Despite significant advances in pharmacotherapy and lifestyle changes, the prevalence of hypertension (HTN) has steadily been on the rise. An estimated 20–30% of hypertensive patients have resistant HTN (rHTN), defined as blood pressure (BP) ≥ 140/90 mm Hg on ≥3 antihypertensive drugs of different classes including one diuretic at optimal doses, or <140/90 on ≥4 drugs [1]. Patients with rHTN have increased risk for cardiovascular events (e.g., coronary artery disease, stroke, and heart failure) [1]. New antihypertensive drugs and interventional procedures (e.g., renal sympathetic denervation, immunization against angiotensin II, and baroreflex activation) have been extensively studied to address the unmet need of controlling BP in this patient population; however, rHTN remains a challenging task for healthcare providers. We report a case of the impact of three antibiotics on BP in a patient with rHTN, which is not related to hypersensitivity reactions and has not been reported in the literature.
A 69-year-old woman with a long history of HTN (44 years), coronary artery disease, arthritis, asthma, obstructive sleep apnea, hyperlipidemia, and diabetes mellitus was diagnosed with rHTN 3 years ago. Her BP was always uncontrolled (>140/90 mm Hg, Fig. 1). At her first cardiology appointment, her BP was 160/90 mm Hg when she was taking amlodipine–benazepril (5–20 mg daily), verapamil (240 mg daily), and valsartan–hydrochlorothiazide (320/12.5 mg daily). Her antihypertensive medication was then adjusted to spironolactone (50 mg daily), valsartan–hydrochlorothiazide (320/25 mg daily), and verapamil (360 mg daily), and her systolic BP was 150 s mm Hg before her knee surgery for her chronic left knee pain. Twenty-one days following the surgery, she exhibited symptoms of early wound infection and received irrigation and debridement and antibiotic treatment [vancomycin (intravenous, 1250 mg, Q12 h), rifampin (oral, 600 mg, Q12 h), and ciprofloxacin (oral, 750 mg, Q12 h) for 42 days]. Thirty days following the initiation of antibiotic therapy, her BP was 130 s/60 s mm Hg at home with hydralazine (25 mg BID) and verapamil (360 mg daily). Two days after the termination of antibiotics, she developed hypotension symptoms and her BP was 70 s/40 s mm Hg with no antihypertensive medication. Her BP was in the 110 s/50 s–60 s range for the following 2 weeks without any antihypertensive drugs. Fourteen days after the termination of antibiotics, her office BP was 154/60 supine, 160/60 sitting, and 140/60 standing with no antihypertensive medication, and initiated verapamil (360 mg daily) treatment. Seventeen days after the termination of antibiotics, her BP at home was 70–80/46–55 mm Hg for about 3 days while she was not on antihypertensive drugs. However, at 1 week follow-up, she awakened with palpitations and her BP was 200/101 mm Hg and began antihypertensive medication, and her BP at home was then relatively stable, with systolic BP 140 s mm Hg with only verapamil 180 mg daily for 4 weeks. At 6 months after termination of antibiotics, her BP gradually elevated and was not controlled by verapamil. Her office BP was 160/88 mm Hg, and lisinopril (20 mg daily) was added to the verapamil (180 mg daily). However, she continued to experience elevated BP with office BP reading 184/91 mm Hg. Taking her continuous elevation of BP and history of rHTN into consideration, spironolactone (25 mg daily) was added to her antihypertensive regimen (verapamil 180 mg daily and lisinopril 20 mg daily).
Fig. 1.
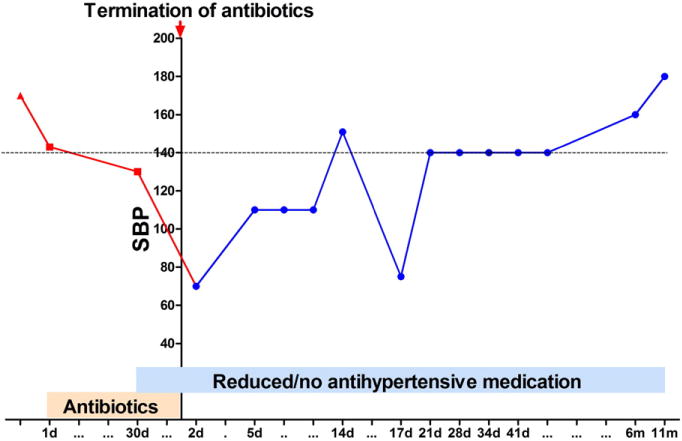
Systolic blood pressure (SBP) during antibiotic and post-antibiotic treatment over a 12-month period. (d: day; m: month).
This case is unique because BP was controlled with no antihypertensive medication for 2 weeks while the patient was taking antibiotics and for 6 months with one category of antihypertensive following the termination of antibiotic treatment. BP lowering effects lasted for several months post-antibiotic treatment, indicating antibiotics initiate underlying mechanisms for BP regulation. Direct effects of antibiotics on gut microbiota (GM) could contribute to their underlying therapeutic effects for rHTN. A delicate balance in the GM composition is critical to maintain intestinal immunity and whole body homeostasis. Any disruption of this balance could cause devastating pathophysiological consequences and has been correlated with many diseases. Notably, GM are suggested to play a role in cardiovascular diseases [2,3], and dysbiosis is associated with HTN [3]. Vancomycin has been shown to reduce the richness of the mucosal and luminal communities, causing a large reduction in Firmicutes and Bacteroidetes and a corresponding dramatic increase in Proteobacteria [4]. Ciprofloxacin altered composition within 3–4 days of administration, and the microbial populations rebounded toward their original composition by 4 weeks after exposure; however, final composition was permanently altered [5]. Thus, treatment with a broad-spectrum antibiotic leads to significant modification to the GM, and these changes in the composition of GM are long-lasting and persist after withdrawal of antibiotic, which possibly contributed to the enduring BP effects observed in this patient.
Increasing evidence suggests that rHTN is accompanied by a chronic inflammation that facilitates end-organ damage and perpetuates the hypertensive state [3,6]. GM have also been shown to modulate the immune system [7]. Chronic inflammation induced by endotoxin from dysbiotic GM contributes to the development of several risk factors for HTN such as obesity and diabetes and increased incidence of vascular complications [3]. Additionally, antibiotics possess anti-inflammatory and immunomodulatory properties [6]. Vancomycin affects tumor necrosis factor α (TNF-α) pathways and regulatory T cells [8]. Rifampin inhibits interleukin-1β (IL1-β)-induced arachidonic acid release and prostaglandin E2 production. Ciprofloxacin enhances IL-3 and GM-CSF production and reduces inflammation mediated by pro-inflammatory cytokines (IL-1, IL-6, and TNF-α). We also observed that minocycline inhibited angiotensin II-stimulated neuroinflammation and significantly reduced BP in animal models of HTN [9], and minocycline progressively decreased BP in nine patients with rHTN over a 36-week period [10].
Collectively, it is indicated that immune-modulatory and GM-related effects of most commonly used antibiotics, coupled with our ever enhancing appreciation of the health-promoting roles of specific populations of microbes, should be considered in the treatment of rHTN. This may influence decision making with regard to which drugs to prescribe or strategies to implement to increase beneficial outcomes in rHTN patients.
Acknowledgments
Sources of funding
2 UM1 HL087366-06, R01 HL056921, HL33610, and UL1 TR000064 from the National Institutes of Health.
Footnotes
Conflict of interest statement
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Muxfeldt ES, de Souza F, Margallo VS, Salles GF. Cardiovascular and renal complications in patients with resistant hypertension. Curr Hypertens Rep. 2014 Sep;16(9):471. doi: 10.1007/s11906-014-0471-7. [DOI] [PubMed] [Google Scholar]
- 2.Howitt MR, Garrett WS. A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 3.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015 Jun;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson C, Young V. Antibiotic administration alters the community structure of the gastrointestinal microbiota. Gut Microbes. 2010 Jul-Aug;1(4):279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl. 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1(1):68–79. [PubMed] [Google Scholar]
- 7.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 8.Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, et al. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol. 2013;33(2):397–406. doi: 10.1007/s10875-012-9801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, Shaw LC, Carnegie D, Caballero S, Li Q, Stitt AW, Raizada MK, Grant MB. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2012 Sep;31(5):481–494. doi: 10.1016/j.preteyeres.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


