Abstract
The Pd-catalyzed coupling of γ-hydroxyalkenes with aryl bromides affords enantiomerically enriched 2-(arylmethyl)tetrahydrofuran derivatives in good yield and up to 96:4 er. This transformation was achieved through the development of a new TADDOL/2-arylcyclohexanol-derived chiral phosphite ligand. The transformations are effective with an array of different aryl bromides, and can be used for the preparation of products bearing quaternary stereocenters.
Keywords: asymmetric catalysis, heterocycles, palladium, enantioselective
Tetrahydrofurans bearing substituents at the C2 position are prominent moieties displayed in many biologically active compounds.[i] As such, the asymmetric construction of tetrahydrofurans is an important challenge in organic synthesis that has attracted considerable attention over the years[ii]
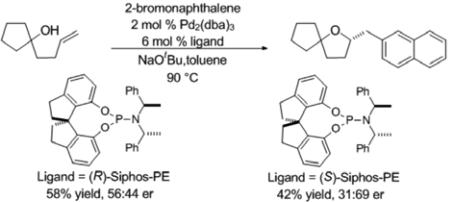
Our group has previously reported the development of Pd-catalyzed alkene carboalkoxylation reactions between γ-hydroxyalkenes and aryl or alkenyl halides for the construction of substituted tetrahydrofurans with high diastereoselectivity.[iii,iv,v] These reactions effect formation of the heterocyclic ring along with a C–O bond, a C–C bond, and 1-2 stereocenters with high diastereoselectivity. However, the successful development of an enantioselective variant of these reactions has remained elusive.[vi,vii,viii,ix] For example, although we have illustrated the chiral phosphoramidite ligands (R)- or (S)-Siphos-PE provided satisfactory results in related asymmetric alkene carboamination reactions of alkenes bearing pendant nitrogen nucleophiles,[x] use of these ligands for the coupling of alcohol 1a with 2-bromonaphthalene led to very low levels of asymmetric induction [Eq. (1)]. Similarly poor results were also obtained with a variety of other chiral phosphine and phosphoramidite ligands.
 |
(1) |
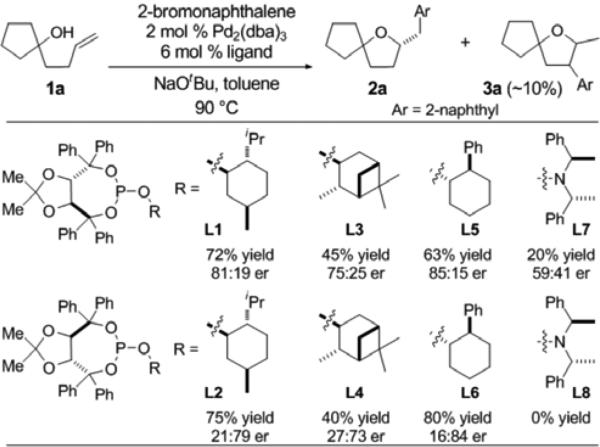
During the course of a rather extensive screen of chiral ligands, we encountered a promising lead result. As shown in Table 1, a chiral phosphite ligand L1 derived from (S,S)-TADDOL and (–)-menthol, afforded the desired product 2a in 72% yield with 81:19 er. We sought to further optimize this result through modification of this ligand, which can easily be prepared from a TADDOL and a chiral alcohol.[xi] We initially investigated changes to the TADDOL backbone, but replacement of the phenyl group or the gem-dimethyl groups with other substituents failed to provide improved results. As such, we turned our attention to the chiral alcohol component. Ligands L2–L6 were synthesized from PCl3 and either (S,S)- or (R,R)-TADDOL along with (−)-menthol, (+)-isopinocampheol, or (+)-2-phenylcyclohexanol. As shown in Table 1, similar enantiomeric ratios were obtained with each pair of ligand diastereomers (e.g., L1 vs L2), although the absolute stereochemistry of the product was reversed. However, improved results were obtained with the 2-phenylcyclohexanol derivatives L5 and L6. All transformations provided small amounts (ca 5–10 %) of side product 3a, which likely derives from competing β-hydride elimination of an intermediate LnPd(Ar)(alkyl) complex in the catalytic cycle.[ivb]
Table 1.
Initial TADDOL ligand screen.[a]
Conditions: 1.0 equiv 1a, 1.8 equiv. 2-bromonaphthalene, 1.8 equiv. NaOtBu, 2 mol % Pd2(dba)3, 6 mol % chiral ligand, toluene (0.2 M), 90 °C, 12–14 h. Reactions were conducted on a 0.10 mmol scale. In all cases regioisomer 3 was formed in ca. 10% yield.
Since L5 afforded the best enantioselectivity for this reaction, we decided to synthesize a variety of chiral 2-arylsubstituted cyclohexanols to test the effect of the aryl group on asymmetric induction.[xii] After some experimentation we discovered that ligand L7 provided slightly improved results (88:12) er, and that use of 1,4-dioxane as solvent in place of toluene resulted in a further increase to 89:11 er (Table 2, entry 2). Further modification of reaction conditions by changing solvent, base, or temperature did not lead to further improvement of selectivity. Similar results were obtained with the use of 2-iodonaphthalene in place of 2-bromonaphthalene.[xiii]
Table 2.
Enantioselective carboalkoxylation reactions.[a]
|
| |||||
|---|---|---|---|---|---|
| entry | R | R1–X | product | yield (%)[b] | er |
| 1c | (CH2)4 (1a) |
|
2a | 62 | 88:12 |
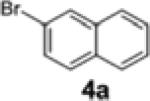
| 2 | 1a | 4a | 2a | 58 | 89:11 |
| 3[c] | 1a |
|
2a | 59 | 87:13 |
| 4 | 1a |
|
2b | 54 | 82:18 |
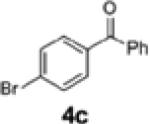
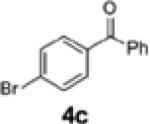
| 5 | H (1b) | 4c | 2c | 23 | 58:42 |
| 6[e] | Ph (1c) |
|
2d | 67 | 95:5 |
| 7[c,d,e] | 1c | 4a | 2d | 60 | 96:4 |
| 8 | 1c |
|
2e | 64 | 92:8 |
| 9c,d | 1c | 4c | 2e | 61 | 95:5 |
| 10 | 1c |
|
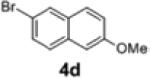
2f | 66 | 95:5 |
| 11 | 1c |
|
2g | 63 | 94:6 |
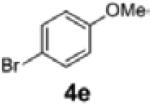
| 12[c,d] | 1c | 4e | 2g | 54 | 95:5 |
| 13 | 1c |
|
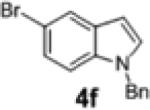
2h | 71 | 93:7 |
| 14 | 1c |
|
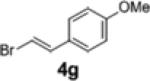
2i | 18 | 79:21 |
Conditions: 1.0 equiv substrate, 1.8 equiv R1–X, 1.8 equiv NaOtBu, 2 mol % Pd2(dba)3, 5 mol % L7, dioxane (0.2 M), 90 °C, 12-14 h. Reactions were conducted on a 0.20 mmol scale. Small amounts (ca 10–15%) of regioismeric products analogous to 3a were also obtained in reactions of substrates 1a and 1b. Product 2a could be easily separated from the regioisomer, whereas the regioisomer could not be separated from 2b and the yield is for the mixture of products.
Isolated yield (average of two or more runs).
The reaction was conducted in toluene solvent.
2 equiv of H2O was added to the reaction mixture.
The reaction was conducted using 1.4 equiv Ar–X.
Having developed a suitable catalyst system and adequate reaction conditions we proceeded to explore the scope of this transformation. As shown in table 2, use of 4-penten-1-ol 1b as a substrate resulted in poor yield and low selectivity (entry 5). However, the transformation of substrate 1c, which contains a gem-diphenyl group at C2, proceeded with higher selectivity (entry 6, 95:5 er) than that of 1a. The reactions of 1c were effective with several different aryl bromides, including electron-rich, electron-poor, and heteroaryl electrophiles. However, use of alkenyl bromide 4g led to low yield and poor enantioselectivity.
Our prior studies on asymmetric Pd-catalyzed alkene carboamination reactions of N-allylureas revealed a surprising positive influence of the addition of water on selectivity.[xb] Thus, we examined the addition of 2 equiv of water to reactions of 1c with different electrophiles (Table 2, entries 7, 9, and 12). In all cases this led to a slight improvement in asymmetric induction. However, these improvements were less significant than those observed in the urea carboamination reactions.
To further explore the scope and potential utility of this method, we elected to examine reactions of substituted alkenes (Table 3). The coupling of substrate 1d with 4-bromobenzophenone proceeded in good yield, but with poor enantioselectivity (entry 1). However, we were gratified to find that related substrate 1e, which contains a gem-diphenyl group rather than a gem-dimethyl group at C2, was transformed in good yield and 95:5 er (Table 3, entry 2). The reactions were effective with the 5-bromoindole derivative 4f and 4-bromophenyl morpholine 4h. However, use of 4-bromobenzonitrile as the electrophile led to the formation of product 2m in a modest 39% yield and 87:13 er. Furthermore, substrates bearing internal alkenes such as 1f and 1g were unreactive under these conditions.
Table 3.
Enantioselective formation of quaternary centers.[a]
| |||||
|---|---|---|---|---|---|
| entry | substrate | Ar–X | product | yield (%)[b] | er |
| 1 | 1d |
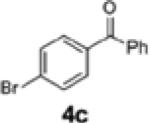
|
2j | 85 | 68:32 |
| 2 | 1e | 4c | 2k | 85 | 95:5 |
| 3 | 1e |
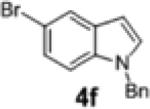
|
2l | 94 | 96:4 |
| 4 | 1e |
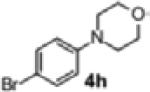
|
2m | 85 | 93:7 |
| 5[c] | 1e |
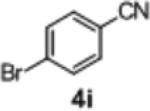
|
2n | 39 | 87:13 |
| 6 | 1f | 4c | – | no rxn | N/A |
| 7 | 1g | 4c | – | no rxn | N/A |
Conditions: 1.0 equiv substrate, 1.8 equiv Ar–X, 1.8 equiv NaOtBu, 2 mol % Pd2(dba)3, 5 mol % L7, dioxane (0.2 M), 90 °C, 12-14 h. Reactions were conducted on a 0.20 mmol scale.
Isolated yield (average of two or more runs).
The reaction proceeded to ca. 75% conversion.
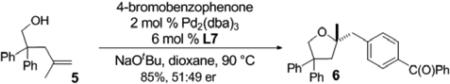
Finally, we briefly explored the reactivity of substrate 5, which contains disubstitution at C2 rather than C1. As shown in [Eq. (2)], the coupling of 5 with 4-bromobenzophenone proceeded in good yield, but afforded product 6 in essentially racemic form. This further illustrates the importance of gem-disubstitution at the C1 position of the substrate.
 |
(2) |
In conclusion, we have developed a new enantioselective synthesis of tetrahydrofurans via asymmetric Pd-catalyzed carboalkoxylation reactions of γ-hydroxyalkenes with aryl bromides. The development and optimization of ligand L7 was key to obtaining high levels of asymmetric induction. Our preliminary studies on the influence of TADDOL-based phosphite ligand structure on enantioselectivity indicate the structure of the phosphite alkoxy group (derived from a chiral alcohol) has the greatest influence on relative levels of asymmetric induction obtained with a structurally related series of ligands. Moreover (and perhaps not surprisingly), large changes in the alkoxy group have a larger impact on selectivity than fine-tuning of closely related structures. These results should help to guide future development of other chiral catalysts for heterocycle-forming alkene difunctionalization reactions.
Supplementary Material
Acknowledgments
The authors acknowledge the NIH-NIGMS (GM098314) for financial support of this work.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- i.a Alali FQ, Liu X-X, McLaughlin JL. J. Nat. Prod. 1999;62:504–540. doi: 10.1021/np980406d. [DOI] [PubMed] [Google Scholar]; b Bermejo A, Figadere B, Zafra-Polo M-C, Barrachina I, Estornell E, Cortes D. Nat. Prod. Rep. 2005;22:269–303. doi: 10.1039/b500186m. [DOI] [PubMed] [Google Scholar]; c Lorente A, Lamariano-Merketegi J, Albericio F, Alvarez M. Chem. Rev. 2013;113:4567–4610. doi: 10.1021/cr3004778. [DOI] [PubMed] [Google Scholar]
- ii.For reviews on the synthesis of tetrahdrofurans, see: Rainier JD. Top. Heterocycl. Chem. 2014;35:1–41.Jalce G, Franck X, Figadere B. Tetrahedron: Asymmetry. 2009;20:2537–2581.Wolfe JP, Hay MB. Tetrahedron. 2007;63:261–290. doi: 10.1016/j.tet.2006.08.105.Muzart J. Tetrahedron. 2005;61:5955–6008.Harmange J–C, Figadere B. Tetrahedron: Asymmetry. 1993;4:1711–1754.
- iii.For reviews on Pd-catalyzed alkene carboamination and carboalkoxylation reactions, see: Wolfe JP. Eur. J. Org. Chem. 2007:517–582.J. P. Wolfe. Synlett. 2008:2913–2937.Schultz DM, Wolfe JP. Synthesis. 2012;44:351–361. doi: 10.1055/s-0031-1289668.Wolfe JP. Top. Heterocycl. Chem. 2013;32:1–38.
- iv.a Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620–1621. doi: 10.1021/ja0394838. [DOI] [PubMed] [Google Scholar]; b Hay MB, Wolfe JP. J. Am. Chem. Soc. 2005;127:16468–16476. doi: 10.1021/ja054754v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hay MB, Hardin AR, Wolfe JP. J. Org. Chem. 2005;70:3099–3107. doi: 10.1021/jo050022+. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ward AF, Wolfe JP. Org. Lett. 2009;11:2209–2212. doi: 10.1021/ol900594h. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ward AF, Wolfe JP. Org. Lett. 2010;12:1268–1271. doi: 10.1021/ol1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.For a related synthesis of epoxides, see: Hayashi S, Yorimitsu H, Oshima K. J. Am. Chem. Soc. 2009;131:2052–2053. doi: 10.1021/ja8084065.
- vi.For examples of asymmetric Cu-catalyzed intramolecular alkene carboalkoxylation reactions of γ-hydroxyalkenes bearing tethered arenes, see: Miller Y, Miao L, Hosseini AA, Chemler SR. J. Am. Chem. Soc. 2012;134:12149–12156. doi: 10.1021/ja3034075.
- vii.For examples of asymmetric Cu-catalyzed intermolecular alkene carboalkoxylation reactions between γ-hydroxyalkenes and styrene derivatives, see: Bovino MT, Liwosz TW, Kendel NE, Miller Y, Tyminska N, Zurek E, Chemler SR. Angew. Chem. 2014;126:6501–6505. doi: 10.1002/anie.201402462.Angew. Chem. Int. Ed. 2014;53:6383–6387. doi: 10.1002/anie.201402462.
- viii.For examples of asymmetric Pd-catalyzed alkene carboalkoxylation reactions between 2-(but-3-enyl)phenols and acrylates, see: Tietze LF, Sommer KM, Zinngrebe J, Stecker F. Angew. Chem. 2005;117:262–264. doi: 10.1002/anie.200461629.Angew. Chem., Int. Ed. 2005;44:257–259.Tietze LF, Spiegl DA, Stecker F, Major J, Raith C, Grosse C. Chem. Eur. J. 2008;14:8956–8963. doi: 10.1002/chem.200800967.
- ix.For examples of asymmetric Pd-catalyzed intermolecular carboalkoxylation reactions between 2-(5-hydroxypent-1-en-1-yl)phenol derivatives and enol ethers, see: Pathak TP, Gligorich KM, Welm BE, Sigman MS. J. Am. Chem. Soc. 2010;132:17471–17482. doi: 10.1021/ja103472a.
- x.a Mai DN, Wolfe JP. J. Am. Chem. Soc. 2010;132:12157–12159. doi: 10.1021/ja106989h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hopkins BA, Wolfe JP. Angew. Chem. 2012;124:10024–10028. [Google Scholar]; Angew. Chem., Int. Ed. 2012;51:9886–9890. doi: 10.1002/anie.201205233. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Babij NR, Wolfe JP. Angew. Chem. 2013;125:9417–9420. [Google Scholar]; Angew. Chem., Int. Ed. 2013;52:9247–9250. doi: 10.1002/anie.201302720. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Hopkins BA, Wolfe JP. Chem. Sci. 2014;5:4840–4844. doi: 10.1039/C4SC01327A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xi.Alexakis A, Burton J, Vastra J, Benhaim C, Fournioux X, van den Heuvel A, Levêque J-M, Mazé F, Rosset S. Eur. J. Org. Chem. 2000:4011–4027. [Google Scholar]
- xii.See the Supporting Information for a table describing the results of chiral ligand screens for the coupling of 1a with 2-bromonaphthalene.
- xiii.In prior studies on asymmetric carboamination reactions of ureas we have found that use of aryl iodides leads to diminished enantioselectivity. See reference 10b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.