater memory impairment for negative events, or greater memory enhancement for positive events, could positively bias a drinker’s memory of past drinking episodes, and this, in turn, may increase the likelihood of alcohol consumption in the future. Thus, it is important to determine how alcohol, through its effects at either encoding or consolidation, affects emotional memories.
The current study examined the effects of alcohol on encoding and consolidation of memory for emotional stimuli in regular social drinkers by testing retrieval in sober participants 2 days later. Participants were assigned to one of three conditions: (a) encoding, in which alcohol was administered before stimulus viewing; (b) consolidation, in which alcohol was administered after viewing; and (c) control, in which placebo was administered both before and after viewing. Exactly 48 hours later, participants performed a surprise memory test in a drug-free state. This included a cued recollection test, which specifically targeted participants’ ability to bring to mind a detailed memory of each studied picture. We also administered a recognition memory test, which required participants to differentiate studied and nonstudied pictures and hence could be based more on a general feeling of familiarity. We hypothesized that alcohol would impair cued recollection and recognition memory in the encoding condition and enhance memory in the consolidation condition. We also hypothesized that alcohol effects on memory would be greater for emotional stimuli than for neutral stimuli.
Method
Design
This study used a two-session, double-blind, between-subjects design with three conditions: an encoding condition (ENC; n = 20), a consolidation condition (CON; n = 20), and a placebo control condition (PLA; n = 19). The between-groups design and group sizes are in line with previous studies of acute alcohol effects on memory encoding and consolidation (Bruce & Pihl, 1997; Bruce et al., 1999b; Knowles & Duka, 2004). Subjects were randomly assigned to the conditions, and at the first session they viewed standardized visual images with positive, negative, or neutral content. The ENC condition received alcohol before viewing the to-be-remembered stimuli, such that their blood alcohol levels were on the rising limb during stimulus viewing (encoding). Subjects in the CON condition received alcohol immediately after viewing the stimuli, such that their blood alcohol levels were ascending during the early stages of consolidation. The PLA condition received placebo both before and after stimulus viewing. Equal numbers of men and women were randomly assigned to the conditions. Exactly 48 hours later, subjects returned to the laboratory for the second session, during which they performed a surprise memory test. This study was approved by the Institutional Review Board of the University of Chicago and was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation.
Participants
Healthy volunteers (n = 59) were recruited from the community through online and printed advertisements. Volunteers were eligible for participation if they consumed an average of 10–30 standard drinks/week, with at least one heavy drinking episode (i.e., 4 or 5 drinks/occasion for women and men, respectively) in the past month. A standard drink was defined as a 12 oz can/bottle of beer, 5 oz glass of wine, 1.5 oz shot of distilled spirits, or mixed drink with 1.5 oz of distilled spirits. These minimum drinking criteria were included to ensure that participants could tolerate the alcohol dose. Additional inclusion criteria included age 21–30 years, body mass index between 19 and 26, at least a high school education, fluency in English, no current or past-year diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994; including substance dependence), no lifetime history of substance dependence, and no serious medical conditions. Participants were excluded if they reported smoking more than five cigarettes per day or daily use of any medication other than birth control, or if they were pregnant, lactating, or planning to become pregnant in the next 3 months. Women who were not on hormonal contraception were tested only in the follicular phase of their menstrual cycle.
Procedure
Participants first attended an orientation session in which they provided informed consent and were familiarized with laboratory procedures and study protocol. Participants were told that the study was investigating the effects of drugs and pictures on mood, and to minimize drug expectancies they were told they could receive one of the following: stimulant, sedative, alcohol, or placebo (Conrad et al., 2012; Gorka et al., 2013; King et al., 2014). They were instructed to consume their normal amounts of caffeine and nicotine but to abstain from drugs, including alcohol, for 24 hours before each session, and to not consume any food after 9 a.m.
Participants then attended two experimental sessions, exactly 48 hours apart. They were tested individually in comfortably furnished rooms. On arrival, compliance with drug abstinence was verified by both self-report and breath and urine screens (testing for amphetamine, cocaine, methamphetamine, opiates, and tetrahydrocannabinol). Baseline (pre-drug) physiological (heart rate and blood pressure) and subjective intoxication measures were obtained.
Session 1: Viewing session.
The viewing session took place from 1 p.m. to 6 p.m. After baseline measures were obtained, participants consumed the first dose of alcohol or placebo. Alcohol and placebo were administered in individual servings of black cherry sugar-free gelatin. The alcohol dose was 0.8 g/kg for men and 0.7 g/kg for women to achieve equivalent breath alcohol concentrations (BrACs) across sex (Fillmore, 2001; Mulvihill et al., 1997). This dose was chosen to produce peak BrACs of 80 mg/100 ml, and alcohol was administered in gelatin for fast consumption and to mask the taste. The alcohol gelatin was prepared with 3 parts 95% alcohol and 5 parts water, mixed together with the gelatin powder and then refrigerated overnight. Placebo gelatin was prepared with 8 parts water. Participants were served individual gelatin servings (5 g alcohol each) in black, opaque 2 oz cups. The number of servings required to reach a dose of 0.8 g/kg alcohol (men) and 0.7 g/kg alcohol (women) was determined for each participant based on body weight and ranged from 10 to 14 for men and 7 to 10 for women. Participants received the same number of servings for both alcohol and placebo gelatin administrations.
Participants in the ENC condition received alcohol before viewing the stimuli, when the CON and PLA conditions received placebo (Table 1). Participants were given 5 minutes to consume the dose. Twenty minutes later, all subjects viewed and rated pictures with labels (see below for picture viewing procedures). Immediately after the last picture, they consumed a second serving, in which the CON group received alcohol and the ENC and PLA groups received placebo. Subjective intoxication measures and BrACs (Alco-Sensor III, Intoximeters, Inc., St. Louis, MO) were assessed multiple times throughout the session. Following the second serving, participants remained in the laboratory for 3.5 hours. During the first 2 hours (i.e., the consolidation period), they listened to nonverbal music (classical or jazz) but were not allowed to watch movies, use the Internet, read, or sleep. We chose a 2-hour window to capture the ascending limb and peak of the blood alcohol curve in the CON group (consistent with Bruce et al., 1999b). After 2 hours they were given a snack and allowed to read or watch movies for the remainder of the session. Those who received alcohol were only allowed to leave after their BrAC had fallen below 40 mg/100 ml. Subjects were not informed during Session 1 that they would be asked to remember the images at Session 2.
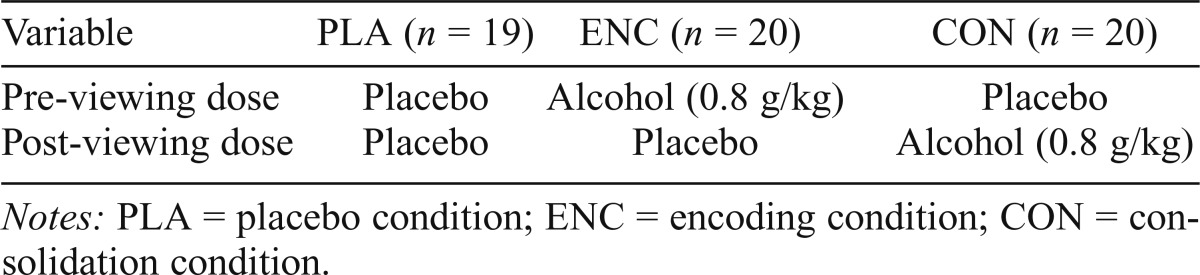
Table 1.
Pre- and post-viewing dose by condition
| Variable | PLA (n = 19) | ENC (n = 20) | CON (n = 20) |
| Pre-viewing dose | Placebo | Alcohol (0.8 g/kg) | Placebo |
| Post-viewing dose | Placebo | Placebo | Alcohol (0.8 g/kg) |
Notes: PLA = placebo condition; ENC = encoding condition; CON = consolidation condition.
Session 2: Retrieval session.
Retrieval sessions took place from 1 p.m. to 3:30 p.m., exactly 2 days after the viewing session, and involved no alcohol or placebo. After compliance testing, participants completed mood questionnaires and performed the cued recollection and recognition tasks (below). They were then debriefed and compensated for their time.
Measures
Cued recollection and recognition tasks: Viewing session.
Memory was assessed using both a cued recollection procedure (cf. Gallo et al., 2009; Weafer et al., 2014) and a picture recognition task. The stimuli were 144 pictures drawn from the International Affective Picture System (IAPS; Lang et al., 1999), as well as 96 alcohol-related images and matched neutral-beverage images. As the focus of the current study is alcohol effects on emotional memory, only data from the IAPS stimuli are presented here. Based on norms (1–9 scale), 48 IAPS images were negative (mean valence = 2.948, mean arousal = 5.660), 48 were neutral (mean valence = 5.306, mean arousal = 3.710), and 48 were positive (mean valence = 7.165, mean arousal = 5.575). Two- or three-word descriptive labels were created for each picture (e.g., “angry man face,” “sailboat on ocean”). From the 240 total pictures, two picture sets (A and B) were created (120 pictures each; 24 of each type of stimuli, based on normative ratings with emotional items matched for arousal), and an equal number of subjects in each condition were assigned to Set A or Set B. During the viewing phase, all participants viewed each of the labels (48 of each stimulus category) in random order. Before each label, a fixation point was presented on the screen. Participants pressed the space bar and a label was presented for 1,500 ms. After each label was presented, participants rated how much they thought they would like a picture associated with that label, on a scale from 1 (not at all) to 5 (very much). For half of the labels (either Set A or Set B), the picture described by the label was presented on the screen for 2000 ms immediately following the associated label. Following the presentation of each picture, participants rated the arousal and valence of the picture on three 5-point scales: affective valence (positive: 1–5; negative: 1–5) and arousal (1–5). For the other half, the labels were not followed by a picture.
Cued recollection and recognition tasks: Retrieval session.
Exactly 48 hours later, participants returned to the laboratory for the retrieval session and performed two surprise memory tasks: a cued recollection task followed by a picture recognition task. For the cued recollection test, participants viewed the labels from the viewing phase in random order. Half of these labels had been associated with a picture during the viewing phase (targets) and half had not (lures). For each label, they were asked whether they remembered seeing a picture that was associated with the label (yes/no). They then rated how confident they were in their decision on a 5-point scale, ranging from not at all to extremely. Because participants viewed all of the test labels during encoding, all of the labels should have been familiar to the participants. As such, the ability to discriminate between targets and lures relied on accurate recollection of the studied pictures associated with the targets (and not lures). Participants then performed the recognition task, in which they viewed the studied pictures (targets) randomly intermixed with pictures that were not seen during the study (but whose labels had been presented, lures). For each picture, they were asked whether they remembered seeing the picture (yes/no). They then rated how confident they were in their decision on a 5-point scale, ranging from not at all to extremely.
Drug Effects Questionnaire.
The Drug Effects Questionnaire was the primary measure of the subjects’ self-rated drug effects. It consisted of two items on a visual analogue scale (0–100 mm) on which participants rated the extent to which they “felt” the drug and “liked” the drug.
Timeline Followback.
At the beginning of the study, participants completed a retrospective timeline calendar of their alcohol consumption for the past month to assess daily patterns of drinking, including the number of heavy drinking episodes. For each day, participants estimated the number of standard drinks they consumed. Any day in which participants consumed five (men) or four (women) or more drinks was considered a heavy drinking episode. The Timeline Followback (Sobell & Sobell, 1992) provided three measures of drinking habits over the past month: (a) drinking days (total number of days alcohol was consumed), (b) total drinks (total number of drinks consumed), and (c) heavy drinking days (total number of heavy drinking episodes).
Data analysis
Cued recollection and recognition accuracy were analyzed by computing signal detection estimates (d'), using the correction described in Snodgrass and Corwin (1988) to avoid ceiling and floor effects. We calculated d' by subtracting the z score of the hit rate (correct responses to targets) from the z score of the false alarm rate (incorrect responses to lures). The acute effects of alcohol on d' estimates for cued recollection and recognition of emotional stimuli were tested by separate 3 (condition: PLA, ENC, CON) × 3 (valence: negative, neutral, positive) mixed-design analyses of variance, in which group was the between-subjects factor and valence was the within-subjects factor. Significant interactions were followed up by one-way analyses of variance testing the effect of beverage condition separately by picture valence. Effect size estimates (partial η2 and Cohen’s d) are provided.
Results
Sample characteristics
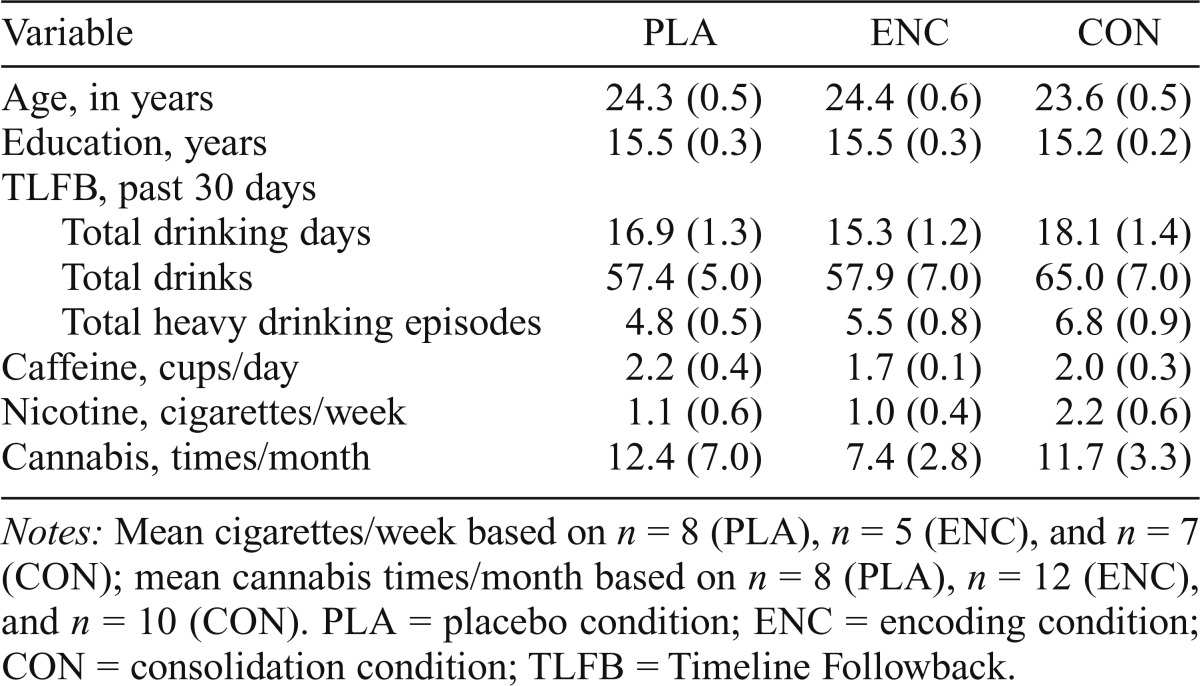
The three groups did not differ on demographic characteristics, current drinking habits, or other drug use (Table 2). Participants were moderate to heavy regular drinkers. The racial makeup of the sample was White (n = 47), African American (n = 5), Asian (n = 3), American Indian/Alaskan (n = 1), and more than one race (n = 3).
Table 2.
Mean (SE) age, education, drinking habits, and drug use by group
| Variable | PLA | ENC | CON |
| Age, in years | 24.3 (0.5) | 24.4 (0.6) | 23.6 (0.5) |
| Education, years | 15.5 (0.3) | 15.5 (0.3) | 15.2 (0.2) |
| TLFB, past 30 days | |||
| Total drinking days | 16.9 (1.3) | 15.3 (1.2) | 18.1 (1.4) |
| Total drinks | 57.4 (5.0) | 57.9 (7.0) | 65.0 (7.0) |
| Total heavy drinking episodes | 4.8 (0.5) | 5.5 (0.8) | 6.8 (0.9) |
| Caffeine, cups/day | 2.2 (0.4) | 1.7 (0.1) | 2.0 (0.3) |
| Nicotine, cigarettes/week | 1.1 (0.6) | 1.0 (0.4) | 2.2 (0.6) |
| Cannabis, times/month | 12.4 (7.0) | 7.4 (2.8) | 11.7 (3.3) |
Notes: Mean cigarettes/week based on n = 8 (PLA), n = 5 (ENC), and n = 7 (CON); mean cannabis times/month based on n = 8 (PLA), n =12 (ENC), and n = 10 (CON). PLA = placebo condition; ENC = encoding condition; CON = consolidation condition; TLFB = Timeline Followback.
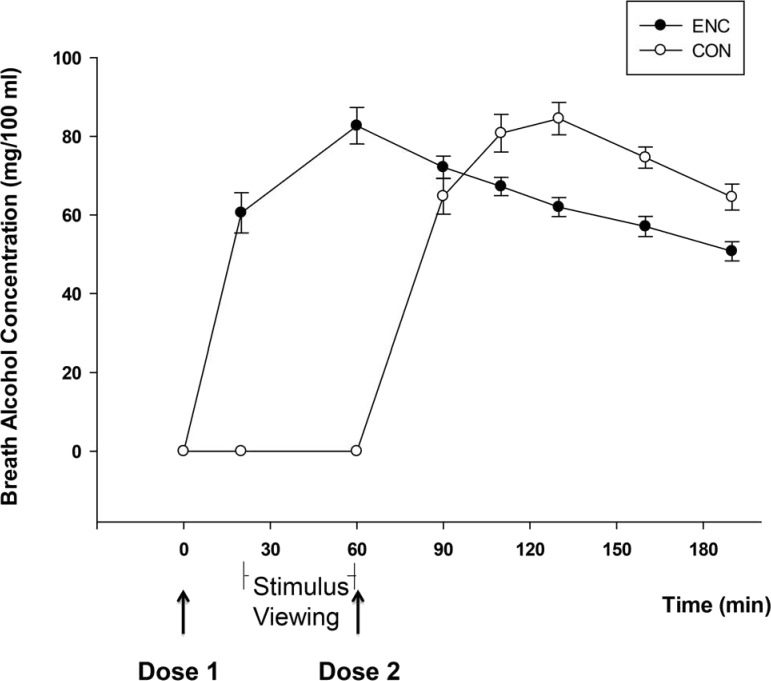
Breath alcohol concentrations
Mean BrACs for the ENC and CON conditions at each time point are presented in Figure 1. The figure shows that BrAC was rising in the ENC condition throughout the stimulus viewing and peaked at 60 minutes after administration, coinciding with the end of the stimulus viewing. In contrast, BrAC in the CON condition rose throughout the consolidation period and peaked 60 minutes after stimulus viewing. No differences in peak BrAC were observed between groups or between men and women (ps > .75).
Figure 1.
Mean (SE) breath alcohol concentration in the encoding (ENC) and consolidation (CON) conditions. Min = minute.
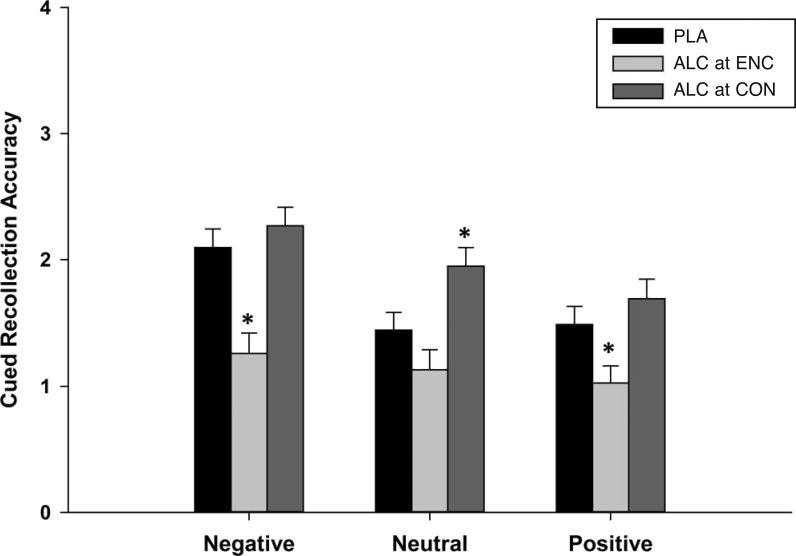
Cued recollection
Figure 2 presents mean cued recollection accuracy (d') by condition, separately for each stimulus valence. The figure shows that overall alcohol before encoding impaired cued recollection, whereas alcohol during consolidation facilitated cued recollection. The figure also shows that these effects differed according to stimulus valence, and this was supported by a Condition × Valence interaction, F(4, 112) = 2.6, p = .04, partial η2 = .09. We tested the effects of beverage condition separately by stimulus valence and observed a significant effect of condition for each valence, Fs(2, 58) > 5.7, ps < .01. For negative and positive stimuli, alcohol impaired cued recollection when administered at encoding (Dunnett t tests comparing ENC with the reference PLA condition for negative stimuli [p = .001] and for positive stimuli [p = .05]) but had no effect in the CON condition (Dunnett t test comparing CON with PLA: ps > .50). For neutral stimuli, alcohol did not affect cued recollection when administered at encoding (Dunnett t test: p = .25) but enhanced cued recollection when administered at consolidation (Dunnett t test: p = .04). Estimates of effect sizes confirmed that the effects of alcohol at encoding were greatest for negative stimuli (Cohen’s d = 1.22), followed by positive (Cohen’s d = 0.74) and then neutral stimuli (Cohen’s d = 0.47). In contrast, alcohol’s improvement of memory consolidation was greater for neutral stimuli (Cohen’s d = 0.79) compared with negative (Cohen’s d = 0.26) and positive (Cohen’s d = 0.31) stimuli.
Figure 2.
Mean (SE) memory accuracy (d') on the cued recollection test in the three conditions presented separately by valence. PLA = placebo condition; ALC at ENC = encoding condition; ALC at CON = consolidation condition. *Indicates Dunnett t test p ≤ .05 (compared with PLA condition).
Recognition
Figure 3 presents the mean recognition accuracy (d') by beverage condition, separately for each picture valence. Contrary to cued recollection, alcohol did not differentially affect recognition accuracy in relation to picture valence (Condition × Valence: p = .91), and so alcohol effects were examined collapsing across valence [main effect of condition, F(2, 56) = 11.6, p < .001, partial η2 = .29]. Alcohol during encoding did not affect recognition accuracy (Dunnett t test comparing ENC with PLA: p = .16), whereas alcohol at consolidation significantly enhanced recognition accuracy (Dunnett t test comparing CON with PLA: p = .008).
Figure 3.
Mean (SE) memory accuracy (d') on the recognition test in the three conditions, presented separately by valence. PLA = placebo condition; ALC at ENC = encoding condition; ALC at CON = consolidation condition. *Indicates Dunnett t test p < .05 (compared with PLA condition).
Stimulus and confidence ratings
Alcohol given before stimulus viewing did not affect ratings of likability, valence, or arousal of the images (ps > .17). No consistent associations between stimulus ratings and memory performance were observed. Alcohol at encoding reduced confidence ratings on the recognition test [main effect of condition, F(1, 37) = 4.6, p = .040, partial η2 = .11]. No other group differences in confidence ratings were observed.
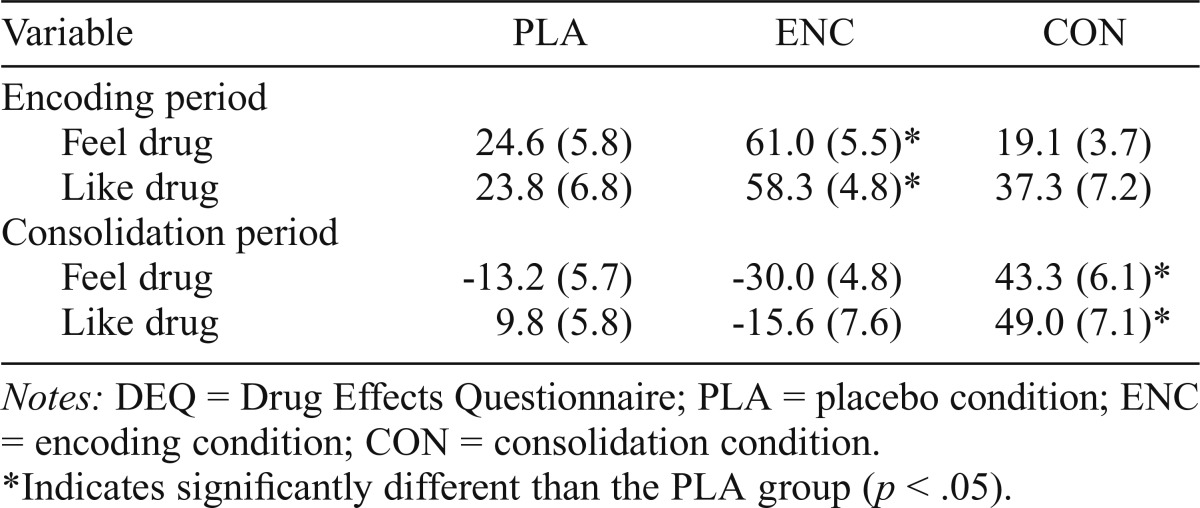
Subjective response (Drug Effects Questionnaire)
Alcohol produced its expected increase in “feel” drug and “like” drug ratings. Table 3 shows that alcohol increased subjective response measures in the ENC condition during the encoding phase (between-groups t tests, ps < .001, ds > 1.3) and increased these ratings in the CON condition during the consolidation phase (ps < .001, ds > 1.3). Subjective ratings of drug effects were not related to memory performance in any condition.
Table 3.
Mean (SE) DEQ ratings (peak change from pre-dose baseline)
| Variable | PLA | ENC | CON |
| Encoding period | |||
| Feel drug | 24.6 (5.8) | 61.0 (5.5)* | 19.1 (3.7) |
| Like drug | 23.8 (6.8) | 58.3 (4.8)* | 37.3 (7.2) |
| Consolidation period | |||
| Feel drug | -13.2 (5.7) | -30.0 (4.8) | 43.3 (6.1)* |
| Like drug | 9.8 (5.8) | -15.6 (7.6) | 49.0 (7.1)* |
Notes: DEQ = Drug Effects Questionnaire; PLA = placebo condition; ENC = encoding condition; CON = consolidation condition.
Indicates significantly different than the PLA group (p < .05).
Discussion
This study replicated previous reports that alcohol impairs memory when administered before encoding but improves memory when administered before consolidation. Using a cued recollection test, we also showed that the effects of alcohol on recollection depend on the nature of the to-be-remembered stimuli. Alcohol impaired memory in the ENC condition for emotional stimuli but not neutral stimuli, whereas alcohol improved memory in the CON condition for neutral stimuli only.
Our findings add to previous reports of alcohol effects on emotional memory. Two previous studies (Knowles & Duka, 2004; Ray et al., 2012) showed that alcohol before encoding impaired memory for both emotional and neutral images, but they tested free recall memory on the same day as encoding, raising questions about whether the drug contributed to the retrieval phase. Here, we found that when retrieval was tested 48 hours later, alcohol before encoding impaired cued recollection for emotional stimuli only. Thus, it could be that after the acute effects of alcohol have subsided, memory impairment is greater for emotional relative to neutral stimuli. Regarding alcohol effects on consolidation, Knowles and Duka (2004) found enhanced free recall memory for emotional compared with neutral images when tested on the same day as encoding, whereas Bruce and Pihl (1997) found no difference in recall for emotional and neutral stimuli when tested 24 hours later, and we found enhanced cued recollection for neutral compared with emotional stimuli when tested 48 hours later. Taken together, these studies suggest that enhanced emotional relative to neutral memory might be observed soon after stimulus presentation but that this emotional boost might fade over time, perhaps to the point where emotional material is remembered with less accuracy than neutral stimuli. Although speculative at this point, future studies examining changes in memory retrieval over time would provide a more direct test of this hypothesis.
The effects of alcohol on memory were more evident using the measure of cued recollection compared with recognition memory. Cued recollection specifically targets explicit recollection of material and thus involves detailed and effortful episodic memory processes, whereas recognition assesses general familiarity with the material and involves faster, relatively automatic memory processes (Yonelinas, 2002). Whereas alcohol at encoding impaired memory overall on both tests, the disproportionately larger effects on emotional compared with neutral stimuli were more pronounced on cued recollection than on recognition testing. Similarly, alcohol at consolidation improved memory overall, but the relatively larger effects on neutral relative to emotional items again were more pronounced on cued recollection than on recognition testing. These patterns suggest that the effortful memory processes targeted by cued recollection are more sensitive to subtle interactions between alcohol and stimulus valence compared with recognition testing.
The effects of cued recollection may be particularly relevant for behavioral outcomes outside the laboratory, as the recollection of past emotional experiences with alcohol might drive future drinking behaviors. Alcohol at encoding disproportionately impaired memory for emotional, and particularly negative, stimuli, which in the nonlaboratory setting might include memory for the negative events that occur during a drinking episode. Such impairment could decrease the potential for negative alcohol-related outcomes to discourage future consumption and could also partially explain the disregard of negative drinking-related consequences observed in alcohol-dependent individuals. On the other hand, the facilitation of neutral over negative memories by alcohol at consolidation could have important implications for learning about stimuli associated with a drinking episode. Theories regarding dependence suggest that drugs of abuse can enhance learning and memory for such stimuli, resulting in enhanced salience attribution (Hyman et al, 2006; Robbins et al., 2008; Torregrossa et al., 2011). Alcohol’s effects on consolidation could be one means through which the drug serves to enhance learning for stimuli encountered just before initiation of a drinking episode. Future studies are needed to test how alcohol effects on these memory processes relate to drinking patterns and the transition to hazardous drinking.
This study had some limitations. First, the cued recollection test was administered immediately before the recognition test. The cued recollection test presented participants with labels describing each of the pictures and required participants to recollect the associated picture. Such mental representation of the stimuli could have potentially influenced responses on the recognition test. In addition, recognition accuracy ranged from approximately 75% to 90%. Although these scores were not quite at the ceiling, they were still high and thus limited room for movement across conditions. Thus, although the same general pattern regarding impairment and facilitation of memory across emotional and neutral stimuli was observed for both the recognition and cued recollection tests, the recognition test was not sensitive to Condition × Valence interactions. Finally, it is important to note that alcohol was present during both encoding and early stage consolidation in the ENC condition. Thus, it is likely that the impairing effects of alcohol observed in this group are attributable to the presence of the drug during both the encoding process and the early stages of consolidation.
In sum, this study provides new evidence regarding the effects of alcohol on encoding and consolidation of memory for emotional stimuli. Results suggest that alcohol administered before encoding impairs memory, preferentially for emotional stimuli, and alcohol administered before consolidation enhances memory, preferentially for neutral stimuli. These findings have potentially important implications for understanding alcohol effects on memory for emotionally salient material, as well as the role of such memory in alcohol abuse and dependence.
Footnotes
This research was supported by National Institute on Drug Abuse Grants DA002812 (to Harriet de Wit), DA031796 (to Harriet de Wit), and F32 DA033756 (to Jessica Weafer).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bruce K. R., Pihl R. O. Forget “drinking to forget”: Enhanced consolidation of emotionally charged memory by alcohol. Experimental and Clinical Psychopharmacology. 1997;5:242–250. doi: 10.1037//1064-1297.5.3.242. doi:10.1037/1064-1297.5.3.242. [DOI] [PubMed] [Google Scholar]
- Bruce K. R., Pihl R. O., Mayerovitch J. I., Shestowsky J. S. Alcohol and retrograde memory effects: Role of individual differences. Journal of Studies on Alcohol. 1999a;60:130–136. doi: 10.15288/jsa.1999.60.130. doi:10.15288/jsa.1999.60.130. [DOI] [PubMed] [Google Scholar]
- Bruce K. R., Shestowsky J. S., Mayerovitch J. I., Pihl R. O. Motivational effects of alcohol on memory consolidation and heart rate in social drinkers. Alcoholism: Clinical and Experimental Research. 1999b;23:693–701. doi:10.1097/00000374-199904001-00016. [PubMed] [Google Scholar]
- Conrad M., McNamara P., King A. Alternative substance paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Experimental and Clinical Psychopharmacology. 2012;20:382–389. doi: 10.1037/a0029261. doi:10.1037/a0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. U., Parker E. S., Weingartner H. Enkephalinergic-dopaminergic “reward” pathways: A critical substrate for the stimulatory, euphoric and memory-enhancing actions of alcohol—a hypothesis. Substance and Alcohol Actions/Misuse. 1984;5:111–119. [PubMed] [Google Scholar]
- Everitt B. J., Dickinson A., Robbins T. W. The neuropsychological basis of addictive behaviour. Brain Research Reviews. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. doi:10.1016/S0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fillmore M. T. Cognitive preoccupation with alcohol and binge drinking in college students: Alcohol-induced priming of the motivation to drink. Psychology of Addictive Behaviors. 2001;15:325–332. doi:10.1037/0893-164X.15.4.325. [PubMed] [Google Scholar]
- Gallo D. A., Foster K. T., Johnson E. L. Elevated false recollection of emotional pictures in young and older adults. Psychology and Aging. 2009;24:981–988. doi: 10.1037/a0017545. doi:10.1037/a0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka S. M., Fitzgerald D. A., King A. C., Phan K. L. Alcohol attenuates amygdala–frontal connectivity during processing social signals in heavy social drinkers: A preliminary pharmaco-fMRI study. Psychopharmacology. 2013;229:141–154. doi: 10.1007/s00213-013-3090-0. doi:10.1007/s00213-013-3090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E., Malenka R. C., Nestler E. J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. doi:10.1146/annurev. neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- King A. C., McNamara P. J., Hasin D. S., Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biological Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. doi:10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. K., Duka T. Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behavioural Pharmacology. 2004;15:111–121. doi: 10.1097/00008877-200403000-00003. doi:10.1097/00008877-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Ludwig A. M. Pavlov’s “bells” and alcohol craving. Addictive Behaviors. 1986;11:87–91. doi: 10.1016/0306-4603(86)90032-8. doi:10.1016/0306-4603(86)90032-8. [DOI] [PubMed] [Google Scholar]
- Mann R. E., Cho-Young J., Vogel-Sprott M. Retrograde enhancement by alcohol of delayed free recall performance. Pharmacology, Biochemistry, and Behavior. 1984;20:639–642. doi: 10.1016/0091-3057(84)90317-4. doi:10.1016/0091-3057(84)90317-4. [DOI] [PubMed] [Google Scholar]
- Mueller C. W., Lisman S. A., Spear N. E. Alcohol enhancement of human memory: Tests of consolidation and interference hypotheses. Psychopharmacology. 1983;80:226–230. doi: 10.1007/BF00436158. doi:10.1007/BF00436158. [DOI] [PubMed] [Google Scholar]
- Mulvihill L. E., Skilling T. A., Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. doi:10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Parker E. S., Birnbaum I. M., Weingartner H., Hartley J. T., Stillman R. C., Wyatt R. J. Retrograde enhancement of human memory with alcohol. Psychopharmacology. 1980;69:219–222. doi: 10.1007/BF00427653. doi:10.1007/BF00427653. [DOI] [PubMed] [Google Scholar]
- Parker E. S., Morihisa J. M., Wyatt R. J., Schwartz B. L., Weingartner H., Stillman R. C. The alcohol facilitation effect on memory: A dose-response study. Psychopharmacology. 1981;74:88–92. doi: 10.1007/BF00431763. doi:10.1007/BF00431763. [DOI] [PubMed] [Google Scholar]
- Ray S., Mun E.-Y., Buckman J. F., Udo T., Bates M. E. Memory for emotional picture cues during acute alcohol intoxication. Journal of Studies on Alcohol and Drugs. 2012;73:718–725. doi: 10.15288/jsad.2012.73.718. doi:10.15288/jsad.2012.73.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. W., Ersche K. D., Everitt B. J. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141:1–21. doi: 10.1196/annals.1441.020. doi:10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Snodgrass J. G., Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. doi:10.1037/0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R. Z., Allen J. P., editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Stacy A. W. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. Journal of Abnormal Psychology. 1997;106:61–73. doi: 10.1037//0021-843x.106.1.61. doi:10.1037/0021-843X.106.1.61. [DOI] [PubMed] [Google Scholar]
- Torregrossa M. M., Corlett P. R., Taylor J. R. Aberrant learning and memory in addiction. Neurobiology of Learning and Memory. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. doi:10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson P. D., Schirmuly M. Memory enhancement after drinking ethanol: Consolidation, interference, or response bias? Physiology & Behavior. 1994;56:933–937. doi: 10.1016/0031-9384(94)90326-3. doi:10.1016/0031-9384(94)90326-3. [DOI] [PubMed] [Google Scholar]
- Weafer J., Gallo D. A., de Wit H. Amphetamine fails to alter cued recollection of emotional images: Study of encoding, retrieval, and state-dependency. PLoS ONE. 2014;9:e90423. doi: 10.1371/journal.pone.0090423. doi:10.1371/journal. pone.0090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. M. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91:921–949. discussion 951–965. doi:10.1046/j.1360-0443.1996.9179212.x. [PubMed] [Google Scholar]
- Yonelinas A. P. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. doi:10.1006/jmla.2002.2864. [Google Scholar]
- Zorumski C. F., Mennerick S., Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. doi:10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]