Abstract
Purpose
Prostate specific antigen velocity is an unreliable predictor of adverse pathology findings in patients on active surveillance for low risk prostate cancer. However, to our knowledge a new concept called prostate specific antigen velocity risk count, recently validated in a screening cohort, has not been investigated in an active surveillance cohort.
Materials and Methods
We evaluated a cohort of men from 1995 to 2012 with prostate cancer on active surveillance. They had stage T1c disease, prostate specific antigen density less than 0.15 ng/ml, Gleason score 6 or less, 2 or fewer biopsy cores and 50% or less involvement of any core with cancer. The men were observed by semiannual prostate specific antigen measurements, digital rectal examinations and an annual surveillance biopsy. Treatment was recommended for biopsy reclassification. Patients with 30 months or greater of followup and 3 serial prostate specific antigen velocity measurements were used in primary analysis by logistic regression, Cox proportional hazards, Kaplan-Meier analysis and performance parameters, including the AUC of the ROC curve.
Results
Primary analysis included 275 of 668 men who met very low risk inclusion criteria, of whom 83(30.2%) were reclassified at a median of 57.1 months. Reclassification risk increased with risk count, that is a risk count of 3 (HR 4.63, 95% CI 1.54–13.87) and 2 (HR 3.73, 95% CI 1.75–7.97) compared to zero. Results were similar for Gleason score reclassification (HR 7.45, 95% CI 1.60–34.71 and 3.96, 95% CI 1.35–11.62, respectively). On secondary analysis the negative predictive value (risk count 1 or less) was 91.5% for reclassification in the next year. Adding the prostate specific antigen velocity risk count improved the AUC in a model including baseline prostate specific antigen density (0.7423 vs 0.6818, p = 0.025) and it outperformed the addition of overall prostate specific antigen velocity (0.7423 vs 0.6960, p = 0.037).
Conclusions
Prostate specific antigen velocity risk count may be useful for monitoring patients on active surveillance and decreasing the frequency of biopsies needed in the long term.
Keywords: prostate, prostatic neoplasms, prostate-specific antigen, biopsy, risk
Although an estimated 241,740 prostate cancer cases were diagnosed in 2012, about 50% were low grade (Gleason score 6 or less), of which greater than 90% were localized.1,2 PSA screening is a major contributor to increased detection of early stage prostate cancer.3 AS is an alternative to immediate intervention for low risk prostate cancer to decrease overtreatment with minimal or no compromise to oncological outcomes but it remains underused, lacking consistent criteria and triggers for intervention.4
PSA kinetics during AS have served as triggers for intervention in several programs and they are an attractive alternative to repeat biopsies.5, 6 However, recent evidence suggests that overall PSAV and PSA doubling time do not reliably predict adverse pathology findings.7–9 Variability in PSA and followup duration may limit the value of a single overall PSAV calculation for patients on AS.
In 2007 a new concept called PSAV RC was proposed in which serial PSAVs are calculated and the number of times that they pass a threshold are counted to tabulate a score. 10,11 PSAV RC was recently validated in a screening cohort, demonstrating an eightfold increased risk of prostate cancer and a greater than fivefold increased risk of Gleason score 8 or greater disease for a RC of 2 vs 1 or zero.12 To our knowledge the usefulness of PSAV RC in patients on AS who already carry a diagnosis of prostate cancer has not been evaluated.
Disease misclassification is a significant concern when determining eligibility for AS. Biopsy criteria and the number of cores are directly related to the sampling error rate.13, 14 Under grading may occur in up to a third of patients.15 The initial diagnostic biopsy may not capture the correct grade or extent of disease, raising the question of whether higher grade disease on subsequent biopsy or prostatectomy is due to true disease progression or misclassification.16 At our institution annual prostate biopsy results are used to determine disease reclassification and recommend curative therapy for very low risk prostate cancer.
We investigated an AS cohort beyond the initial misclassification period to determine whether PSAV RC is associated with biopsy reclassification of disease and whether it might represent a clinically useful measure for monitoring patients. We also evaluated the prognostic usefulness of the temporally earliest PSA information and compared model performance with that in addition to PSAV RC.
Materials and Methods
Study Cohort
Since January 1995, older men with very low risk prostate cancer who present to our institution have been advised that AS is an alternative to immediate intervention.17 As previously described by Epstein et al, inclusion criteria for very low risk prostate cancer include clinical stage T1c disease, PSAD less than 0.15 ng/ml and favorable characteristics on needle biopsy (Gleason score 6 or less, 2 or fewer biopsy cores with cancer and 50% or less involvement of any core with cancer).18 With institutional review board approval and appropriate informed consent from all participants we observed men by semiannual serum PSA measurements and digital rectal examinations as well as annual extended 12-core or greater surveillance biopsy. Curative therapy was recommended upon disease reclassification, defined as surveillance biopsy with unfavorable pathology findings.
The primary analysis included patients on AS for at least 30 months without biopsy reclassification in whom 3 serial PSAVs were calculated using linear regression (fig. 1).19,20 We selected 30 months to allow for time for 2 surveillance biopsies in all patients to minimize biopsy misclassification and enable sufficient followup for 3 serial PSAV calculations. Secondary analysis was done in patients with less than 30 months of followup, which allowed for only 1 or 2 serial PSAVs to be calculated. Finally, we analyzed early PSA data while on AS, looking at the first 2, 12-month and 24-month windows after diagnosis in patients with at least 24 and 48 months of followup, respectively.
Figure 1.
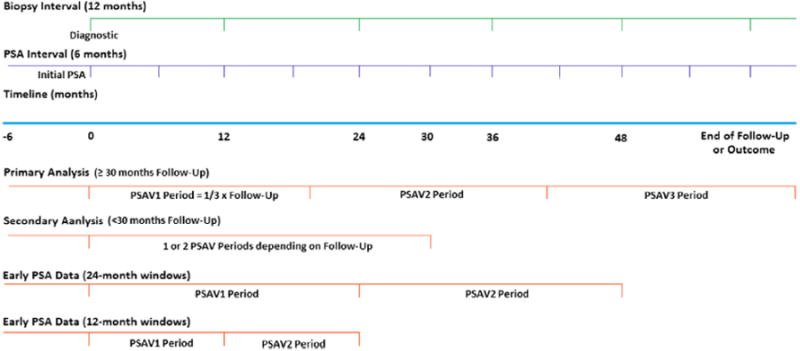
Study design shows timing of biopsies, PSA testing and periods for successive PSAV calculations 1 to 3 (PSAV1, PSAV2 and PSAV3, respectively) in Johns Hopkins AS Program from 1995 to 2012.
Variables and Outcomes
Clinical characteristics recorded in all patients at diagnosis and during followup included age, race, PSA, PSAD, whether cancer was present on surveillance biopsy, number of biopsy cores with cancer, maximum percent core involvement with cancer and Gleason score. Men on 5α-reductase inhibitors were excluded from analysis. Two outcomes were defined. The first criterion was biopsy reclassification of disease by any of the 3 criteria mentioned. The second criterion was reclassification based on Gleason score only (greater than 6) since a prior group suggested that Gleason score on biopsy may be a better predictor of prostate cancer specific mortality than disease volume.21
Statistical Analysis
For the primary analysis we divided followup into 3 approximately equal time periods for each individual. A minimum of 6 months was required between the first and last measurements in each period. We tabulated the PSAV RC as the number of times that PSAV exceeded a threshold of 0.4 ng/ml per year based on prior research.11,12
The Student t-test (equal variances), Welch t-test (unequal variances) and chi-square test (categorical variables) were used to compare men who met study inclusion criteria and had 3 vs fewer than 3 calculated serial PSAVs. NPV and PPV were determined. Univariate and multivariable logistic regression, and Cox proportional hazards regression were used to assess the association of PSAV RC with biopsy reclassification. The proportional hazards assumption was globally tested with Schoenfeld residuals, resulting in nonsignificant deviations in the Cox models. The Kaplan-Meier method was used to estimate the incidence of biopsy reclassification by RC. Improvement in model performance was assessed by the AUC of ROC curve analysis and the Harrell c rank parameter. The AUC was directly compared between models by the z-test. Statistical analysis was done using STATA®, version 12.0.
Supplemental Methods
The AS program is primarily recommended to older men but younger men and those who do not meet some criteria may elect to enroll due to other competing health concerns or personal preference if cancer has a Gleason score of 6 or less. However, only men who met all very low risk criteria were included in the current study. Disease reclassification on surveillance biopsy due to no longer meeting favorable characteristics on needle biopsy specifically refers to a Gleason score of greater than 6, more than 2 biopsy cores with cancer or greater than 50% involvement of any core with cancer. The initial PSA value was considered PSA at prostate cancer diagnosis or within 6 months before diagnostic biopsy. The first PSAV was calculated using this initial PSA measurement and all measurements up to the end of the first period (first third of followup). The second PSAV was calculated using the last PSA measurement from the first period and all measurements up to the end of the second period. The third PSAV was calculated similarly.
Model improvement was done to assess the addition of PSAV RC to a base model controlling for baseline and first surveillance biopsy characteristics, including age, race, PSAD at diagnosis and cancer on the first surveillance biopsy. The addition of PSAV RC was compared to the addition of overall PSAV. Adjustment for overall PSAV during followup was tested as a continuous and a categorical variable, dichotomized with a cutoff of 0.4 ng/ml per year. We used the AUC from ROC analysis to assess discrimination and the Hosmer-Lemeshow goodness of fit test to assess logistic regression model calibration. The Harrell c rank parameter was applied to evaluate discrimination in the Cox regression models.
Results
As of September 2012, 870 men had enrolled in the AS program, of whom 668 met all very low risk inclusion criteria. Of men excluded from analysis 166 had PSAD greater than 0.15 ng/ml, 27 had an unknown volume at diagnosis and 9 did not have T1c or less disease while 85 who met inclusion criteria had insufficient followup or PSA measurements spaced appropriately to calculate any PSAV. Table 1 compares the 275 men with 3 serial PSAV calculations on primary analysis to the 308 with only 1 or 2 calculations. The latter were older, had higher PSA and PSAD, and were more likely to have cancer on first surveillance biopsy. A total of 83 men (30.2%) with 3 serial PSAVs experienced biopsy reclassification by any criteria at a median followup of 57.1 months vs 156 (50.7%) with fewer than 3 serial PSAVs at a mean followup of 17.1 months (p <0.01). Successive PSAVs generally increased but there was substantial variability (fig. 2).
Table 1. Demographics, biopsy reclassification and followup in 668 of 870 men on AS in Johns Hopkins AS Program from 1995 to 2012.
| All Inclusion Criteria | 3 PSAVs | 1 or 2 PSAVs | p Value (t-test or chi-square test) | |
|---|---|---|---|---|
| No. pts | 668 | 275 | 308 | |
| Mean ± SD age | 65.2 ± 5.7 | 64.2 ± 5.4 | 66.3 ± 5.6 | <0.01 |
| No. ethnicity (%): | ||||
| White | 598 (89.5) | 252 (91.6) | 270 (87.7) | 0.294 |
| Black | 41 (6.1) | 14 (5.1) | 23 (7.5) | |
| Other | 29 (4.3) | 9 (3.3) | 15 (4.9) | |
| Mean ± SD diagnosis (ng/ml): | ||||
| PSA | 4.5 ± 2.3 | 4.1 ± 2.2 | 4.7 ± 2.2 | <0.01 |
| PSAD | 0.089 ± 0.032 | 0.085 ± 0.033 | 0.09 ± 0.031 | 0.038 |
| At 1st AS biopsy: | ||||
| Mean ± SD PSA (ng/ml) | 4.7 ± 2.9 | 4.0 ± 2.4 | 5.2 ± 3.0 | <0.01 |
| Mean ± SD PSAD (ng/ml) | 0.095 ± 0.073 | 0.083 ± 0.055 | 0.102 ± 0.084 | <0.01 |
| No. Ca (%) | 462 (55.5) | 108 (39.3) | 207 (67.7) | <0.01 |
| Overall biopsies: | ||||
| No. pts (%) | 3.8 (1.9) | 5.5 (1.8) | 2.5 (0.6) | <0.01 |
| No. cores (%) | 13.2 (1.5) | 13.1 (1.2) | 13.3 (1.9) | 0.139 |
| Mean ± SD interval (mos) | 12.9 ± 3.6 | 13.9 ± 3.5 | 12.1 ± 3.1 | <0.01 |
| Mean ± SD No. followup PSAs | 6.3 ± 4.2 | 10 ± 3.8 | 4.1 ± 1.5 | <0.01 |
| No. reclassified (%): | ||||
| No. any criteria (%):* | 257 (38.5) | 83 (30.2) | 156 (50.7) | <0.01 |
| Gleason score | 107 (41.6) | 37 (44.6) | 66 (42.3) | |
| Greater than 2 cores | 169 (65.8) | 52 (62.7) | 104 (66.7) | |
| Greater than 50% any core | 71 (27.6) | 24 (28.9) | 44 (28.2) | |
| No. Gleason score (%): | 120 (18.0) | 42 (15.3) | 72 (23.4) | 0.014 |
| 3 + 4 =7 | 78 (65.0) | 32 (76.2) | 42 (58.3) | |
| 4 + 3 =7 | 27 (22.5) | 5 (11.9) | 20 (27.8) | |
| 8 or Greater | 15 (12.5) | 5 (11.9) | 10 (13.9) | |
| Mean ± SD followup (mos): | – | |||
| Any criteria | 35.9 ± 29.0 | 62.8 ± 25.3 | 17.2 ± 6.3 | |
| Gleason score | 38.2 ± 30.4 | 65.4 ± 27.2 | 19 ± 9.6 |
Gleason score 7 or greater, more than 2 cores with cancer or greater than 50% cancer involvement in any core.
Figure 2.
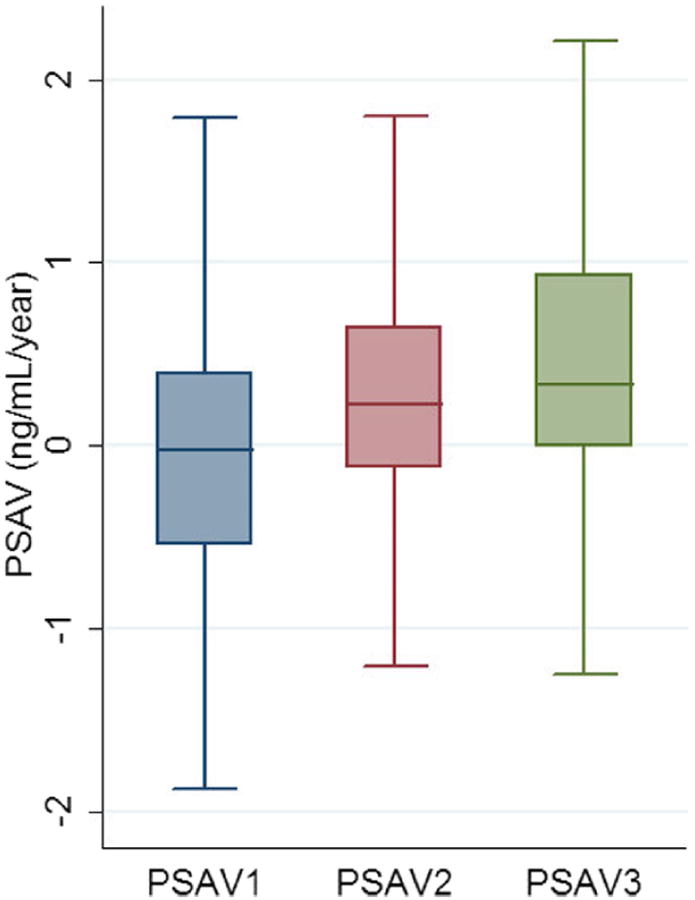
PSAV in men with 3 serial PSAV measurements (PSAV1, PSAV2 and PSAV3, respectively) in Johns Hopkins AS Program from 1995 to 2012.
On primary analysis patients with a higher RC experienced a greater proportion of biopsy reclassification events by any criteria and by Gleason score (p <0.01 and 0.017, respectively, table 2). Using the Kaplan-Meier method the estimated 5-year probability of biopsy reclassification by any criteria was 9.7% (95% CI 4.4–20.3), 18.7% (95% CI 12.1–28.2) and 39.5% (95% CI 28.2–53.2) for a RC of zero, 1 and 2 or greater, respectively (log rank test p <0.01, fig. 3). At a RC cutoff of 2 or greater PPV was 50.0% and NPV was 78.2%. Final adjusted logistic regression and Cox proportional hazards models yielded similar results with ORs generally overestimating HRs (supplementary table, http://jurology.com/). After adjustment RCs of 3 (HR 4.63, 95% CI 1.54–13.87) and 2 (HR 3.73, 95% CI 1.75–7.97) were associated with a significantly increased risk of biopsy reclassification by any criteria compared to a RC of zero. Parallel results were obtained for the association of RCs of 3 (HR 7.45, 95% CI 1.60–34.71) and 2 (HR 3.96,95% CI 1.35–11.62) with the specific outcome of Gleason score reclassification.
Table 2. Biopsy reclassification associations with RC in men with 3 PSAV calculations in Johns Hopkins AS Program from 1995 to 2012.
| RC | No. Pts | No. Reclassified by Any Criteria (%)* | No. Reclassified to Gleason Score 7 or Greater (%) |
|---|---|---|---|
| 0 | 78 | 13 (16.7)† | 7 (9.0)‡ |
| 1 | 115 | 29 (25.2) | 15 (13.0) |
| 2+ | 82 | 41 (50.0) | 20 (24.4) |
Gleason score 7 or greater, more than 2 cores with cancer or greater than 50%cancer involvement in any core.
Chi-square p <0.01.
Chi-square p = 0.017.
Figure 3.
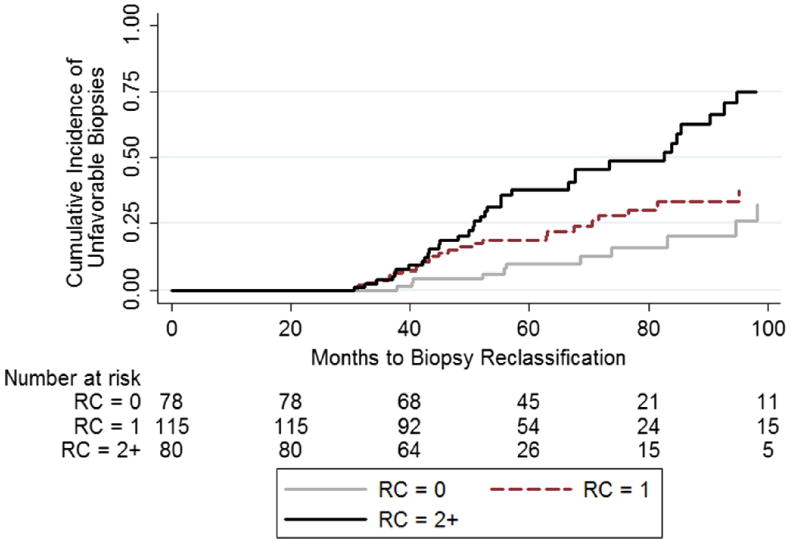
Cumulative incidence of unfavorable biopsies by RC during followup in men with 3 serial PSAV calculations in Johns Hopkins AS Program from 1995 to 2012.
Logistic regression models were also adjusted for period duration. Additional analysis revealed no association of overall followup, period duration or number of PSA measurements with reclassification in patients on the primary analysis. Of 1,237 surveillance biopsies in the cohort an estimated 518 (42%) could have been avoided based on patients with a RC of 1 or less. Of 35 men (12.7%) treated with prostatectomy 24 (68.6%) underwent it due to reclassification and 1 (2.9%) experienced biochemical recurrence (PSA greater than 0.2 ng/ml). This patient had a RC of 2 and Gleason 4 + 3 = 7 on surgical pathology findings. Of men with pathology data available 5 of 17 (29.4%) with a RC of 1 or less had Gleason 7 or greater disease compared to 11 of 16 (68.8%) with a RC of greater than 1.
Secondary analysis of patients with less than 30 months of followup showed some association of RC with biopsy reclassification but it was not statistically significant in men with 2 serial PSAV calculations (supplementary table, http://jurology.com/). Patients with 1 PSAV calculation were at increased risk for biopsy reclassification by any criteria and by Gleason score. Analysis of early PSA data calculating 2 serial PSAVs in the 12 and24-month windows did not show a statistically significant association of RC with biopsy reclassification during the full followup (table 3). However, RC in 2, 24-month windows predicted reclassification in the next year by any criteria (HR 4.99, 95% CI 1.08–23.00) and it suggested an association with reclassification by Gleason score (HR 7.80, 95% CI 0.54–113.25). In this group a RC cutoff of 2 resulted in a PPV of 23.5% and a NPV of 91.5%. Figure 4 shows the ROC analysis of adding total PSAV RC vs adding overall PSAV to the base model. Adding overall PSAV resulted in minimal and nonsignificant AUC improvement over the base model (p = 0.365). Adding total RC resulted in more substantial AUC improvement, which was significant when compared directly to the base model and the PSAV model for biopsy reclassification by any criteria (p = 0.025 and 0.037, respectively). Total RC also led to higher Harrell c rank parameters compared to the base model, PSAV model and models including individual RCs from each period.
Table 3. Cox proportional hazards regression and logistic regression analysis of association of RC calculated from 2 serial PSAVs in 12 and 24-month windows from beginning of AS with biopsy reclassification during full patient followup or immediate next year in Johns Hopkins AS Program from 1995 to 2012.
| Full Followup | Next Yr | |||
|---|---|---|---|---|
|
|
|
|||
| RC vs 0 | HR (95% CI)* | p Value | HR (95% CI)* | p Value |
| 12-Mo window (318 pts) | ||||
| Any criteria: | ||||
| 1 | 1.32 (0.82–2.15) | 0.256 | 0.99 (0.42–2.34) | 0.982 |
| 2 | 1.27 (0.65–2.48) | 0.483 | 2.27 (0.85–6.06) | 0.101 |
| Gleason score reclassification: | ||||
| 1 | 1.60 (0.77–3.32) | 0.209 | 0.48 (0.12–2.00) | 0.313 |
| 2 | 2.29 (0.85–6.14) | 0.101 | 2.09 (0.49–8.87) | 0.32 |
| 24-Mo window (168 pts) | ||||
| Any criteria: | ||||
| 1 | 1.10 (0.55–2.18) | 0.792 | 1.30 (0.36–4.74) | 0.689 |
| 2 | 1.90 (0.61–5.90) | 0.266 | 4.99 (1.08–23.00) | 0.039 |
| Gleason score reclassification: | ||||
| 1 | 0.42 (0.14–1.28) | 0.127 | 1.03 (0.13–8.04) | 0.975 |
| 2 | 1.22 (0.21–7.16) | 0.822 | 7.80 (0.54–113.25) | 0.132 |
Adjusted for age at diagnosis, race, PSAD at diagnosis, overall PSAV during followup and cancer on first surveillance biopsy.
Figure 4.
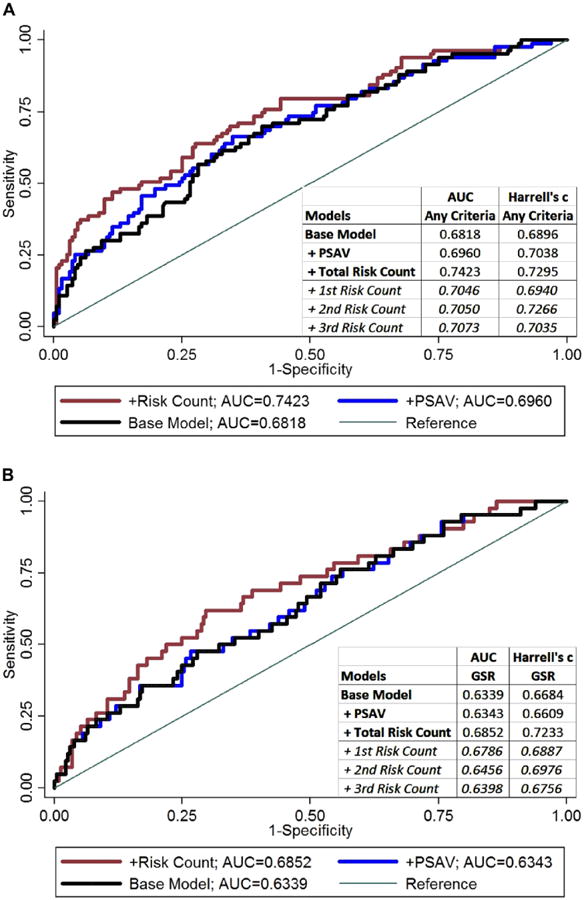
ROC analysis of base logistic regression model vs models with addition of overall PSAV or PSAV RC for outcomes of biopsy reclassification by any criteria (A) and specifically Gleason score reclassification (GSR) (B) in Johns Hopkins AS Program from 1995 to 2012.
Discussion
In our cohort of men with very low risk prostate cancer PSAV RC was associated with an increased risk of biopsy reclassification due to any unfavorable pathology finding as well as the specific criterion of Gleason score reclassification. The association was less robust in patients with less than 30 months of followup, in whom few serial PSAVs could be calculated and the potential for biopsy misclassification was greatest. Furthermore, using only the earliest PSA data to calculate RCs was unreliable to predict biopsy reclassification during the full patient followup. However, it revealed an association with reclassification in the immediate next year, indicating the importance of continuing to tabulate the RC with time. PSAV RC significantly improved a base model incorporating baseline PSAD and it was better than a model that also accounted for overall PSAV. These data suggest that PSAV RC has the potential to serve as a clinically useful measure to monitor patients on AS after they pass an initial period of a high reclassification rate when biopsy appears critical.
The interest in monitoring patients on AS is especially relevant in the context of recent updates to large, randomized, controlled trials of PSA screening.22,23 Adding organized screening had no benefit in a population in which widespread opportunistic screening had already occurred while screening in Europe required treatment of 37 cancers to prevent 1 death from cancer at a median followup of 11 years. Because almost half of all men diagnosed with prostate cancer may be eligible, multiple organizations, including the American Urological Association, recommend that all discussions of treatment options include AS.2,24 Maximizing the effectiveness and minimizing the invasiveness of monitoring are priorities.
Controversy surrounds the use of PSA kinetics for prostate cancer screening as well as for monitoring patients on AS.7–9,25–28 PSAV RC was recently validated in a screening cohort but it is a different question whether it may be useful in an AS cohort, in which patients have already been diagnosed with prostate cancer.11,12 As PSA increases with time, the rate of that increase also appears to increase with time and a unique transition point was described where the PSA curve transitions from a linear to an exponential increase.20 However, the mathematics involved limit the clinical application of this method of calculating PSAV.
In a setting where urologists are familiar with calculating overall PSAV one can think of PSAV RC as a clinically feasible approximation and an advance from the idea of a transition point. It considers all PSA measurements during followup in windows of time to capture the extra dimension of acceleration. RC in 2, 24-month windows had a 91.5% NPV for reclassification within the next year that applied to 89% of patients. It suggests that increasing the interval between biopsies could be considered in patients with low total RCs. PPV was low at 23.5% for reclassification within the next year. However, this was still a clinically relevant value since it showed that only 11% of patients might need to continue the annual biopsy schedule with the expectation that almost a quarter of this subset would be reclassified at the next surveillance biopsy.
Several factors are important for risk stratifying patients on AS.29,30 At diagnosis or first surveillance biopsy PSAD is a pertinent factor as well as part of the criteria of Epstein et al.18 We controlled for baseline PSAD rather than PSA at diagnosis or first surveillance biopsy because these measures were highly correlated and including PSAD led to a more conservative association. Cancer at first surveillance biopsy is also an independent predictor of biopsy reclassification, likely due to the mentioned concern for misclassification.29 Our results show that the model was significantly improved by adding RC even after adjusting for baseline PSAD, cancer at first biopsy and overall PSAV during followup. Therefore, as the data support, PSAV RC may be most clinically relevant in patients followed on AS for 30 months or greater. However, the ideal interval for calculating RC in prospective fashion requires further evaluation.
The current study has several important limitations. 1) Because the cohort represents a carefully selected, very low risk population of men with prostate cancer, the study may not be generalizable to cohorts with higher risk characteristics. 2) Only PSA measurements during AS were used and associations with PSA measurements before enrollment were not evaluated. 3) While our sample size was relatively large compared to other cohorts, secondary analysis in patients with only 2 serial PSAV calculations and less than 30 months of followup was underpowered (102 patients), requiring a large detectable difference to achieve significance. 4) We addressed variations in period duration for RC calculation using restriction, survival analysis, adjusted logistic regression and additional analysis of associations of period duration or number of PSA measurements with reclassification. However, the inherent requirement of calculating serial PSAVs for different period durations in each individual may still have introduced some bias. Also, comparing cross-institutional results might be difficult without a standard interval for measuring PSA. Notwithstanding the limitations our analysis reveals a strong association of PSAV RC with biopsy reclassification of disease in patients with very low risk prostate cancer on AS.
Conclusions
PSAV RC was associated with disease reclassification by unfavorable biopsy characteristics in men on AS with very low risk prostate cancer. A RC of 2 or 3 compared to zero was independently associated with a fourfold increased risk of any unfavorable pathology results on biopsy and a fourfold to sevenfold risk of Gleason score 7 or greater disease in men with 3 serial PSAV measurements. RC also improved model discrimination. RC was less reliable during the initial 2 to 3 years on AS, given the potential for misclassification and limited PSA data, underscoring the importance of annual surveillance biopsies during this period. Validation of this association in other cohorts may support a role for PSAV RC for monitoring patients on AS and decrease the frequency of biopsies in the long term.
Supplementary Material
Acknowledgments
Supported by the Predoctoral Clinical Research Training Program and Johns Hopkins Institute for Clinical and Translational Research, and the Prostate Cancer Foundation (Johns Hopkins Active Surveillance Program).
Abbreviations and Acronyms
- AS
active surveillance
- NPV
negative predictive value
- PPV
positive predictive value
- PSA
prostate specific antigen
- PSAD
PSA density
- PSAV
PSA velocity
- RC
risk count
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Djenaba JA, Soman A, et al. Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001-2007. Prostate Cancer. 2012;691380 doi: 10.1155/2012/691380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 4.Glass AS, Cooperberg MR, Meng MV, et al. Role of active surveillance in the management of localized prostate cancer. J Natl Cancer Inst Monogr. 2012;2012:202. doi: 10.1093/jncimonographs/lgs032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz L. Active surveillance: the Canadian experience. Curr Opin Urol. 2012;22:222. doi: 10.1097/MOU.0b013e328352598c. [DOI] [PubMed] [Google Scholar]
- 6.Soloway MS, Soloway CT, Williams S, et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101:165. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 7.Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810. doi: 10.1200/JCO.2009.25.7311. [DOI] [PubMed] [Google Scholar]
- 8.Whitson JM, Porten SP, Hilton JF, et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185:1656. doi: 10.1016/j.juro.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Iremashvili V, Manoharan M, Lokeshwar SD, et al. Comprehensive analysis of post-diagnostic prostate-specific antigen kinetics as predictor of a prostate cancer progression in active surveillance patients. BJU Int. 2013;111:396. doi: 10.1111/j.1464-410X.2012.11295.x. [DOI] [PubMed] [Google Scholar]
- 10.Carter HB, Morrell CH, Pearson JD, et al. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 1992;52:3323. [PubMed] [Google Scholar]
- 11.Carter HB, Kettermann A, Ferrucci L, et al. Prostate-specific antigen velocity risk count assessment: a new concept for detection of life-threatening prostate cancer during window of curability. Urology. 2007;70:685. doi: 10.1016/j.urology.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb S, Metter EJ, Kan D, et al. Prostate-specific antigen velocity (PSAV) risk count improves the specificity of screening for clinically significant prostate cancer. BJU Int. 2012;109:508. doi: 10.1111/j.1464-410X.2011.10900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ploussard G, Xylinas E, Salomon L, et al. The role of biopsy core number in selecting prostate cancer patients for active surveillance. Eur Urol. 2009;56:891. doi: 10.1016/j.eururo.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Abouassaly R, Lane BR, Jones JS. Staging saturation biopsy in patients with prostate cancer on active surveillance protocol. Urology. 2008;71:573. doi: 10.1016/j.urology.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 15.Motamedinia P, RiChard JL, McKiernan JM, et al. Role of immediate confirmatory prostate biopsy to ensure accurate eligibility for active surveillance. Urology. 2012;80:1070. doi: 10.1016/j.urology.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Stackhouse DA, Sun L, Schroeck FR, et al. Factors predicting prostatic biopsy Gleason sum under grading. J Urol. 2009;182:118. doi: 10.1016/j.juro.2009.02.127. [DOI] [PubMed] [Google Scholar]
- 17.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368. [PubMed] [Google Scholar]
- 19.Connolly D, Black A, Murray LJ, et al. Methods of calculating prostate-specific antigen velocity. Eur Urol. 2007;52:1044. doi: 10.1016/j.eururo.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Kettermann AE, Ferrucci L, Trock BJ, et al. Interpretation of the prostate-specific antigen history in assessing life-threatening prostate cancer. BJU Int. 2010;106:1284. doi: 10.1111/j.1464-410X.2010.09363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 25.Ulmert D, Serio AM, O'Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835. doi: 10.1200/JCO.2007.13.1490. [DOI] [PubMed] [Google Scholar]
- 26.Orsted DD, Bojesen SE, Kamstrup PR, et al. Long-term prostate-specific antigen velocity in improved classification of prostate cancer risk and mortality. Eur Urol. 2013;64:384. doi: 10.1016/j.eururo.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Carter HB, Ferrucci L, Kettermann A, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb S, Sutherland DE, D'Amico AV, et al. PSA velocity is associated with Gleason score in radical prostatectomy specimen: marker for prostate cancer aggressiveness. Urology. 2008;72:1116. doi: 10.1016/j.urology.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 29.Tseng KS, Landis P, Epstein JI, et al. Risk stratification of men choosing surveillance for low risk prostate cancer. J Urol. 2010;183:1779. doi: 10.1016/j.juro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Francisco IF, Werner L, Regan MM, et al. Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol. 2011;185:471. doi: 10.1016/j.juro.2010.09.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


