Abstract
In order to engage in purposeful behavior, it is important to make plans, which organize subsequent actions. Most studies of planning involve “look-ahead” puzzle tasks that are unrelated to personal goals. We developed a task to assess autobiographical planning, which involves the formulation of personal plans in response to real-world goals, and examined autobiographical planning in 63 adults during fMRI scanning. Autobiographical planning was found to engage the default network, including medial temporal lobe and midline structures, and executive control regions in lateral prefrontal and parietal cortex and caudate. To examine how specific qualitative features of autobiographical plans modulate neural activity, we performed parametric modulation analyses. Ratings of plan detail, novelty, temporal distance, ease of plan formulation, difficulty in goal completion, and confidence in goal accomplishment were used as covariates in six hierarchical linear regression models. This modeling procedure removed shared variance among the ratings, allowing us to determine the independent relationship between ratings of interest and trial-wise BOLD signal. We found that specific autobiographical planning, describing a detailed, achievable, and actionable planning process for attaining a clearly envisioned future, recruited both default and frontoparietal brain regions. In contrast, abstract autobiographical planning, plans that were constructed from more generalized semantic or affective representations of a less tangible and distant future, involved interactions among default, sensory-perceptual, and limbic brain structures. Specific qualities of autobiographical plans are important predictors of default and frontoparietal control network engagement during plan formation and reflect the contribution of mnemonic and executive control processes to autobiographical planning.
Keywords: Default mode network, episodic future thinking, prospection, executive function, cognitive control, frontoparietal control network
The ability to mentally represent the future, or prospection, is a broad concept that has been used to characterize a wide variety of future-oriented cognitions (e.g., Gilbert & Wilson, 2007; Seligman, Railton, Baumeister, & Sripada, 2013). Four modes of future thinking have been identified that encapsulate the bulk of research on prospective cognition: simulation, prediction, intention, and planning (Szpunar, Spreng & Schacter, 2014). These modes of future thinking range from the initial conception of a possible future event to the process of attaining a goal. Planning involves the identification and sequencing of steps toward achieving a goal state. Autobiographical planning in particular involves the identification and organization of steps needed to arrive at a specific autobiographical future event or outcome. The process of autobiographical planning combines elements of autobiographical memory with goal-directed planning operations. Several studies have shown that autobiographical planning engages synchronized activity of medial temporal lobe memory structures as well as frontal executive regions (Gerlach, Spreng, Madore & Schacter, 2014; Spreng et al., 2010; Spreng & Schacter, 2012). This work has emphasized the coordinated activation of large-scale brain systems; specifically, of the default and frontoparietal control networks.
The default network is a set of functionally connected brain regions engaged by self-generated thought and active across multiple functional domains including memory, future-thinking, and social cognition (Andrews-Hanna, Smallwood, & Spreng, 2014; Buckner, Andrews-Hanna & Schacter, 2008; Spreng, Mar & Kim, 2009). The network includes the medial prefrontal cortex (PFC), medial parietal cortex, including posterior cingulate cortex (PCC) and retrosplenial cortex (RSC), the posterior inferior parietal lobule (IPL), medial temporal lobes (MTL), and lateral temporal cortex.
Default network activity has been implicated in future-oriented episodic simulation, which involves spatiotemporal unfolding of imagined events (Schacter, Addis & Buckner, 2008; Schacter et al., 2012). Specific qualitative features of simulated events have been found to modulate brain activity. The richness and specificity of episodic detail during such simulations has been associated with increasing left (Addis & Schacter, 2008) and right anterior hippocampal activation (Addis et al., 2011), as well as left amygdala and right frontal polar regions (Addis & Schacter, 2008). Imagining more temporally distant future events results in greater hippocampal activity bilaterally (Addis & Schacter, 2008), and activation of medial prefrontal cortex has also been observed while envisioning distal emotional events (D’Argembeau et al., 2008). Simulation of events more proximal in time has also been associated with activation in default network brain regions (Tamir & Mitchell, 2011). Decreasing probability of an event occurring in the future has been linked with increasing right anterior hippocampal activity, controlling for effects of temporal distance, amount of detail, and emotionality (Weiler et al., 2010). Finally, optimism about future event occurrence has been shown to modulate ventromedial prefrontal cortex (Sharot et al., 2007). While much work has been done to elucidate these qualitative aspects of episodic simulation, it remains unclear what qualitative features of autobiographical plans may modulate neural activity.
There is an extensive body of literature investigating the neuropsychological and neurophysiological correlates of planning as a domain of executive functioning (see Owen 1997, for a review). This work has typically employed laboratory-based, problem-solving paradigms that require individuals to formulate, sequence, and implement a series of steps towards attainment of a target goal state (c.f. Tower of London task (TOL), Owen et al., 1990; 1996), although efforts have been made to assess planning capacity in more ecologically valid settings (c.f. Multiple Errands Test; Shallice and Burgess, 1991). Planning capacity has been associated with lateral prefrontal and parietal cortex activations (e.g., Owen et al., 1996; Spreng et al., 2010; van den Heuvel et al., 2003; Wager et al., 2006), as well as subcortical structures, including associative striatum (e.g., Monchi et al., 2006; van den Heuvel et al., 2003; Wunderlich, Dayan & Dolan, 2012). Lateral prefrontal cortex, the anterior extent of the inferior parietal lobule, dorsal anterior cingulate, and anterior insula comprise regions of an extended frontoparietal control system broadly involved in executive control (Niendam et al., 2012; Vincent et al., 2008). In a recent study, regions of the dorsal attention network, including the frontal eye fields and superior parietal cortex, coupled with a frontoparietal control network during performance of the TOL task (Spreng et al., 2010). Critically, during performance of an autobiographical planning analog of the TOL task in the same scanning session, network coupling shifted such that the frontoparietal control network was more closely coupled with regions of the default network. This finding suggests that generating plans for one’s personal future requires engagement of both default network brain regions, to simulate personal future goal states, as well as the frontoparietal control network, to implement the control processes necessary to guide actions towards goal attainment.
While the general network architecture supporting autobiographical planning is beginning to come into focus, the role of specific regions within these networks, and the ways in which they are modulated by discrete planning features, have yet to be determined. The aim of the present study is to identify the distributed pattern of brain regions involved in autobiographical planning and to investigate how these are modulated by plan detail, novelty, temporal distance, ease of plan formulation, perceived difficulty in goal attainment, and confidence in plan completion. In light of the previous observations discussed above, we predicted that autobiographical planning would additionally engage both default and frontoparietal control network regions and further hypothesized that contributions of regions in both networks would be modulated by their qualitative features. Specifically, we predict that the modulation effect of qualitative features during episodic future event simulation, associated with default brain regions, would be replicated during autobiographical planning. Moreover, we suggest that greater specificity in planning processes will be associated with activity in the frontal parietal control brain regions as control processes are engaged to formulate a detailed path to a readily envisioned future. The results provide the first comprehensive assessment of how specific qualities of autobiographical plans are linked with engagement of default and frontoparietal control networks during plan formation.
Methods
Participants
Sixty-three healthy young adults (Mage = 22.5y ± 2.6; range = 18-30y; 40 women) consented to participate in this study approved by the Harvard Institutional Review Board. The present study is based on a novel analysis of previously published data (Spreng et al., 2010; Spreng & Schacter, 2012; Spreng et al., 2013).
Task
Only a brief description of the paradigm is provided here; for a full description, refer to Spreng et al. (2010). Autobiographical planning was assessed by a novel task that required participants to devise personal plans in order to meet specific goals. For example, “freedom from debt” constituted one of the goals in the autobiographical planning task. Participants viewed the goal and then saw two steps they could take toward achieving that goal (“good job” and “save money”) as well as an obstacle they needed to overcome in order to achieve the goal (“have fun”). Participants were instructed to integrate the steps and obstacles into a cohesive personal plan that would allow them to achieve the goal. Participants also performed a baseline counting task, which involved the sequential counting of vowels within random letter sequences. Performance on a Tower of London task was also scanned but not included in the current analysis. All stimuli were visually matched (see Spreng et al., 2010 for details).
Study A included 20 participants who generated 30 autobiographical plans (Spreng et al., 2010). Study B included 18 participants who generated 24 autobiographical plans (Spreng & Schacter, 2012). Study C included 25 participants who generated 20 autobiographical plans (R.N. Spreng, A.W. Gilmore, & D.L. Schacter, unpublished observations). All participants rated the extent of detail included in their plan immediately following each trial in the scanner. After the scan, participants were interviewed about their autobiographical plans. They rated each plan for novelty (i.e., how much the plan had been given prior consideration before participating in the study), ease of formulating the plan in the scanner, and foreseeable difficulty in accomplishing the goal. Ratings of confidence in achieving the goal were collected in Study B and C. All characteristics of the autobiographical plans were rated on a Likert-scale ranging from one to four (or five, for detail in Study C). Due to some Likert scaling differences between studies and for ease of interpretation, ratings were subsequently rescaled from one to 100 prior to analysis. Participants also estimated the time to goal completion (number of days, months and/or years). These values were calculated as a function of days from the present and log transformed for subsequent analysis to correct for positive skew in the distribution (see Spreng & Levine, 2006). See Table 1.
Table 1.
Mean within-subject correlations among the ratings and descriptive statistics
| Detail | Novelty | Temporal distance | Ease of formulation | Difficulty in goal attainment | Confidence in completion | |
|---|---|---|---|---|---|---|
| Detail | — | |||||
| Novelty | −.28 (−.32)** | — | ||||
| Temporal distance | −.02 (.21) | .05 (.25) | — | |||
| Ease of formulation | .24 (.33)** | −.35 (.34)** | .04 (.25) | — | ||
| Difficulty in goal attainment | −.13 (.26)** | .12 (.33)* | −.04 (.26) | −.57 (.44)** | — | |
| Confidence in completion | .02 (.22) | .03 (.24) | −.40 (.25)** | 0.0 (.26) | .03 (.22) | — |
| M | 65 | 31 | 4m 10d | 68 | 42 | 79 |
| SD | 14 | 12 | 10d | 15 | 15 | 10 |
Note:
SD of the within subject correlations are in parentheses. Ratings are presented on a scale of 1 – 100.
p < .01;
p < .001;
m = months;
d = days.
In order to examine the association among the behavioral ratings, trial-wise within-subject correlations were computed. To determine which associations were significant, the within-subject correlations were submitted to a Fisher’s r-to-z transform and tested by a simple t-test (test criteria = 0, no correlation). Mean z-scores were then converted back to r-values for interpretation purposes (Table 1).
Neuroimaging
Neuroimages were acquired on a Siemens Trio 3 Tesla scanner with a 12- or 32- channel head coil. BOLD functional scans were acquired with a T2*- weighted EPI pulse sequence (TR = 2500 ms; TE = 30 ms; 3×3×3mm voxels). Details of the scanning parameters for Study A (Spreng et al, 2010) and Study B (Spreng and Schacter, 2012) can be found in the original published reports. For Study C, anatomical scans and five 10min 15sec BOLD functional scans were acquired with the same imaging parameters as Spreng and Schacter (2012). All fMRI data were subjected to standard preprocessing steps, including slice-timing and motion correction, atlas registration to the MNI template, and spatial smoothing with a 6mm Gaussian kernel at full width at half maximum, as detailed in Spreng et al. (2010).
In the present study, neuroimaging data were analyzed with SPM8. First, brain activity during autobiographical planning was examined relative to counting. We generated a general linear model for each participant, modeling cognitive events with the canonical hemodynamic response function, its temporal derivative, and its dispersion derivative, mean and linear drift for each functional run, and the six motion parameters. Cognitive tasks comprised autobiographical planning, counting, and the Tower of London. Study C modulated autobiographical load by including three or six items to integrate into the plan. The present analysis merged across these trials, and load was included as a covariate of no interest. The t-contrast image for autobiographical planning > counting was used in a second-level, random-effects analysis, which included study and head coil type as second-level regressors. This whole-brain contrast was corrected for multiple comparisons using the False Discovery Rate (FDR) significance threshold of p < .05 and a required cluster size of k > 20.
Although there are significant associations among the qualitative autobiographical plan ratings (see Behavior Results), statistically independent relationships between these ratings and brain activity during planning were determined. In order to examine unique brain activity associated with each of the ratings, six hierarchical linear models were built, such that associations with the rating of interest were orthogonalized with respect to the other ratings. This modeling procedure removed the shared variance among the different ratings, allowing us to determine the independent relationship between the rating of interest and the BOLD signal during autobiographical planning. In order to assess how brain activity during autobiographical planning was modulated according to the ratings, we generated a hierarchical linear model for each participant, modeling the cognitive tasks with the canonical hemodynamic response function, its temporal derivative, and its dispersion derivative, mean and linear drift for each functional run, the six motion parameters, as well as the parametric regressors. Participants’ in-scan rating for detail and post-scan ratings of novelty, confidence, temporal distance, ease of formulating the plan in the scanner, and difficulty in accomplishing the goal in life were included as parametric regressors in six distinct models. Four subjects were excluded due to collinearity among the ratings. The resulting parametric t-contrast images for the ratings were then used in a second-level, random-effects analyses, which included study and scanner type as second-level regressors. To examine how neural activity was modulated as a function of the parametric regressors, we performed a simple t-test, masked by the autobiographical planning > counting contrast image, and again a significance threshold of p < .05, FDR-corrected and k > 20. For a priori investigation into the temporal distance effect on hippocampal modulation, the cluster extent criteria was relaxed to k > 10 (c.f. Addis et al., 2008).
Results
Behavior
On average, participants generated moderately detailed plans. As anticipated, many of the plans were personally meaningful and had been thought of previously, as indicated by low novelty ratings. Participants did not experience difficulty formulating their plans in the scanner. They felt that their plans were modestly challenging and achievable. On average, participants predicted that they would accomplish their goals in a little more than four months; however, there was substantial variability across goals, ranging from one day to 50 years. See Table 1 for means and standard deviations.
We observed significant associations among the ratings for autobiographical plans (See Table 1 for all means and standard deviations of correlations among ratings). Plans made with greater detail were associated with lower novelty (t = −6.74, p < .001), greater ease in formulating the plan in the scanner (t = 5.59, p < .001), and less difficulty to accomplish in the world (t = −3.85, p < .001). The less novel the plan, the easier it was to formulate in the scanner (t = −8.13, p < .001), and the less difficult it was perceived to accomplish in the world (t = 2.67, p < .01). Ease in formulating plans in the scanner was associated with decreased difficultly in accomplishing those plans in the world (t = −10.88, p < .001). Temporally distant goals were associated with lower confidence in completion (t = −10.50, p < .001). No other correlations reliably differed from zero across participants.
Brain
Whole brain results
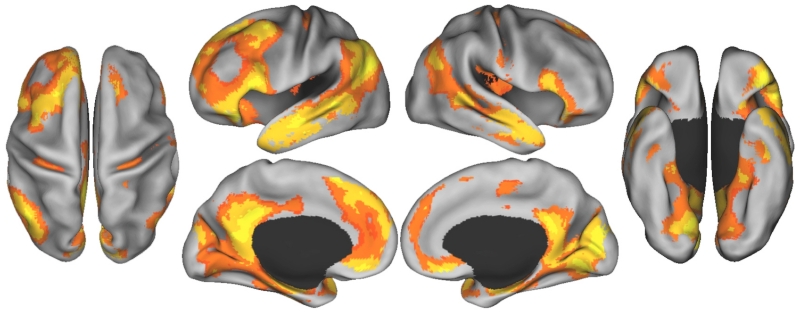
In examining the neural correlates of autobiographical planning, we first determined whole brain activity relative to a counting baseline task. The results of this whole brain contrast (Figure 1) were consistent with the previous reports that utilized a multivariate method, partial least squares, to contrast task conditions (Spreng et al., 2010; Spreng & Schacter, 2012; Spreng et al., 2013). Autobiographical planning, relative to counting, engaged a number of regions associated with cognitive control in the left hemisphere, including rostral and caudal aspects of middle frontal gyrus, the anterior extent of the IPL, dorsal anterior cingulate, and the anterior insula. Autobiographical planning also robustly engaged the default network bilaterally, including medial PFC, superior and inferior frontal gyri, posterior IPL, lateral temporal cortex, posterior cingulate cortex, retrosplenial cortex, and the medial temporal lobes, including the amygdala and hippocampus. Additional activity was also observed in medial occipital cortex, ventral temporal cortex, and the posterior insula.
Figure 1.
Brain regions associated with autobiographical planning relative to counting. Results images are FDR corrected, p < .05, k > 20, and displayed on an inflated surface map (population average landmark surface: PALS-B12) using CARET software (Van Essen, 2005).
Modulation by qualitative plan features
Next, the relationship between the ratings and brain activity during autobiographical planning was determined. Positive and negative modulation was examined for plan detail, novelty, temporal distance, ease of formulating the plan in the scanner, difficulty in accomplishing the goal, and confidence in completing the goal. The results of each hierarchical linear regression analysis are presented in turn.
Detail
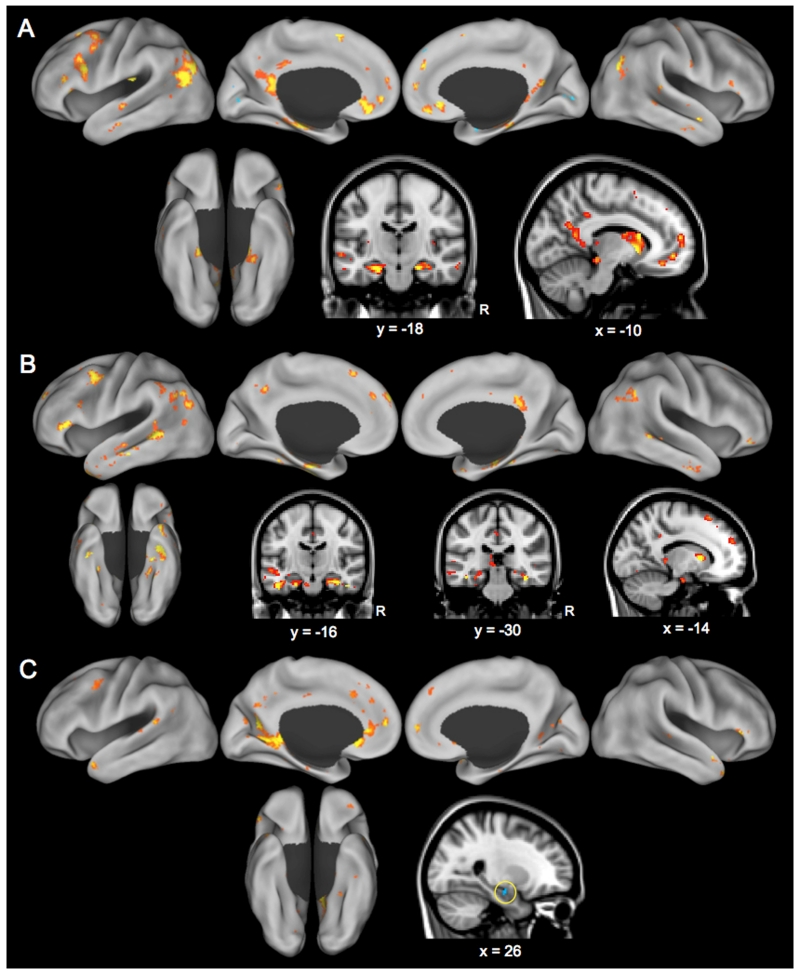
Modulation of activity in a number of brain structures was observed for autobiographical plan detail (see Figure 2A, Table 2). On the lateral surfaces, increasing detail was associated with greater activity in lateral temporal cortex, inferior parietal cortex, inferior frontal and middle frontal gyrus. On the medial surface, increasing detail was associated with greater activity in retrosplenial, posterior cingulate and medial prefrontal cortex. Highly detailed plans also increased activity in thalamus, caudate, and the hippocampus. Lower levels of plan detail were associated with increasing right temporal pole as well as bilateral cuneus and lingual gyrus activity.
Figure 2.
Modulated autobiographical planning activity I. (A) Detail. More detailed autobiographical plans were associated with default and frontoparietal brain structure activity in cortex and subcortically in hippocampus and caudate, depicted in warm colors. Cool colors depict low detailed plans. (B) Novelty. More novel autobiographical plans were associated with default and frontoparietal brain structure activity in cortex and subcortically in hippocampus and caudate. No regions were associated with low novelty. (C) Temporal distance. Modulation of activity for temporally proximal goals were associated with greater activity in warm colors. Distant goals were associated with right anterior hippocampal activity (circled) in cool colors. Results images are FDR corrected, p < .05, k > 20, and displayed on an inflated surface map (population average landmark surface: PALS-B12) using CARET software (Van Essen, 2005) or displayed in the volume image to depict subcortical structures.
Table 2. Detail.
| Positive modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| L | Middle frontal gyrus | −34 | 6 | 52 | 4.38 | 409 |
| L | Temporal parietal junction/angular gyrus | −46 | −68 | 24 | 4.18 | 1016 |
| L | Anterior medial prefrontal cortex | −16 | 52 | 8 | 4.10 | 382 |
| L | Ventromedial prefrontal cortex | −2 | 36 | −14 | 3.96 | 384 |
| L | Caudate | −2 | 6 | −2 | 3.93 | 290 |
| L | Thalamus | −2 | −6 | 8 | 3.82 | 82 |
| R | Temporal pole | 48 | −4 | −24 | 3.81 | 115 |
| R | Angular gyrus | 46 | −72 | 34 | 3.68 | 221 |
| L | Hippocampus | −22 | −16 | −20 | 3.62 | 534 |
| R | Retrosplenial cortex | 16 | −42 | 6 | 3.61 | 635 |
| R | Hippocampus | 20 | −16 | −20 | 3.53 | 236 |
| L | Inferior frontal gyrus | −42 | 10 | 22 | 3.48 | 355 |
| L | Medial superior prefrontal cortex | −6 | 8 | 60 | 3.46 | 78 |
| L | Supramarginal gyrus | −34 | −30 | 50 | 3.32 | 22 |
| L | Middle frontal gyrus | −22 | 40 | 22 | 3.30 | 126 |
| L | Posterior insula | −36 | −26 | 22 | 3.22 | 49 |
| L | Pons | −6 | −24 | −26 | 3.07 | 20 |
| L | Posterior cingulate cortex | −2 | −46 | 38 | 2.98 | 168 |
| R | Anterior medial prefrontal cortex | 12 | 56 | 8 | 2.96 | 52 |
| R | Medial prefrontal cortex | 10 | 48 | 30 | 2.94 | 57 |
| R | Posterior superior tempotal sulcus | 42 | −42 | 4 | 2.93 | 116 |
| L | Superior frontal gyrus | −18 | 20 | 54 | 2.79 | 213 |
| L | Inferior frontal gyrus | −48 | 28 | 4 | 2.76 | 131 |
| R | Caudate | 10 | 14 | 6 | 2.75 | 32 |
| L | Superior tempotal sulcus | −60 | −16 | −6 | 2.68 | 82 |
| R | Middle temporal gyrus | 64 | −40 | 0 | 2.60 | 26 |
| L | Middle temporal gyrus | −60 | −12 | −24 | 2.57 | 27 |
| L | Superior tempotal sulcus | −44 | −36 | 2 | 2.55 | 31 |
| L | Medial prefrontal cortex | −2 | 34 | 24 | 2.54 | 31 |
| R | Inferior frontal gyrus | 60 | 18 | 8 | 2.53 | 20 |
| R | Superior temporal gyrus | 66 | −38 | 12 | 2.52 | 31 |
| R | Precentral gyrus | 32 | −22 | 60 | 2.49 | 32 |
| R | Middle temporal gyrus | 62 | −18 | −18 | 2.44 | 27 |
| Negative modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Temporal pole | 36 | 16 | −22 | 3.18 | 27 |
| R | Cuneus | 20 | −76 | 20 | 2.90 | 56 |
| L | Cuneus | −14 | −78 | 16 | 2.86 | 36 |
| R | Lingual gyrus | 18 | −88 | 6 | 2.72 | 96 |
| L | Lingual gyrus | −12 | −76 | −2 | 2.32 | 36 |
Novelty
Many brain regions increased in activity as a function of higher novelty, or of the extent to which the plan had been given prior consideration before participating in the study (Figure 2B, Table 3). Greater novelty was associated with increasing bilateral activity in lateral and ventral temporal cortex, inferior parietal cortex, left lateral prefrontal cortex, and dorsomedial prefrontal cortex. Higher novelty was also associated with greater activity in precuneus, right posterior cingulate cortex, as well as bilateral medial temporal lobes and caudate. No regions of the brain showed more activity for decreasing levels of novelty.
Table 3. Novelty.
| Positive modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Parahippocampus | 38 | −28 | −20 | 4.42 | 369 |
| R | Hippocampus | 30 | −18 | −20 | 3.53 | - |
| R | Caudate | 14 | 6 | 12 | 4.20 | 39 |
| L | Inferior frontal gyrus | −54 | 30 | 4 | 4.13 | 154 |
| L | Ventral temporal lobe | −38 | −10 | −36 | 4.10 | 810 |
| L | Hippocampus | −18 | −10 | −26 | 3.09 | - |
| L | Middle frontal gyrus | −32 | 26 | 30 | 3.90 | 219 |
| L | Anterior superior frontal gyrus | −8 | 64 | 28 | 3.86 | 162 |
| L | Fusiform gyrus | −54 | −46 | −12 | 3.84 | 278 |
| L | Parahippocampus | −42 | −32 | −16 | 3.81 | 162 |
| L | Middle temporal gyrus | −60 | −44 | 2 | 3.73 | 231 |
| L | Caudate Body | −16 | 10 | 6 | 3.60 | 56 |
| R | Parahippocampal Gyrus | 16 | −40 | 6 | 3.34 | 20 |
| L | Inferior parietal lobule | −48 | −48 | 26 | 3.25 | 370 |
| L | Middle frontal gyrus | −36 | 2 | 40 | 3.21 | 221 |
| R | Middle temporal gyrus | 66 | −44 | 4 | 2.99 | 87 |
| L | Posterior cingualte cortex | 2 | −36 | 40 | 2.97 | 259 |
| L | Anterior superior frontal gyrus | −20 | 54 | 30 | 2.94 | 180 |
| L | Inferior frontal gyrus | −42 | 36 | −12 | 2.91 | 41 |
| R | Medial frontal gyrus | 12 | 50 | 34 | 2.80 | 25 |
| L | Superior frontal gyrus | −8 | 24 | 52 | 2.72 | 160 |
| R | Angular gyrus | 56 | −56 | 38 | 2.71 | 162 |
| R | Temporal pole | 30 | 14 | −30 | 2.67 | 24 |
| L | Temporal pole | −48 | −2 | −26 | 2.64 | 59 |
| L | Posterior hippocampus | −10 | −36 | 2 | 2.62 | 52 |
| R | Superior frontal gyrus | 20 | 24 | 48 | 2.55 | 70 |
| L | Posterior cingualte cortex | −12 | −42 | 34 | 2.51 | 20 |
| L | Precuneus | 0 | −68 | 46 | 2.35 | 23 |
| Negative modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| none | ||||||
Temporal distance
Goals that were situated nearer in time demonstrated an extended pattern of increasing activity in medial prefrontal cortex, retrosplenial cortex, temporal pole, lateral temporal cortex, angular gyrus, as well as left parahippocampus and left hippocampus. Proximal temporal distance also modulated aspects of posterior lateral prefrontal cortex and dorsal anterior cingulate, as well as the cuneus and intracalcarine cortex. Planning for goals that were temporally more distant, in contrast, engaged the right hippocampus. See Figure 2C, Table 4.
Table 4. Temporal Distance.
| Positive modulation (distal) | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Hippocampus | 26 | −10 | −14 | 2.33 | 15 |
| Negative modulation (proximal) | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| L | Ventromedial prefrontal cortex | −4 | 24 | −10 | 3.73 | 391 |
| L | Retrosplenial cortex | −12 | −44 | 2 | 3.71 | 818 |
| R | Temporal pole | 50 | 14 | −22 | 3.54 | 48 |
| R | Posterior superior temporal sulcus | 48 | −42 | 2 | 3.36 | 149 |
| R | Inferior frontal gyrus | 54 | 30 | −4 | 3.37 | 45 |
| L | Middle frontal gyrus | −36 | 4 | 42 | 3.36 | 265 |
| R | Superior frontal gyrus | 16 | 24 | 48 | 3.26 | 37 |
| R | Superior frontal gyrus | 16 | 38 | 38 | 2.99 | 56 |
| L | Inferior frontal gyrus | −60 | 14 | 26 | 2.99 | 24 |
| R | Anterior medial prefrontal cortex | 14 | 60 | 6 | 2.96 | 38 |
| L | Dorsomedial prefrontal cortex | −8 | 36 | 46 | 2.95 | 74 |
| R | Temporal pole | 42 | 20 | −34 | 2.85 | 24 |
| L | Parahippocampus | −28 | −36 | −12 | 2.82 | 35 |
| L | Dorsal anterior cingulate | −2 | 18 | 42 | 2.74 | 96 |
| L | Temporal pole | −50 | 12 | −28 | 2.72 | 25 |
| R | Cuneus | 14 | −70 | 22 | 2.72 | 302 |
| L | Superior frontal gyrus | −18 | 34 | 40 | 2.71 | 75 |
| L | Angular gryus | −50 | −54 | 28 | 2.69 | 35 |
| R | Cuneus | 16 | −90 | 16 | 2.68 | 21 |
| R | Middle temporal gyrus | 68 | −32 | 0 | 2.60 | 35 |
| R | Retrosplenial cortex | 18 | −48 | 10 | 2.51 | 72 |
| L | Superior temporal gyrus | −62 | −44 | 16 | 2.50 | 26 |
| L | Hippocampus | −24 | −18 | −22 | 2.45 | 23 |
| L | Medial prefrontal cortex | −10 | 38 | 30 | 2.41 | 32 |
| R | Intracalcarine cortex | 22 | −68 | 4 | 2.37 | 29 |
| L | Superior temporal gyrus | −62 | −28 | 8 | 2.30 | 21 |
| R | Medial prefrontal cortex | 4 | 46 | 36 | 2.20 | 31 |
| R | Medial prefrontal cortex | 4 | 54 | 14 | 2.18 | 23 |
Ease of formulating the plan in the scanner
The process of planning in the scanner revealed differential activity by perceived difficulty. Plans that were easier to form showed greater activity in the temporal poles bilaterally as well left-lateralized activity in inferior temporal gyrus, fusiform, and anterior lateral prefrontal cortex (BA 9). Plans that were more difficult to form in the scanner, in contrast, revealed more activity in right fusiform and cuneus, in addition to left anterior insula. See Table 5 and Supplemental Figure 1.
Table 5. Ease of formulating plan.
| Positive modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Temporal pole | 44 | −4 | −32 | 3.08 | 77 |
| L | Temporal pole | −54 | 0 | −24 | 2.75 | 20 |
| L | Fusiform gyrus | −28 | −32 | −24 | 2.74 | 21 |
| L | Inferior temporal gyrus | −52 | −14 | −28 | 2.68 | 54 |
| L | Anterior superior frontal gyrus | −8 | 64 | 30 | 2.64 | 22 |
| L | Temporal pole | −44 | 8 | −30 | 2.61 | 77 |
| Negative modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Occipital fusiform gyrus | 22 | −78 | −10 | 2.55 | 64 |
| L | Anterior insula | −38 | 22 | 4 | 2.24 | 29 |
| R | Cuneus | 16 | −66 | 14 | 2.21 | 30 |
Difficulty accomplishing the goal
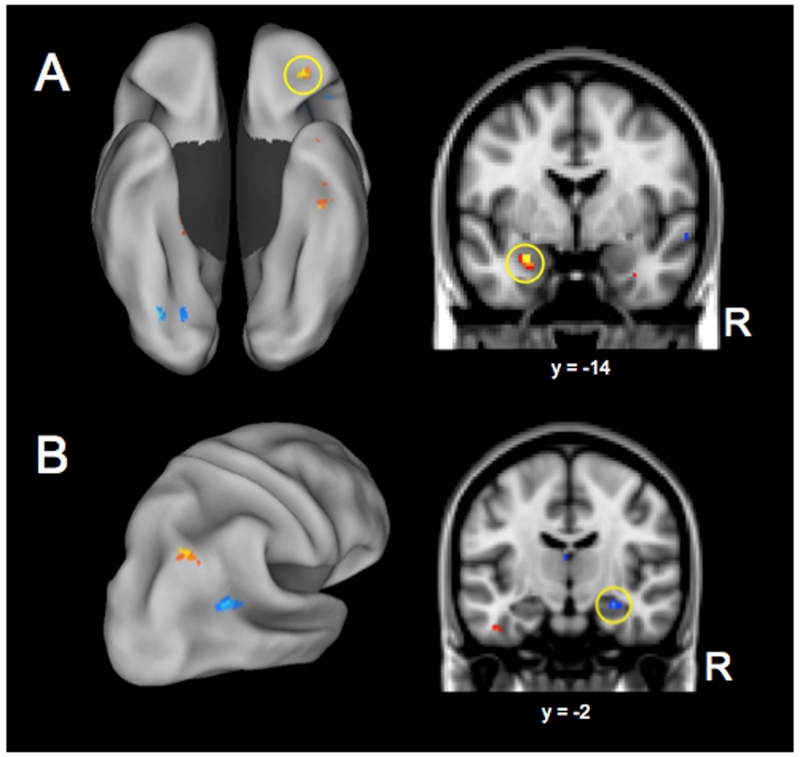
Plans that were foreseen to be more difficult to accomplish were associated with increasing activity in the left amygdala, orbitofrontal cortex, and ventral temporal cortex, as well as right parahippocampal cortex. Plans that were foreseen to be easier to accomplish were associated with increasing activity in precuneus, posterior superior temporal sulcus, left anterior insula, and occipital cortex. See Figure 3A, Table 6 and Supplemental Figure 1.
Figure 3.
Modulated autobiographical planning activity II. (A) Perceived difficulty in fulfilling the goal. High difficulty is depicted in warm colors, including the amygdala and orbitofrontal cortex (both circled); Low difficulty is depicted in cool colors. Left panel is ventral view of the brain’s surface (B) Confidence in fulfilling the goal. High confidence is depicted in warm colors; Low confidence in goal fulfillment is depicted in cool colors, including the hippocampus (circled). Left panel is a posterior view of the right hemisphere. Results images are FDR corrected, p < .05, k > 20, and displayed on an inflated surface map (population average landmark surface: PALS-B12) using CARET software (Van Essen, 2005) or displayed in the volume image to depict subcortical structures.
Table 6. Difficulty accomplishing goal.
| Positive modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| L | Amygdala | −28 | −2 | −20 | 3.09 | 25 |
| L | Orbitofrontal cortex | −32 | 36 | −12 | 2.98 | 30 |
| L | Ventral temporal cortex | −48 | −18 | −28 | 2.59 | 23 |
| R | Parahippocampus | 20 | −26 | −20 | 2.43 | 22 |
| Negative modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| L | Temporal parietal junction | −58 | −54 | 12 | 3.27 | 53 |
| R | Ventral occipital cortex | 32 | −70 | −16 | 3.00 | 117 |
| L | Anterior insula | −32 | 20 | −2 | 2.75 | 97 |
| L | Precuneus | −6 | −46 | 44 | 2.58 | 50 |
| R | Lingual gyrus | 16 | −68 | 6 | 2.53 | 25 |
| L | Cuneus | −10 | −84 | 32 | 2.52 | 29 |
| R | Precuneus | 4 | −44 | 38 | 2.50 | 21 |
| R | Middle temporal gyrus | 56 | −22 | −12 | 2.49 | 21 |
| R | Lateral parietal | 42 | −64 | 42 | 2.42 | 62 |
Confidence in completion
Plans for which participants had high confidence in eventually fulfilling resulted in greater activity in the inferior temporal gyrus, superior lateral occipital cortex, and posterior cingulate cortex. Plans for which participants had lower confidence in fulfilling led to greater activity in the right hippocampus, angular gyrus and retrosplenial cortex. Lower confidence plans were also associated with greater ventral occipital and temporal cortex activity, as well as activity in the inferior frontal gyri. See Figure 3B, Table 7 and Supplemental Figure 1.
Table 7. Confidence.
| Positive modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| L | Inferior temporal gyrus | −42 | −10 | −36 | 3.25 | 31 |
| R | Superior lateral occipital cortex | 44 | −64 | 48 | 2.60 | 23 |
| L | Posterior cingulate cortex | −4 | −40 | 24 | 2.33 | 25 |
| Negative modulation | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | k | |
| R | Occipital fusiform gyrus | 24 | −66 | −6 | 3.48 | 116 |
| L | Lingual gyrus | −16 | −54 | −10 | 3.26 | 49 |
| R | Angular gyrus | 54 | −52 | 20 | 2.85 | 147 |
| L | Inferior frontal gyrus | −38 | 44 | −18 | 2.78 | 24 |
| R | Inferior frontal gyrus | 48 | 26 | 6 | 2.76 | 26 |
| R | Hippocampus | 28 | −14 | −16 | 2.76 | 27 |
| R | Retrosplenial cortex | 16 | −40 | 0 | 2.70 | 40 |
| R | Temporal pole | 40 | 8 | −34 | 2.68 | 82 |
| L | Lingual gyrus | −14 | −74 | 0 | 2.55 | 26 |
| L | Cuneus | −10 | −88 | 26 | 2.54 | 90 |
| R | Inferior frontal gyrus | 34 | 18 | −22 | 2.38 | 20 |
| R | Cuneus | 22 | −70 | 14 | 2.38 | 39 |
| R | Lingual gyrus | 26 | −52 | 4 | 2.30 | 39 |
| L | Inferior frontal gyrus | −46 | 16 | −2 | 2.28 | 21 |
| R | Cuneus | 22 | −88 | 24 | 2.25 | 25 |
Discussion
In the last several years, substantial attention has been given to the cognitive and neural processes associated with episodic future simulation – the capacity to draw upon the constructive nature of memory to flexibly reconstitute past experiences into coherent simulations of a personal future event (Schacter & Addis, 2007). In contrast, researchers are just beginning to investigate the processes involved in guiding our actions towards realizing this personal future, what we have termed ‘autobiographical planning’. Constructing future simulations is closely associated with default network activity, as details of personal episodic events are recombined into an imagined future. We predicted that planning for that future would additionally engage frontoparietal control regions commonly associated with performance on standard laboratory-based measures of planning. Consistent with previous studies investigating the specific qualities of personal episodic simulation (e.g. Addis et al., 2008), we further hypothesized that contributions of specific brain regions in both default and frontoparietal control networks would be modulated by plan detail, novelty, ease of plan formulation, perceived difficulty, confidence in goal completion and temporal proximity.
Consistent with predictions and previous multivariate analyses (Spreng et al., 2010; Spreng & Schacter, 2012; Spreng et al., 2013), whole brain analyses revealed robust engagement of default and frontoparietal control network regions during autobiographical planning relative to a counting control task. Results of the hierarchical regression analyses provided further support for this default-executive model of autobiographical planning. Novel plans that were richly detailed, comparatively easy to construct in mind, judged to be readily and confidently achievable and were targeted toward attainment of a shorter term goal, robustly and concurrently engaged regions of both the default and executive control networks. In contrast, repeated plans or those that were sparsely detailed, difficult to formulate and accomplish, and were directed towards a more distant goal state engaged regions of the default network – but failed to reliably engage brain regions associated with executive control processing.
Detailed autobiographical planning for novel goals that were perceived to be easier and more readily achievable, was associated with a broad pattern of cortical activity encompassing both default and frontoparietal control regions bilaterally. This finding is consistent with evidence linking default network brain regions with episodic simulation (Schacter et al., 2012) and imagining personal, versus non-personal, future events (D’Argembeau et al., 2010). Similarly, engagement of frontoparietal control network regions, including dorsolateral prefrontal cortex as well as lateral parietal cortices is consistent with activation patterns observed in an independent sample of subjects engaged in autobiographical planning (Gerlach et al., 2014), during goal-directed future thinking (Stawarczyk & D’Argembeau, 2015), and during standardized, in-lab planning tasks (Owen et al 1997; van den Heuvel et al., 2003).
Co-activation of default and frontoparietal control brain regions during detailed autobiographical planning is consistent with a recent report demonstrating that prospective mind-wandering, commonly associated with default network activity, often involves future planning. Critically, the extent of this association between mind-wandering and planning was dependent upon individual differences in cognitive control capacity (Baird et al., 2011). Providing additional behavioral evidence for a link between future thinking and executive function, individual differences in working memory predicted future episodic specificity, even after controlling for autobiographical memory specificity (Hill & Emery, 2013). A recent meta-analytic review directly investigating patterns of brain activity during mind-wandering observed activation in both default network and frontoparietal control regions (Fox et al., 2015). Taken together, these findings are consistent with our observations that autobiographical planning requires both default network engagement to project into one’s personal future and frontoparietal control network involvement to construct a viable way forward. Further, these patterns of brain activity, linking personal relevance to planning, extend beyond the neocortex to sub-cortical brain structures. Detailed plans for novel versus rehearsed goals were associated with robust hippocampal activation, consistent with reports demonstrating that medial temporal lobe structures are actively engaged during detailed and novel episodic simulations (Addis & Schacter, 2008; Addis et al., 2011; Gaesser et al., 2013; Martin et al., 2011). Similarly, detailed planning in novel contexts was also associated with activity in the caudate nucleus, a region commonly associated with future planning for novel actions on standardized planning tasks (e.g. Jankowski et al., 2009; Monchi et al., 2006; Wunderlich, et al., 2012).
Planning for more temporally remote goals, which was considered by our participants to be more difficult and had lower probability of success, engaged default network brain regions but was not associated with activity in the frontoparietal control network. Planning for less immediate and more intangible personal goals engaged the right hippocampus, which has also been associated with episodic simulation of low-probability events (Weiler et al., 2010). Planning for personal goals that were considered more difficult to accomplish, were harder to plan in the scanner, or where confidence in completion was low, which were also associated with temporal distance, preferentially engaged posterior default network regions, visual cortices, as well as affective and reward processing regions, including the amygdala and orbitofrontal cortex. This observation suggests that autobiographical planning for a personally distant future may involve more affective and perceptually-based projections as opposed to the controlled construction of detailed plans to a more proximal and tangible goal state.
Based on these findings, we suggest that autobiographical planning requires access to detailed representations of one’s personal past, mediated by default network brain regions, as well as the control processes necessary to update, reconfigure, inhibit and flexibly recombine these representations to forge a mental pathway towards personal goal attainment. However, the analyses of qualitative plan features also hint at a more complex model of autobiographical planning – one in which there may be multiple paths to planning our personal futures. In our recently proposed taxonomy of future thinking (Szpunar et al., 2014), we argued that planning (as well as other modes of future thinking) varies along a gradient from specific, episodic planning that involves organizing steps needed to achieve a particular autobiographical future outcome, to abstract, semantic planning needed for some general state of the world to arise in the future. In a similar spirit, we suggest that specific autobiographical planning describes a detailed, achievable, and actionable planning process for attaining a clearly envisioned future, whereas abstract autobiographical planning refers to plans that may be constructed from more generalized semantic or affective representations of a less tangible and distant future. In this model, abstract autobiographical plans would be associated with default network structures as well as posterior and limbic brain regions linked to perceptually- or affectively-based holistic representations of a more generalized future self.
This distinction between specific and abstract autobiographical planning mirrors recent evidence that future simulations may vary by level of construal (Trope & Liberman, 2010). Construal theory predicts that prospection may consist of both richly detailed and more abstract representations of the future. Our data suggest that autobiographical planning may reflect a similar distinction. Specific autobiographical planning may require engagement and interaction among default and frontoparietal brain regions to both instantiate the goal-state and to shape the detailed means to its attainment. In contrast, abstract autobiographical planning engages default network regions to instantiate the desired, albeit more distal goal state; however, specific control processes may give way to more sensory and affective responses to a less detailed or determined future.
We observed the hippocampus to be involved in facets of both abstract and specific autobiographical planning. We observed predominantly right anterior hippocampus activity associated with more abstract features of autobiographical planning, including greater temporal distance (y = −10), low confidence in plan completion (y = −14) and high novelty (y = −18). In contrast, specific planning features were associated with left anterior hippocampal activity, including high detail plans (y = −14) and close temporal proximity (y = −18). However, laterality does not cleanly separate abstract and specific planning processes: detailed planning was also associated with the right anterior hippocampus (y = −18) and left posterior hippocampus (y = −36) was engaged for higher novelty plans. Parametric modulation of the hippocampus for both greater detail and temporal distance have been observed previously in research on future episodic event simulation effects (Addis & Schacter, 2008), likely reflecting the contribution of the hippocampus to both generating specific details for simulated experiences and recombining those details into a coherent event. Addis and Schacter (2008) proposed that detail recombination may be more difficult for distant, more abstract future events than for proximal, more concrete events, and hence require greater hippocampal contributions; this account fits with our observations concerning autobiographical planning. Overall, the modulation effects we observed here may reflect the multifaceted functions of the hippocampus, including the accessing of episodic detail, recombination of details, and/or encoding of plans (c.f. Addis & Schacter, 2012 for a review).
In this study we were able to demonstrate that autobiographical planning involves co-activation of default and frontoparietal brain regions, consistent with our hypothesis that envisioning a personal future and devising a means to its attainment is critically dependent upon cross-talk between these two brain networks. Further, our analysis of the qualitative features of autobiographical planning suggests that it is not a unitary process but may be differentiated, both in its phenomenology and neural instantiation, depending upon on the psychological distance that must be bridged between the present and an imagined future.
Supplementary Material
Acknowledgements
We thank Adrian Gilmore and Clifford Robbins for assistance with stimulus preparation and data collection, Tom Miller and David Orama for help with data processing, and the Harvard Center for Brain Science Neuroimaging Core and the Harvard Neuroinformatics Research Group for imaging support. This work was supported by NIMH grant MH060941 to D.L.S.
References
- Addis DR, Cheng T, Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011;21:1045–1052. doi: 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Effects of detail and temporal distance of past and future events on the engagement of a common neural network. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Schooler JW. Back to the future: autobiographical planning and the functionality of mind-wandering. Consciousness & Cognition. 2011;20:1604–1611. doi: 10.1016/j.concog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, Maquet P, Salmon E. The neural basis of personal goal processing when envisioning future events. Journal of Cognitive Neuroscience. 2010;22:1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Renaud R, Van der Linden M. Frequency, characteristics, and functions of future-oriented thoughts in daily life. Applied Cognitive Psychology. 2011;25:96–103. [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL. Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus. 2013;23:1150–1161. doi: 10.1002/hipo.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience. 2014;9:1942–1951. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson T. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Hill PF, Emery L. Episodic future thought: Contributions from working memory. Consciousness & Cognition. 2013;22:677–683. doi: 10.1016/j.concog.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Hüppe C, Boecker H. Distinct striatal regions for planning and executing novel and automated movement sequences. NeuroImage. 2009;44:1369–1379. doi: 10.1016/j.neuroimage.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding future simulations. Proceedings of the National Academy of Sciences USA. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [11C] raclopride PET study. NeuroImage. 2006;33:907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive Affective Behavioral Neuroscience. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM. Cognitive planning in humans: neuropsychological, neuroanatomical and neuropharmacological perspectives. Progress in Neurobiology. 1997;53:431–450. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JD, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in Man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory examined with positron emission tomography (PET) European Journal of Neuroscience. 1996;8:353–364. doi: 10.1111/j.1460-9568.1996.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society (B) 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and application. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Railton P, Baumeister RF, Sripada C. Navigating into the future or driven by the past. Perspectives on Psychological Science. 2013;8:119–141. doi: 10.1177/1745691612474317. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Memory & Cognition. 2006;34:1644–1651. doi: 10.3758/bf03195927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cerebral Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain J, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;31:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, D’Argembeau A. Neural correlates of personal goal processing during episodic future thinking and mind-wandering: an ALE meta-analysis. Human Brain Mapping. 2015 doi: 10.1002/hbm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Spreng RN, Schacter DL. A taxonomy of prospection: Introducing an organizational framework of future-oriented cognition. Proceedings of the National Academy of Sciences USA. 2014;111:18414–18421. doi: 10.1073/pnas.1417144111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. The default network distinguishes construals of proximal versus distal events. Journal of Cognitive Neuroscience. 2011;23:2945–2955. doi: 10.1162/jocn_a_00009. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Construal Level Theory of Psychological Distance. Psychological Review. 2010;117:440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Huevel OA, Groenewegen HJ, Barkhof F, Lazeron RHC, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of the Tower of London task. NeuroImage. 2003;8:367–374. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlosser RGM. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: Occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Dayan P, Dolan RJ. Mapping value based planning and extensively trained choices in the human brain. Nature Neuroscience. 2012;15:786–791. doi: 10.1038/nn.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.