Abstract
Our recent studies showed that total body irradiation (TBI) induces long-term bone marrow (BM) suppression in part by induction of hematopoietic stem cell (HSC) senescence through NADPH oxidase 4 (NOX4)-derived reactive oxygen species (ROS). Therefore, in the present study we examined if resveratrol (3,5,4’-trihydroxy-trans-stilbene), a potent antioxidant and a putative activator of Sirtuin 1 (Sirt1), can ameliorate TBI-induced long-term BM injury by inhibiting radiation-induced chronic oxidative stress and senescence in HSCs. Our results showed that pretreatment with resveratrol not only protected mice from TBI-induced acute BM syndrome and lethality but also ameliorated TBI-induced long-term BM injury. This later effect is likely attributed to resveratrol-mediated reduction of chronic oxidative stress in HSCs, because resveratrol treatment significantly inhibited TBI-induced increase in ROS production in HSCs and prevented mouse BM HSCs from TBI-induced senescence, leading to a significant improvement of HSC clonogenic function and long-term engraftment after transplantation. The inhibition of TBI-induced ROS production in HSCs is likely attributable to resveratrol-mediated down-regulation of NOX4 expression and up-regulation of Sirt1, superoxide dismutase 2 (SOD2), and glutathione peroxidase 1 (GPX1) expression. Furthermore, we showed that resveratrol increased Sirt1 deacetylase activity in BM hematopoietic cells; and Ex527, a potent Sirt1 inhibitor, can attenuate resveratrol-induced SOD2 expression and the radioprotective effect of resveratrol on HSCs. These findings demonstrate that resveratrol can protect HSCs from radiation at least in part via activation of Sirt1. Therefore, resveratrol has the potential to be used as an effective therapeutic agent to ameliorate TBI-induced long-term BM injury.
Keywords: Ionizing radiation, Oxidative stress, Hematopoietic stem/progenitor cells, Radioprotection, Resveratrol
Introduction
Bone marrow (BM) suppression is one of the common side effects of conventional cancer therapy using ionizing radiation (IR) and chemotherapy and the primary cause of death after accidental exposure to a high dose of total body irradiation (TBI) [1, 2]. Acute BM suppression resulting from the induction of apoptosis in the rapidly proliferating hematopoietic progenitor cells (HPCs) and to a lesser degree in the relatively quiescent hematopoietic stem cells (HSCs) by IR, can cause high mortality and morbidity, but have been better managed in the clinic by the use of hematopoietic growth factors [2, 3]. However, the long term BM damage induced by IR, manifested by a defect in HSC self-renewal and a decrease in HSC reserves, is latent, long-lasting, and shows little tendency for recovery [4]. Moreover, an effective treatment to ameliorate the injury has not been developed yet.
Several studies have shown that IR induces long-term BM injury primarily via induction of HSC senescence [2, 3], resulting in a persistent loss of the proliferative capacity of HSCs. Our recent studies showed that exposure of mice to a sublethal dose of TBI induced a persistent increase in reactive oxygen species (ROS) production in HSCs in part via up-regulation of NADPH oxidase 4 (NOX4). The induction of chronic oxidative stress in HSCs was associated with sustained increases in oxidative DNA damage, p16Ink4a (p16) expression [2, 5], inhibition of HSC clonogenic function, and induction of HSC senescence but not apoptosis. Several recent studies also showed that induction of oxidative stress was primarily responsible for the loss of HSC self-renewal and premature exhaustion of HSCs in mice with mutations in the ATM [6] and deletion of Forkhead box O3 (FoxO3) [7]. These findings suggest that IR-induced long-term BM injury may be ameliorated by the treatment with a potent antioxidant.
Resveratrol (trans-3,5,4’-trihydroxystilbene), a polyphenolic compound primarily found in grapes, nuts, fruits and red wine, is a potent antioxidant and a putative activator of Sirtuin 1 (Sirt1) [8, 9]. Accumulating reports have shown that resveratrol can prevent or slow the progression of a wide variety of diseases, including cancer, cardiovascular disease and Alzheimer’s disease, as well as enhance stress resistance and extend the lifespans of various organisms from yeast to vertebrates [10]. As a polyphenolic compound, resveratrol has been shown to be a scavenger of hydroxyl, superoxide, and metal-induced radicals [11]. However, the direct antioxidant effects of resveratrol are rather poor. The protective effects of resveratrol against oxidative injury are likely to be attributed to the up-regulation of endogenous cellular antioxidant systems rather than the direct ROS scavenging activity of the compound. Treatment of rat cardiac cells and human vein endothelial cells increases the activity of cellular antioxidant enzymes including superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPX) [12, 13]. Resveratrol also eliminates intra-cellular ROS by inhibiting the expression of NOX4, a pro-oxidative enzyme, in human vein endothelial cells [12]. Some of these effects of resveratrol have been attributed to the activation of Sirt1 which can regulate the expression of various genes through downstream transcriptional factors including FoxO3 [14, 15]. Because of the remarkable therapeutic potential of resveratrol, we examined its effect on TBI-induced long-term BM suppression in our well-established and characterized mouse model in the present study. The data from this studies showed that resveratrol can ameliorate TBI-induced long-term BM injury by inhibiting IR-induced chronic oxidative stress and cellular senescence in HSCs at least in part via Sirt1.
MATERIALS AND METHODS
Reagents
Anti-Mouse-CD117 (c-kit)-Alexa Fluor 700 (clone ACK2), anti-Mouse-Ly-6A/E (Sca-1)-PE (clone D7); biotin-conjungated anti-Mouse-CD4 (clone GK1.5), anti-Mouse-CD8 (clone 53-6.7), anti-Mouse-CD45R/B220 (clone RA3-6B2), anti-Mouse-Ly6G/Gr-1 (clone RB6-8C5), anti-Mouse-CD11b (clone M1/70), anti-Mouse-Ter-119 (clone Ter-119); and APC-Cy7-conjugated streptavidin were obtained from eBioscience (San Diego, CA). Anti-Mouse-CD45.1-FITC (clone A20, Ly5.1), Anti-Mouse-CD45.2-PE (clone 104, Ly5.2), Anti-Mouse-Ly6G/Gr-1-PE/Cy7 (clone RB6-8C5), anti-Mouse-CD45R/B220-PerCP (clone RA3-6B2), anti-Mouse-CD11b- PE/Cy7 (clone M1/70), and anti-Mouse-CD3-APC (clone 145-2C11) were obtained from Biolegend (San Diego, CA). 2’,7’-Dichlorofluorescin diacetate (DCFDA) were obtained from Sigma (St. Louis, MO). Rabbit anti-acetylated p53 (ab61241) and FITC-conjugated goat anti-rabbit antibodies were obtained from Abcam Biotechnology (Cambridge, MA). Resveratrol was kindly provided by Dr. Qi Hou at the Institute of Materia Medica of the Peking Union Medical College (PUMC, Beijing, China).
Mice
Male C57BL/6-Ly-5.1 (Ly5.1) mice were purchased from the Institute of Laboratory Animal Sciences (PUMC, Beijing, China). C57BL/6-Ly-5.2 (Ly5.2) and C57BL/6-Ly-5.1/5.2 (Ly5.1/5.2) mice were bred at the certified animal care facility in the Institute of Radiation Medicine of PUMC. They received food and water ad libitum. All of the mice were used at approximately 8–10 weeks of age. The Institutional Animal Care and Use Committee of PUMC approved all experimental procedures used in this study.
TBI and Resveratrol Administration
Ly5.1 mice were divided into three groups randomly: (a) Control; (b) Vehicle + TBI; and (c) Resveratrol + TBI. Resveratrol was dissolved in 96% ethanol to a concentration of 50 mg/ml as stock solution. Prior to gavage, the stock solution was diluted as follows: 0.1 ml of stock solution in 1.9 ml distilled water for a final concentration of 2.5 mg/ml and protected from light by covering the tube with an aluminum foil. Individual mice in Resveratrol + TBI group received a dose of 20 mg/kg resveratrol administered by gavage every day for 7 days prior to irradiation and then 30 days after irradiation. For control, the mice in Vehicle + TBI group received intragastric administration of the same volume of vehicle (4.8% ethanol) for the same frequency and duration as those in Resveratrol + TBI group. The mice in both Vehicle + TBI and Resveratrol + TBI groups were exposed to a sublethal dose (6.0 Gy) or a lethal dose (7.2 Gy, for the survival study only) of TBI in an Exposure Instrument Gammacell-40 137Cesium-irradiator (Atomic Energy of Canada Lim) at a dose rate of 0.76 Gy/min. Control mice were sham irradiated as irradiated mice.
Peripheral blood cell and BM nucleated cell (BMNs) counts
Blood was obtained from anesthetized mice via the orbital sinus and was collected in ethylenediaminetetra-acetic acid (EDTA·K3) tubes. Complete blood cell counts were obtained within 30 min of blood collection using a pocH-100i Hematology Analyzer (Sysmex, Japan). The cell counts included white blood cells (WBC), red blood cells (RBC), platelets (PLT), and percentage of lymphocytes (LYM%). BM cells were flushed from the bones as described previously [1], and BMNs were counted using the Hematology Analyzer.
Isolation of BM mononuclear cells (BM-MNCs), HPC and HSC
BM-MNCs were isolated from BMNs as described previously [2, 3]. They were incubated with biotin-conjugated antibodies specific for murine CD4, CD8, CD11b, CD45R/B220, Ter-119, and Gr-1 and then stained with streptavidin-APC-Cy7, anti-Sca-1-PE, and anti-c-kit-Alexa Fluor 700. HPCs (Lin−c-kit+Sca-1−) and HSCs (Lin−c-kit+Sca-1+) were analyzed and sorted using a BD Aria FACS II cell sorter (BD Bioscience, San Jose, CA).
Cobblestone area-forming cell (CAFC) assay
Competitive repopulation assay (CRA)
Competitive repopulation assays were performed using the Ly5 congenic mice as described previously [16, 17]. Specifically, donor BMNs were harvested from Ly5.1 mice after receiving various treatments. They (1×106 BMNs) were mixed with 2×105 competitive BMNs pooled from six Ly5.1/Ly5.2 hybrid mice. The mixed cells were transplanted into lethally irradiated (9.0 Gy TBI) Ly5.2 recipient mice (9 mice/group) by lateral canthus-vein injection. For analysis of engraftment, peripheral blood were obtained from the medial canthus using heparin-coated micropipets (Drummond Scientific, Broomall, PA) at 2 months after transplantation from all the recipients. After red blood cells had been lysed by 0.15 M NH4Cl solution, the blood samples were stained with FITC conjugated anti-CD45.1, PE conjugated anti-CD45.2, PerCP conjugated anti-B220, APC conjugated anti-CD3, and PE/Cy7 conjugated Anti-Gr-1 and CD11b and were analyzed by a LSR II flow cytometer (BD Bioscience, San Jose, CA).
Analysis of the levels of intracellular ROS
BM lineage negative hematopoietic cells (Lin− cells) were isolated as we previously described [16, 17]. They were stained with anti-Sca-1-PE and anti-c-kit–Alexa Fluor 700A antibodies and then incubated with DCFDA (10 µM) for 20 min at 37°C. The levels of intracellular ROS in HPCs and HSCs were analyzed by measuring the mean fluorescence intensity (MFI) of 2`,7`-dichlorofluorescein (DCF) using a flow cytometer as described previously [16, 17]. The specificity of this assay to detect intracellular ROS in HPCs and HSCs was validated in our recently reported studies [16, 17]. For each sample, a minimum of 100,000 Lin− cells were acquired and the data were analyzed using FlowJo 7.6.1 software (Tree-Star Inc., Ashland, OR). In all experiments, PE and Alexa Fluor 700A isotype controls and other positive and negative controls were included as appropriate.
Quantification Real-time PCR assays
Total RNA was extracted from sorted 10,000 HPCs and 2,000 HSCs using TRIzol reagent (Life Technologies, Grand Island, NY) and Glycogen (Roche, Indianapolis, IN) following the manufacturer’s protocol. First-strand cDNA was synthesized from total RNA using a RNA PCR Kit (AWV) Ver3.0 (Takara Co., Japan) according to the manufacturer’s protocol. PCR primers for the SOD2, GPX1, NOX4, SIRT1 and the housekeeping gene GAPDH were obtained from Sangon Biotech (Shanghai, China). The sequences of primers used in this study were: NOX4, 5'-GAT TTC TGG ACC TTT GTG CCT TT-3' (forward) and 5'-TGA TGG TGA CAG GTT TGT TGC T-3' (reverse); SOD2, 5'-ATT AAC GCG CAG ATC ATG CA -3' (forward) and 5'-TGT CCC CCA CCA TTG AAC TT-3' (reverse); GPX1, 5'-TGC TCA TTG AGA ATG TCG CGT CTC-3' (forward) and 5'-AGG CAT TCC GCA GGA AGG TAA AGA-3' (reverse); SIRT1, 5'-TTG GCA CCG ATC CTC GAA C -3' (forward) and 5'-CCC AGC TCC AGT CAG AAC TAT-3' (reverse); GAPDH, 5'-TGA AGG TCG GTG TGA ACG GAT TTG GC-3' (forward) and 5'-CAT GTA GGC CAT GAG GTC CAC CAC-3' (reverse). cDNA samples were mixed with primers and SYBR Master Mix (Life Technologies) in a total volume of 25 µl. All samples were analyzed in triplicate using an ABI Prism 7500 Sequence Detection System (Applied Biosystems-Life Technologies). The threshold cycle (CT) values for each reaction were determined and averaged using the TaqMan SDS analysis software (Applied Biosystems-Life Technologies). The changes in the expression of a target gene were calculated by the comparative CT method (fold changes =2[−ΔΔCT]) as described previously [18].
Analysis of p16 mRNA expression in HSCs
These experiments were performed as we previously reported [1, 16].
Analysis of enzymatic activity of SOD2 and GPX1
SOD2 and GPX1 enzymatic activities in BM-MNCs were analyzed using a SOD2 Assay Kit and a Cellular Glutathione Peroxidase 1 Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China) following the manufacturer’s instructions, respectively.
Immunofluorescent staining for acetylated p53
Approximately 500 sorted HSCs were cytospun onto a slide and fixed in 4% paraformaldehyde solution for 10 min at room temperature. Cells were permeabilized with 0.2% Triton X-100 on ice and blocked with 5% goat serum before incubation with anti-acetylated p53 antibodies (1:200) overnight at 4°C. After being washed three times with PBS (5 min each), the immunostaining of acetylated p53 in the cells was visualized with FITC-conjugated goat anti-rabbit IgG (1:200). Nuclei were counterstained with DAPI and slides were mounted in Vectashield as described previously [16].
Single-cell colony assay
Lin− cells were incubated with resveratrol (1 µM), resveratrol (1 µM) plus EX527 (10 µM), or vehicle (0.2% dimethylsulfoxide) at 37°C for 60 min prior to exposure to 4 Gy IR and sham-irradiation. Individual HSCs were seeded into wells of 96-well round-bottom microplates by the BD Aria FACS II cell sorter at one cell/well. The cells were cultured in 200 µL IMDM medium supplemented with 10% FCS, 1% BSA, 2 mM L-Glutamin, 50 µM 2-β-mercaptoethanol, and 10 ng/ml of stem cell factor, thrombopoietin, and IL-3 as described previously [19]. After 14 days of culture, colonies of cells ≥ 50 cells/well were scored under an inverted microscope. The results are expressed as the number of colonies per 20 wells.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc. USA). In the event that ANOVA justified post hoc comparisons between group means, these were conducted using the Student–Newman–Keuls test for multiple comparisons. Differences were considered significant at p<0.05.
RESULTS
Resveratrol increases survival rate of mice exposed to a lethal dose of TBI
In this study, mice were exposed to a lethal dose (7.2 Gy) of TBI and treated with resveratrol or vehicle as illustrated in Fig. 1A. Their survival was monitored for 30 days after TBI. As shown in Fig. 1B, Kaplan-Meier analysis of survival indicated that no statistical difference was identified between TBI mice treated with resveratrol or vehicle before day 12. From day 15 to day 30, the survival rate of TBI mice treated with resveratrol was significantly higher than that of vehicle-treated TBI mice. These data suggests that resveratrol increases the survival after mice were exposed to a lethal dose (7.2 Gy) of TBI.
Figure 1. Resveratrol administration increases survival.
A. Mice were treated daily with vehicle or resveratrol (20 mg/kg) by gavage for 7 days prior to exposure to a lethal dose (7.2 Gy) TBI and then continuously for 30 days after TBI as illustrated in the diagram. A group of un-irradiated mice was included as control. B. Kaplan-Meier analysis of animal survival after exposure to the lethal dose of TBI. N = 12 mice/group. ap<0.01 vs. control; bp<0.05 vs vehicle + TBI.
Resveratrol ameliorates ionizing radiation induced reduction in BM hematopoietic cells
It has been well established that the survival of mice after exposed to a moderate lethal dose of TBI depends on the recovery of the hematopoietic system [20, 21]. To determine whether resveratrol may protect mice from TBI-induced lethality by inhibiting IR-induced BM injury, we exposed mice to a sublethal dose of TBI (6.0 Gy). So, the mice can survive from the radiation injury for analyses of hematopoietic function. As shown in Fig. 2, vehicle-treated mice exhibited a substantial reduction in various blood cell indices and the numbers of BMNs even 30 days after TBI. Treatment of the irradiated mice with resveratrol significantly attenuated the reduction, particularly the reduction of WBC and BMNs. This finding demonstrates that resveratrol can effectively inhibit IR-induced BM injury.
Figure 2. Resveratrol attenuates TBI-induced myelosuppression.
Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as those illustrated in Fig. 1A. A. The numbers of red blood cells (RBC), white blood cells (WBC) and platelets (PLT) in peripheral blood and percentage of lymphocytes in WBC were quantified after the mice were euthanized 30 days after exposure to TBI. B. The numbers of bone marrow nucleated cells (BMN) were counted 30 days after TBI. The data are presented as mean ± SE. N = 8 mice/group. ap<0.01 vs. control; bp<0.05 vs vehicle + TBI.
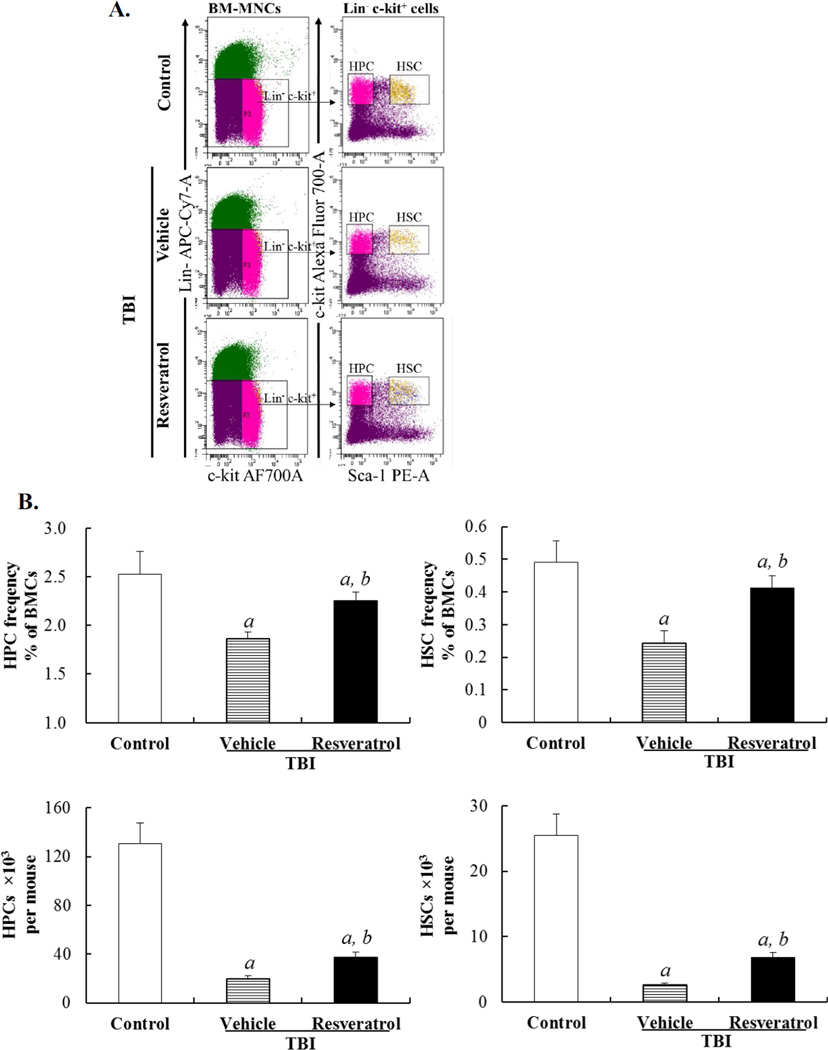
Resveratrol attenuates TBI-induced long-term BM injury
Next, we examined if resveratrol can also inhibit IR-induced long-term BM injury. First, we analyzed the frequencies and total numbers of HPCs and HSCs in BM from control mice and irradiated mice treated with vehicle or resveratrol 30 days after TBI. It was found that TBI caused a sustained reduction in both HPCs and HSCs in mice treated with vehicle compared to control mice (Fig. 3). This reduction was associated with a considerable suppression of HPC and HSC clonogenic functions measured by the day-14 and day-35 CAFC assays, respectively (Fig. 4A & B). More importantly, the HSCs from the irradiated mice treated with vehicle exhibited about 70% of reduction in long-term and multi-lineage engraftment after transplantation (Fig. 4C). These findings demonstrate that exposure of mice to a sublethal dose of TBI (6.0 Gy) not only induces acute BM damage but also causes long-term BM injury as shown in previous studies [2, 16, 20]. Treatment of the irradiated mice with resveratrol resulted in a significant quantitative recovery of HPCs and HSCs in BM (Fig. 3) and an improvement of their clonogenic functions (Fig. 4A & B). More importantly, the engraftment capability of the irradiated HSCs was almost back to normal level after resveratrol treatment (Fig. 4C), indicating resveratrol can ameliorate TBI-induced long-term BM injury.
Figure 3. Resveratrol attenuates TBI-induced reduction of HPCs and HSCs.
Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as those illustrated in Fig. 1A. The frequencies and numbers of HPCs and HSCs in BM were analyzed by flow cytometry after the mice were euthanized 30 days after exposure to TBI. A. A representative gating strategy of HSC and HPC analysis by flow cytometry. B. The frequencies of HSCs and HPCs in BMNs and absolute numbers of HSCs and HPCs per mouse are presented as means ± SE of three independent experiments. ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI.
Figure 4. Resveratrol reduces TBI-induced suppression of HPC and HSC clonogenic function and HSC long-term engraftment after transplantation.
Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as those illustrated in Fig. 1A. They were euthanized 30 days after exposure to TBI to harvest BM-MNCs. The cells were analyzed as described below: A. Analysis of the clonogenic function of HPCs in BM-MNCs by day-14 CAFC assay. B. Analysis of the clonogenic function of HSCs by day-35 CAFC assay. The data are presented in A & B as mean ± SE of the frequencies of day-14 CAFCs and day-35 CAFCs per 105 BM-MNCs from three independent assays. ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI. C. Analysis of donor cell engraftment 2 months after transplantation. The data are expressed as means ± SE of percent of donor-derived leukocytes (CD45.1+ cells), T cells (CD45.1+CD3+ cells), B cells (CD45.1+B220+ cells), and myeloid cells (CD45.1+CD11b+ and/or Gr-1+ granulocyte–monocyte–macrophage) in the peripheral blood (N = nine recipient mice/group).
Resveratrol inhibits TBI-induced chronic oxidative stress and senescence in HSCs
Our recent studies have shown that exposure of mice to a sublethal dose of TBI causes long-term BM suppression primarily by induction of HSC senescence via NOX4-derived ROS [16]. Resveratrol is a potent antioxidant that has the ability to inhibit oxidative stress-induced tissue damage in various pathological conditions not only by scavenging ROS but also by modulating the expression of various antioxidant enzymes [22, 23]. Therefore, we examined if resveratrol can ameliorate TBI-caused long-term BM suppression by inhibiting IR-induced chronic oxidative stress and senescence in HSCs. As shown in Fig. 5A & B, the production of ROS was more significantly elevated in HSCs than in HPCs from vehicle-treated mice even 30 days after TBI compared to that of non-irradiated cells as shown in our previous study [16, 17], confirming that exposure to a sublethal dose of TBI induces chronic oxidative stress in HSCs. The increase in ROS production led to the induction of HSC senescence as they expressed an elevated level of p16 mRNA (Fig. 5C). Increased expression of p16 has been widely used as a senescence biomarker and it also functions as an important mediator of cellular senescence induction [24, 25]. Treatment with resveratrol abrogated TBI-induced increases in ROS production in HSCs and HPCs and significantly reduced HSC expression of p16 mRNA. This finding suggests that resveratrol ameliorates TBI-caused long-term BM suppression probably by inhibiting IR-induced chronic oxidative stress and senescence in HSCs.
Figure 5. Resveratrol inhibits TBI-induced increases in ROS production in HSCs and HPCs and p16 mRNA expression in HSCs.
Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as illustrated in Fig. 1A. They were euthanized 30 days after exposure to TBI to harvest BM-MNCs. The production of ROS in HPCs and HSCs and expression of p16 mRNA were analyzed. A. A representative analysis of ROS production in HPCs and HSCs by flow cytometry. B. The levels of intracellular ROS in HSCs and HPCs were presented as means ± SE of DCF MFI from three independent experiments. ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI. C. The levels of p16 mRNA expression in sorted HSCs were analyzed by qRT-PCR and are expressed as means ± SE of fold changes compared to that of control (N = 3). ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI.
Resveratrol moderates NOX4, SOD2, and GPX1 expression perturbed by TBI in HSCs and HPCs
Increased production of ROS by irradiated cells has been largely attributed to the dysfunction of mitochondria [26, 27]. SOD2 and GPX-1 are two primary oxidative stress defense enzymes in mitochondria. SOD2 convert superoxide radical (O2·−) into hydrogen peroxide (H2O2), whereas GPX1 along with catalase convert H2O2 into water. However, an increasing body of evidence demonstrates that cells can also produce ROS through activation and/or induction of NOXs [28, 29]. Our previous studies demonstrated that the induction of chronic oxidative stress in HSCs by TBI is probably attributable to the up-regulation of NOX4 [16]. To learn the mechanisms of action by which resveratrol inhibits IR-induced chronic oxidative stress in HSCs after TBI, we measured the expression of NOX4, SOD2 and GPX1 mRNA in HSCs and HPCs from irradiated mice treated with vehicle or resveratrol. As shown in Fig. 6A & B, TBI up-regulated the expression of NOX4 mRNA in HSCs and HPCs by 32.2% and 13.3% but down-regulated the expression of SOD2 and GPX1 mRNA in these cells to a variable degree compared with their respective controls. Administration of resveratrol not only reversed the effects of IR on the expression of NOX4, SOD2 and GPX1 mRNA in HSCs and HPCs, but in fact significantly suppressed the expression of NOX4 mRNA in these cells to a level that was even lower than that in control un-irradiated cells while increasing the expression of SOD2 and GPX1 mRNA. The modulation of SOD2 and GPX1 expression by TBI and resveratrol in BM hematopoietic cells was also confirmed by SOD2 and GPX1 enzymatic assays shown in Fig. 6C. These results suggest that resveratrol inhibits TBI-induced chronic oxidative stress in HSCs and HPCs at least in part via down-regulation of NOX4 expression and up-regulation of SOD2 and GPX1 expression.
Figure 6. Resveratrol inhibits TBI-induced expression of NOX4 and increases the expression and activity of SOD2 and GPX1 in hematopoietic cells.
Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as illustrated in Fig. 1A. They were euthanized 30 days after exposure to TBI to harvest BM-MNCs. HPCs and HSCs were isolated from these cells by cell sorting and analyzed for the expression of NOX4, SOD2, and GPX1 mRNA by qRT-PCR. Enzyme activity of SOD2 and GPX1 in BM-MNCs were analyzed using a SOD2 Assay Kit and a Cellular GPX1 Assay Kit, respectively. A. Expression of NOX4, SOD2, and GPX1 mRNA in HPCs. B. Expression of NOX4, SOD2, and GPX1 mRNA in HSCs. C. Enzyme activity of SOD2 and GPX1 in BM-MNCs. The levels of NOX4, SOD2, and GPX1 mRNA expression are expressed as means ± SE of fold changes compared to those of control (N = 3). ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI.
Resveratrol protects HSCs against IR in part via activation of Sirt1
Resveratrol is a putative activator of Sirt1 [9]. Sirt1 is a NAD+-dependent deacetylase that has many biological functions [8, 14, 15, 30]. Its activation can promote longevity in yeast and mammalian cells and protect cells from oxidative stress-induced damage in part via deacetylation of several transcriptional factors to regulate the expression of various genes including SOD2 [8]. To gain a better understanding of the mechanisms by which resveratrol protects HSCs from radiation injury, we investigated the effects of resveratrol treatment on Sirt1. As shown in Fig. 7A, TBI reduced the expression of Sirt1 mRNA in HSCs by 74.5%. This reduction was associated with a significant increase in the acetylation of p53, indicating that TBI also decreased Sirt1 deacetylase activity in HSCs (Fig. 7B). These effects of TBI on Sirt1 were abrogated by the treatment of resveratrol, suggesting that resveratrol may protect HSCs from IR in part via activation of Sirt1. To test this hypothesis, we first examined whether resveratrol can upregulate the expression of SOD2 in BM cells in a Sirt1-dependent manner, because SOD2 is one of the most important intracellular antioxidants that can protect cells from radiation injury. As shown in Fig. 7C, BMCs incubated with resveratrol exhibited a significant increase in SOD2 mRNA expression. The increase was completely inhibited by the addition of Ex527, a potent Sirt1 inhibitor that does not inhibit histone deacetylase (HDAC) or other members of the Sirt deacetylase family [31]. More importantly, we found that resveratrol could protect the clonogenic function of HSCs against IR and the protective effect was significantly attenuated by Ex527 (Fig. 7D). Collectively, these findings suggest that resveratrol protects HSCs from radiation at least in part via activation of Sirt1.
Figure 7. Resveratrol protects HSCs against IR in part via activation of Sirt1.
A. Mice were sham-irradiated as control or sublethally irradiated with 6.0 Gy TBI after receiving vehicle or resveratrol treatment in a similar manner as illustrated in Fig. 1A. They were euthanized 30 days after exposure to TBI to isolate HSCs by cell sorting. The levels of SIRT1 mRNA expression in irradiated HSCs are expressed as means ± SE (N = 3) of fold changes compared to those in unirradiated control HSCs. ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI. B. Analysis of immunostaining of acetylated p53 in HSCs from sham-irradiated or sublethally irradiated mice as described above. The left panel: Representative photomicrographs of DAPI nuclear staining and acetylated p53 immunostaining in isolated HSCs are shown. The right panel: the percentages of acetylated p53 positive HSCs are presented as mean ± SE (n = 3). ap<0.05 vs. control; bp<0.05 vs. vehicle + TBI. C. Resveratrol upregulates SOD2 mRNA expression in BM-MNCs in a Sirt1-dependent manner. BM-MNCs isolated from normal C57BL/6 mice were incubated with vehicle (control), 1 µM resveratrol (Res), or resveratrol (1 µM) plus Ex527 (10 µM) (Res+Ex527) at 37°C, 5% CO2, and 100% humidity for 24 h. The expression of SOD2 mRNA in these cells was analyzed by qRT-PCR and are expressed as means ± SE of fold changes compared to that of control (N = 3). ap<0.05 vs. control; bp<0.05 vs. Res. D. Resveratrol protects HSCs against IR in vitro in a Sirt1-dependent manner. The clonogenic function of single HSCs were determined after the cells were exposed to 4 Gy IR in the presence of vehicle, 1 µM resveratrol (Res), or resveratrol (1 µM) plus Ex527 (10 µM) (Res+Ex527) in comparison with that of unirradiated control HSCs (control). The results are presented as means ± SE of colonies per 20 wells or cells from three independent assays. ap<0.01 vs. control; bp<0.01 vs. vehicle + IR; cp<0.05 vs. Res + IR.
Discussion
In spite of an extensive use of resveratrol as a health care product to prevent and treat various human diseases [9, 10, 32, 33], the therapeutic potential of resveratrol as a radiation protectant or mitigator has not been well investigated. In this study, we examined if resveratrol can inhibit IR-induced BM toxicity in a TBI mouse model. Our results showed that treatment with resveratrol not only protected mice from IR-induced acute BM syndrome and lethality but also ameliorated TBI-induced long-term BM injury. The effects of resveratrol on IR-induced acute and long-term BM injury are likely attributed to its antioxidant properties. However, resveratrol is not a regular antioxidant that inhibits oxidative stress mainly by scavenging free radicals, because it can also regulate the redox of a cell by differentially affecting the expression of various oxidases and antioxidant enzymes [9, 34]. As shown in our study, we found that resveratrol treatment effectively inhibited TBI-induced chronic oxidative stress, p articularly in BM HSCs. This effect was associated with down-regulation of NOX4 and up-regulation of SOD2 and GPX1. Therefore, resveratrol may be more efficacious than other commonly used antioxidants as a radiation medical countermeasure, particularly considering that resveratrol is a natural product that is inexpensive and low in toxicity and has been widely used as a food supplement for various human health causes. The dose (20 mg/kg/day) of resveratrol used in our study is safely achievable in humans, because the dose of 100 mg/kg/day of resveratrol in mice is equivalent to 2 mg/kg/day in humans [35, 36] and a clinical study showed that resveratrol did not cause any toxicity or side effects in humans after they were given 25 mg to 5 g of resveratrol every day [37]. However, resveratrol can only functions as a radiation protectant to reduce TBI-induced lethality, because we found that post TBI treatment with resveratrol had no significant effect on the survival of mice exposed to a lethal dose of TBI (Supplementary figure 1).
The mechanisms by which resveratrol differentially regulates the expression of NOX4, SOD2 and GPX1 in HSCs to protect them from radiation injury have yet to be investigated. Resveratrol is a well-known putative activator of Sirt1 [8, 9]. It has been shown that resveratrol can extend lifespan of yeast, worms, Drosophila melanogaster and Caenorhabditis elegans [38, 39], and improve health and survival of high-calorie diet mice [40], in part by activation of Sirt1. Therefore, we examined whether Sirt1 plays a role in mediating the effects of resveratrol on HSCs. Our studies showed that TBI reduced the expression of Sirt1 mRNA in HSCs and the reduction was associated with a significant decrease of Sirt1 deacetylase activity. These effects of TBI on Sirt1 were abrogated by the treatm ent of resveratrol, suggesting that resveratrol may protect HSCs from IR in part via activation of Sirt1. This suggestion is supported by the findings that resveratrol can upregulate the expression of SOD2 in BM cells and protect HSCs from IR in a Sirt1-dependent manner in vitro.
As a NAD+-dependent histone deacetylase, Sirt1 can regulate gene expression by modulating the chromatins [8]. In addition, multiple non-histone targets have also been described for Sirt1. These include some transcription factors or cofactors such as FoxO3, nuclear factor κB (NF-κB), and peroxisome proliferator-activated receptor-co-activator 1a (PGC-1a) [15, 41]. Which of these factors and cofactors may be involved in the regulation of NOX4, GPX1 and SOD2 expression by resveratrol in HSCs reported in the present study have yet to be studied. In addition, nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that also plays an important role in regulation of the expression of several antioxidant enzymes [42]. Nrf-2 knockout animals are sensitive to oxidative stress and radiation-induced tissue injury [43]. Resveratrol is known to disrupt the Nrf-2/Keap1 interaction, which can activate Nrf-2 to induce of the expression of many antioxidants being proposed as protectants against IR [44, 45]. It is worthy to study the role of Nrf2 in resveratrol attenuating TBI-induced long-term BM injury. Finally, resveratrol has the ability to modulate insulin sensitivity, reduce the level of insulin-like growth factor-1 (IGF-I), increase AMP-activated protein kinase (AMPK) and PGC-1a activity [40, 46], all of which could impact on HSC self-renewal by regulating HSC senescence. It will be interesting to determine whether these activities of resveratrol play a role in inhibition of TBI-induced HSC senescence and long-term BM injury.
It has been well established that increase in ROS production is the underlying mechanism whereby IR causes genetic instability [17] that can lead to the induction of leukemia and cancer. Since resveratrol can protect hematopoietic stem and progenitor cells (HSPCs) from radiation injury in part by inhibiting IR-induced oxidative stress, it is unlikely that the treatment with resveratrol will increase the risk of IR-induced secondary malignancies. In fact, resveratrol has been considered as one of the most potent natural antioxidants for cancer prevention including gastric, colorectal, lung, breast, prostate, esophageal, and thyroid carcinomas [32]. Therefore, treatment of mice with resveratrol may provide dual benefits again IR, e.g. protecting HSPCs while inhibiting IR-induced genomic instability and malignancies.
In addition, exposure to IR not only induces long-term BM injury but also causes late tissue damages such as lung fibrosis and enteropathy [47–50]. It has been shown that chronic oxidative stress is also an underlying cause by which IR produces the late tissue damages. Therefore, it will be also interesting to examine whether resveratrol may have the potential to be used as a therapeutic for other IR-induced late tissue damages.
Supplementary Material
Research Highlight.
Resveratrol can ameliorate irradiation (IR)-induced long-term bone marrow injury.
Activation of Sirt1 by resveratrol plays a role in protecting HSCs from IR.
These effects are likely attributed to the inhibition of IR-induced oxidative stress.
Inhibition of oxidative stress suppresses IR-induced HSC senescence.
Acknowledgments
This study was supported by National Program on Key Basic Research Project (973 Program, 2011CB964800-G), National Natural Science Foundation of China (No 81129020 & 81072237), Tianjin Natural Science Foundation (11JCZDJC19100), and National Institutes of Health of United States (R01AI080421 and R01CA122023). We thanks for the excellent work of Song Huang and Zhubo Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meng A, Wang Y, Brown SA, Van Zant G, Zhou D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol. 2003;31:1348–1356. doi: 10.1016/j.exphem.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng A, Wang Y, Van Zant G, Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res. 2003;63:5414–5419. [PubMed] [Google Scholar]
- 4.Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5:101–110. [PubMed] [Google Scholar]
- 5.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature medicine. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell stem cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 11.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovascular drug reviews. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 12.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- 13.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 14.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 15.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Wang Y, Pazhanisamy SK, Shao L, Batinic-Haberle I, Meng A, Zhou D. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med. 2011;51:30–37. doi: 10.1016/j.freeradbiomed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Li J, Wang YY, Meng AM, Liu Q, Wang L, Chen FH, Wang XC, Zhai ZB, Fu Y, Wang Q. Retinoblastoma 94 enhances radiation treatment of esophageal squamous cell carcinoma in vitro and in vivo. Journal of radiation research. 2012;53:117–124. doi: 10.1269/jrr.11051. [DOI] [PubMed] [Google Scholar]
- 19.Ema H, Morita Y, Yamazaki S, Matsubara A, Seita J, Tadokoro Y, Kondo H, Takano H, Nakauchi H. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nature protocols. 2006;1:2979–2987. doi: 10.1038/nprot.2006.447. [DOI] [PubMed] [Google Scholar]
- 20.Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, Deeg HJ. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. International journal of radiation oncology, biology, physics. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP, Zhang L, Yu J, Zambetti GP, Cheng T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun. 2008;372:254–259. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Mokni M, Elkahoui S, Limam F, Amri M, Aouani E. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem Res. 2007;32:981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- 24.Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 27.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer metastasis reviews. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 28.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 29.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napper AD, Hixon J, McDonagh T, Keavey K, Pons JF, Barker J, Yau WT, Amouzegh P, Flegg A, Hamelin E, Thomas RJ, Kates M, Jones S, Navia MA, Saunders JO, DiStefano PS, Curtis R. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. Journal of medicinal chemistry. 2005;48:8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- 32.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carsten RE, Bachand AM, Bailey SM, Ullrich RL. Resveratrol reduces radiation-induced chromosome aberration frequencies in mouse bone marrow cells. Radiation research. 2008;169:633–638. doi: 10.1667/RR1190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. International immunopharmacology. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Timmer S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging. 2012 doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mechanisms of ageing and development. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mechanisms of ageing and development. 2010;131:261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vakhrusheva O, Braeuer D, Liu Z, Braun T, Bober E. Sirt7-dependent inhibition of cell growth and proliferation might be instrumental to mediate tissue integrity during aging. J Physiol Pharmacol. 2008;59(Suppl 9):201–212. [PubMed] [Google Scholar]
- 42.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, Freeman ML. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med. 2011;51:1175–1183. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, Wang L, Szklarz G, Bi Y, Ma Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the Nrf2 pathway. The Journal of pharmacology and experimental therapeutics. 2012 doi: 10.1124/jpet.112.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochimica et biophysica acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Meng A, Lang H, Brown SA, Konopa JL, Kindy MS, Schmiedt RA, Thompson JS, Zhou D. Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res. 2004;64:6240–6246. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- 48.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. British journal of cancer. 1998;78:993–1003. doi: 10.1038/bjc.1998.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22:37–47. discussion 52-33. [PubMed] [Google Scholar]
- 50.Terasaki Y, Ohsawa I, Terasaki M, Takahashi M, Kunugi S, Dedong K, Urushiyama H, Amenomori S, Kaneko-Togashi M, Kuwahara N, Ishikawa A, Kamimura N, Ohta S, Fukuda Y. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. American journal of physiology. Lung cellular and molecular physiology. 2011;301:L415–L426. doi: 10.1152/ajplung.00008.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.