Supplemental Digital Content is available in the text.
Keywords: influenza, infant, cohort studies, pneumonia, hospitalization
Abstract
Background:
Influenza is a major public health problem worldwide; however, relatively little is known about influenza in tropical regions, especially for infants. Additional information is required to inform public health policy making, in particular vaccination guidelines.
Methods:
Between September 2011 and July 2013, we enrolled newborns into the Nicaraguan Birth Cohort Study. Infants were provided primary medical care and actively followed for reverse-transcription polymerase chain reaction–confirmed influenza virus infection when presenting with influenza-like illness or undifferentiated fever. This report presents data pertaining to the first year of life.
Results:
Of the 518 children enrolled in the study, 441 participated throughout their first year of life, 71 were withdrawn, and 6 died. Overall, 13% of the participants experienced at least 1 laboratory-confirmed influenza virus infection. The overall incidence of influenza was 15.5 cases per 100 person-years [95% confidence interval (CI): 12.2–19.5]. Infants aged 6–11 months experienced significantly higher rates of laboratory-confirmed influenza than infants aged 0–5 months (incidence rate ratio: 2.1; 95% CI: 1.3–3.4). The overall incidence of pneumonia was 52.6 cases per 100 person-years (95% CI: 46.3–59.6). Three percent of the pneumonia cases were influenza associated, and the incidence of influenza-associated pneumonia and hospitalization was 1.7 (95% CI: 0.9–3.5) and 0.22 (95% CI: 0.03–1.55) cases per 100 person-years, respectively.
Conclusions:
We found a significant burden of influenza and influenza-associated severe respiratory outcomes in infants. Our results support the need to explore the potential value of vaccinating pregnant women and infants aged >6 months, as recommended by the World Health Organization in 2012.
Influenza is a cause of significant morbidity in children and infants. Infants have the highest rates of hospitalization among all children1–5; however, data on influenza infection rates in the first year of life are relatively limited, especially from developing countries where the burden of pneumonia is the highest.6 In 2012, the Strategic Advisory Group of Experts (SAGE) on Immunization of the World Health Organization (WHO) updated their position on influenza vaccination, identifying pregnant women as the highest priority group and children aged 6–23 months as a priority risk group.7 In order to prioritize both the purchase and the distribution of influenza vaccine, developing countries need more data on the incidence of influenza illness in high-risk groups, particularly infants.
In the Americas, influenza vaccination coverage has been rapidly expanded since 2004.8 In December 2014, the WHO Technical Burden of Disease Working group established that there are insufficient data about the influenza incidence among very young children and that more evidence is needed to bolster SAGE recommendations.7 Data on the burden of influenza in young children in Latin America are limited. A study we performed in Nicaraguan children aged 2–14 years showed high attack rates for pandemic H1N1 influenza as well as seasonal influenza A and B.9 A study in Guatemala identified children aged 6 months to 5 years as a priority group for vaccination for pandemic influenza in 2009.10 A study in El Salvador found on average 3.2 influenza-associated severe pneumonia cases per 1000 person-years in children >5 years of age over multiple influenza seasons.11 Thus, data are needed on influenza particularly in infants <12 months of age.
In 2011, we established the Nicaraguan Influenza Birth Cohort Study to characterize the burden of influenza in children aged 0 to 2 years in Managua, Nicaragua, to examine the risk factors for influenza virus infection and disease severity and to investigate sequential influenza virus infections. This report focuses on the burden of influenza in the first year of life.
MATERIALS AND METHODS
Ethics Statement
This study was conducted in collaboration between the Sustainable Sciences Institute, the Nicaraguan Ministry of Health, the University of California, Berkeley (UCB), the University of Michigan, Ann Arbor and the US Centers for Disease Control and Prevention (CDC). The study was approved by the Institutional Review Boards (IRBs) of the Nicaraguan Ministry of Health, University of Michigan and UCB. The CDC’s IRB relied on the UCB IRB for approval. Written informed consent was obtained from a parent/guardian of all participants.
Study Site and Population
The Nicaraguan Influenza Birth Cohort Study is an ongoing prospective cohort study based at the Health Center Socrates Flores Vivas (HCSFV), a primary health care facility run by the Nicaraguan Ministry of Health. The HCSFV serves a population of ≈61,500, in the low-to-middle income District II of the city of Managua. For inclusion in the study, a participant must (1) have been ≤4 weeks of age at enrollment, (2) lived in the HCSFV catchment area, (3) had no plan to leave the study area in the next 2 years and (4) have been willing to attend the HCSFV for all medical needs. Infants with immunocompromising conditions and premature infants who required ≥4 weeks of hospitalized care directly after birth were excluded. Infants were recruited when parents/guardians brought them to their first well-baby appointment or through house visits. Participants were enrolled in the study until the age of 2 years, unless they voluntarily withdrawn before reaching that age. Recruitment into the study began in September 2011. Every month, ≈20–24 children were enrolled.
Enrollment, Medical Care and Annual Surveys
At enrollment, socioeconomic, risk factor and breastfeeding questionnaires were administered by study personnel. Participants’ health status was followed through parent self-reporting on a standardized symptom diary and medical visits at the HCSFV. Parents/guardians were instructed to fill out a simple daily symptom diary for the infant collecting information on the presence of fever, cough and runny nose. Diaries were collected weekly from the participant’s home by a study field worker who was instructed to refer participants with current symptoms to the HCSFV. Parents/guardians were encouraged to seek medical care for all participant illnesses at the HCSFV and to present early in case of a febrile illness. Infants were identified as study participants at chart reception and routed to the study area of the HCSFV. Study physicians were available to all participants 24 hours/day, 7 days/week and information pertaining to clinical visits was systematically recorded on a standardized case report form. Children requiring hospitalization were transferred to a hospital by study staff.
Every year in March/April, length and weight of healthy infants were measured and questionnaires completed at enrollment were readministered by study staff to examine risk factors for influenza virus infection and outcome. A blood sample of 3 mL was collected from infants aged 6 months or older in March/April and banked for future analysis.
Respiratory Sampling and Case Follow-Up
Respiratory samples were collected from children presenting with (1) influenza-like illness (ILI), defined as fever (≥37.8°C) or history of fever and rhinorrhea and/or cough12; (2) fever or history of fever without a defined focus or (3) severe respiratory symptoms (stridor, nasal flaring, wheezing, chest indrawing and/or central cyanosis) regardless of the presence of fever. Participants identified >96 hours after symptom onset were not sampled. Study staff obtained oropharyngeal swabs from infants <6 months as this is the least invasive sampling method. Combined nasal and oropharyngeal swabs were obtained from older infants. After sample collection and depending on the severity of the illness, participants were asked to return to the HCSFV for at least 1 follow-up visit. Daily occurrence of fever and respiratory symptoms, as observed by the physician or as reported by the parent, were recorded in a separate form. Pneumonia and severe pneumonia were clinically assessed using the WHO’s Integrated Management of Childhood Illnesses guidelines.13
Influenza Virus Detection
Swab specimens were placed in viral transport media, stored at 4°C, and transported within 72 hours to the National Virology Laboratory at the National Center for Diagnosis and Reference of the Nicaraguan Ministry of Health. RNA was extracted (QIAamp Viral RNA Mini Kit, Qiagen) and tested by real-time reverse-transcription polymerase chain reaction (RT-PCR) for influenza A and B, using primers, probes and protocols supplied by the CDC. Samples positive for influenza A, were subtyped for seasonal influenza A H1N1pmd09 and H3N2, and lineage-specific PCR for both influenza B Victoria and Yamagata were run for influenza B–positive samples.14
Statistical Analysis
Follow-up time was calculated as the time between enrollment and the infant’s first birthday or withdrawal from the study. For those lost to follow-up, person-years were calculated as the time between enrollment and last contact with study personnel, plus one-half the time between the last contact and the date recorded as lost to follow-up. A Poisson distribution was used to calculate 95% CIs for incidence rates. Incidence rate ratios (IRRs) were calculated using generalized estimating equations with a Poisson distribution and robust standard errors. Statistical analyses were performed in STATA 13 (StataCorp LP).
RESULTS
The Nicaraguan Influenza Birth Cohort Study
Enrollment began on September 8, 2011 and continued through July 15, 2013 with ≈20 newborns, aged 4 weeks or less, enrolled monthly. Most (97%) of the parents/guardians were invited to participate in the study agreed to enroll their children.
This report focuses on the first year of life. All infants born on or before July 15, 2013 were included in this analysis (n = 518). A total of 441 (85%) infants were followed until their first birthday, whereas 77 (15%) were withdrawn. Forty-six families moved outside of the study area and were lost-to-follow-up. Six infants died while enrolled in the study, 5 with severe pneumonia that was accompanied with heart and/or lung congenital malformations in 2 of the 5 deaths. On average, the time of participation in the study was 0.9 years (10.5 months) per child. Fifty-eight (11%) women were vaccinated against influenza during pregnancy (Table 1). A total of 23 (4%) children were vaccinated between 6 months and 1 year of age.
Table 1.
Characteristics of study participants at enrollment

A total of 23,654 symptom diaries from 517 (99.8%) of the 518 study infants were collected by field workers, with most (96%) collected in the week after their completion. The average number of medical visits per participant was 13 (range: 0–50), and 504 (97%) participants attended at least 1 medical visit. The total number of medical visits attended was 6552 with 4577 (70%) initial visits (ie, medical visits for a new illness) and 1975 (30%) follow-up visits.
ILI and Respiratory Sample Collection
A total of 1286 ILI were identified using the symptoms diaries completed by the parent/guardians of study participants and 1053 ILI events were medically attended. Three hundred ninety-eight (77%) participants experienced at least 1 medically attended ILI event. The average number of medically attended ILI events per infant was 2.0 (range: 0–12). The incidence of medically attended ILI was 229.6 cases per 100 person-years and was similar in both genders (Table 2). The medically attended ILI incidence was higher in infants aged ≥6 months when compared with younger infants (Table 2). Of the 1053 medically attended ILI cases, 1021 presented in the first 96 hours from symptom onset and thus met the testing definition. Of these, 1019 (99.8%) had a respiratory sample collected immediately after presentation. Moreover, an additional 165 samples were collected from infants presenting with fever without a defined focus and 29 from infants with severe respiratory symptoms in the absence of fever.
Table 2.
Incidence of ILI, laboratory-confirmed influenza and influenza-associated pneumonia*

Incidence of Influenza
Of the 1213 collected samples, 74 (6%) tested positive for influenza by RT-PCR. Three infants had 2 respiratory samples that tested positive for the same influenza virus type/subtype within 14 days and were considered a single influenza infection, leaving a total of 71 influenza positive cases. Of the 518 infants, 68 (13%) tested positive at least once, 3 children experienced 2 infections. Of the influenza cases (n=71), 49 were attributed to influenza A and 22 to influenza B. Of the 49 influenza A cases, 12 were H1N1pdm09 and 37 were H3N2. All influenza B cases tested were positive for the Victoria lineage. Influenza occurred predominantly during June to November in 2012 and June to October in 2013 (Figure 1).
FIGURE 1.

Incidence of ILI and influenza by study month. Monthly incidence of ILI and laboratory-confirmed influenza.
Seven (10%) influenza cases did not meet the ILI definition and presented with fever without a defined focus. No infant with severe respiratory symptoms in the absence of fever tested positive for influenza. Three (0.6%) participants experienced 2 influenza infections during their first year of life. Two participants exhibited 2 influenza A infections (in both cases, 1 H1N1pdm09 and 1 H3N2), and 1 experienced an influenza A H1N1pdm09 infection and an influenza B infection. The mean number of days between infections was 49 (range: 24–268).
The incidence of laboratory-confirmed influenza illness among children meeting the testing definition was 15.5 cases per 100 person-years (95% CI: 12.2–19.5) (Table 2). Consistent with the ILI data, influenza incidence was higher in children aged 6 months or older. This increased incidence in infants aged 6 months or older compared with younger infants was significant for both influenza A (IRR: 1.87, 95% CI: 1.04–3.37) and influenza B (IRR: 2.65, 95% CI: 1.04–6.78).
Clinical Presentation of ILI and Influenza
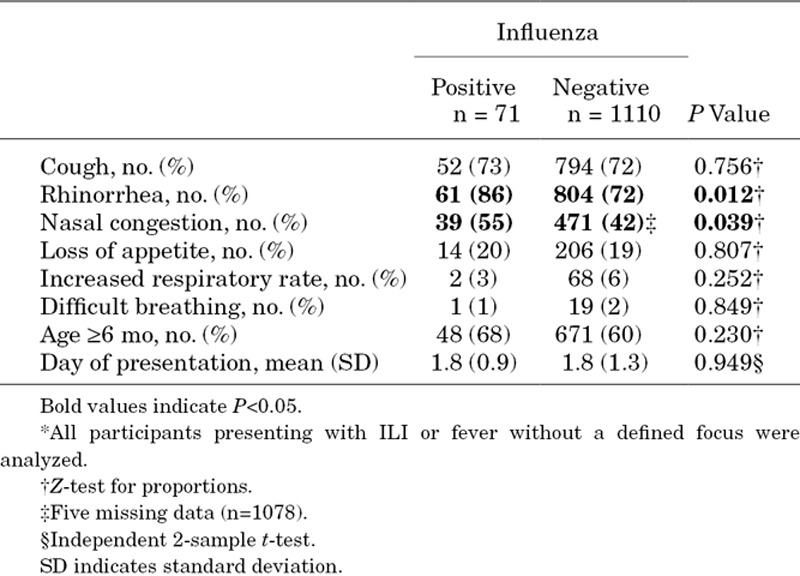
We then compared influenza-positive and influenza-negative cases in terms of clinical signs at presentation. For this analysis, participants who were sampled because they presented with severe respiratory symptoms in the absence of fever were excluded (n=29). The proportion of children who presented with rhinorrhea and nasal congestion was significantly higher in influenza-positive cases (Table 3). On the contrary, the proportion of children with cough, increased respiratory rate, difficulty in breathing or loss of appetite was not different in influenza-positive versus influenza-negative cases (Table 3). Clinical symptoms throughout the episodes were also compared using the daily information collected during the initial and follow-up medical consults in the follow-up forms. Follow-up consults were attended by 763 (63%) of the participants who were sampled. The number of days with clinical symptoms was not different in influenza-positive versus influenza-negative cases (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/C290).
Table 3.
Symptoms at presentation in influenza-positive versus influenza-negative cases*
Severe Manifestations of Influenza Virus Infection
We examined the contribution of influenza virus infection to severe respiratory outcomes. A total of 241 pneumonia cases were identified in the study, yielding an incidence of 52.6 cases per 100 person-years (95% CI: 46.3–59.6). Eight (3%) had a positive influenza test at presentation or in the 28 days before onset of pneumonia and were considered influenza associated. These 8 influenza-associated pneumonia cases were distributed as follows: 4 presented with an initial diagnosis of pneumonia and were influenza positive at presentation (time between symptom onset and presentation: 1 day for all 4 cases); 2 presented with ILI, tested positive for influenza, and developed pneumonia during follow-up (time between ILI and pneumonia onset for both cases: 3 days) and 2 presented with ILI, tested positive for influenza, and developed pneumonia after the initial illness resolved (time between ILI and pneumonia onset: 16 and 18 days). Furthermore, 1 of 60 (1.7%) severe pneumonia and 1 of 81 (1.2%) hospitalizations for respiratory causes were influenza associated. The influenza-associated hospitalization was because of severe pneumonia. The incidence of influenza-associated pneumonia and hospitalization was 1.7 (95% CI: 0.9–3.5) and 0.22 (95% CI: 0.03–1.55) cases per 100 person-years, respectively (Table 2). None of the 6 infants who died during the course of the study had an influenza infection in the month before their death.
DISCUSSION
We actively followed a cohort of infants through their first birthday in order to ascertain clinical influenza infection rates. Infants in our cohort had a substantive risk of developing influenza illness: 13% experienced one or more laboratory-confirmed influenza illnesses. One in 65 also developed laboratory-confirmed influenza illness associated with pneumonia. In our study, infants aged 6 months and older had a higher incidence of both influenza A and B, than younger children.
The incidence of laboratory-confirmed influenza among children with ILI or fever without a defined focus seeking care in our study [ie, 15.5 cases per 100 person-years (95% CI: 12.2–19.5)] is similar to or higher than what has been found in other studies both within the region and worldwide. A study of influenza illness among infants in Bangladesh found a rate of 10.2 influenza infections per 100 person-year through community-based surveillance of children <5 years.15 A second study in Bangladesh observed 6 (95% CI: 3–12) and 20 (95% CI: 14–28) influenza illnesses per 100 person-years in children below 6 months of age and infants aged 6 to 12 months, respectively.16 In the US, rates of medically attended influenza illness in infants have ranged from 2.8 (95% CI: 0.7–11.1) to 5.9 (95% CI: 2.8–12.8) cases per 100 person-years in infants <6 months, and from 5.2 (95% CI: 3.0–9.0) to 12.5 (95% CI: 8.7–17.9) cases per 100 person-years in children aged 6 to 23 months.17 In the placebo arm of a randomized-controlled trial in Bangladesh, an incidence of 22.1 influenza cases per 100 person-years (95% CI: 13.5–36.0) was observed in infants between 0 and 24 weeks of age.18 In a similar study in South Africa, an influenza attack rate of 3.6% (95% CI: 2.6–5.0) was calculated in the same age group.19 Differences in influenza rates may be because of differences in case definitions, respiratory sampling algorithms, laboratory assays among other study design issues, as well as yearly variation in influenza virus circulation and transmission.
Importantly, 3% of clinical pneumonia cases in our study, as defined by Integrated Management of Childhood Illnesses guidelines,13 were attributable to influenza infection. Lower proportions of severe pneumonia (1.7%) and hospitalization for respiratory causes (1.2%) were attributable to influenza infection, and we did not document any influenza-associated deaths. Overall, our rate of influenza-associated hospitalizations (0.22 per 100 person-years; 95% CI: 0.03–1.55) was similar to other studies of children aged 5 years and younger, for example, 0.12 in Oregon20; 0.12 in Kiel, Germany21; and 0.09 (95% CI: 0.08–0.11) in 3 counties in the US.17 To estimate the actual burden of influenza-associated hospitalizations, our study was based in the community and tested infants for influenza virus infections before hospitalization, which should result in a more accurate estimate of the hospitalization rates than studies of hospitalized influenza, which typically test for influenza after the child has been hospitalized and may no longer be shedding influenza virus. Also, our study was restricted to infants, who are at higher risk for influenza-associated hospitalization. In fact, a study reported influenza-associated estimated excess rates of hospitalization during periods of influenza circulation to be 1.34 (95% CI: 0.88–1.80) and 1.03 (95% CI: 0.47–1.58) in California and Seattle, respectively.22 Finally, regional differences probably contributed to the observed differential risk of influenza-associated hospitalization.23,24
Since 2012, the SAGE on Immunization of the WHO has recommended that pregnant women be the top priority group and that young children be a priority for influenza vaccination in the community.7 In Nicaragua, pregnant women and children aged 6–23 months are prioritized during vaccination campaigns. We found that ≈1 in 10 pregnant women in our study population were vaccinated against influenza during their pregnancy. Infant vaccination rates were particularly low in our study population with only 1 infant in 20 receiving the influenza vaccine. These low vaccination rates could be because of the limited availability of the vaccine in the country. More research is needed on the cost effectiveness of the vaccination among pregnant women and in young infants to inform vaccination policy.
One limitation of our study is that it was conducted in a single location during 2 years. Influenza incidence is known to vary by year and location, so longer term follow-up in several locations is ideal. In addition, differences in our age-specific respiratory sampling protocol (ie, oropharyngeal swab only in infants aged below 6 months) may have contributed to the difference in incidence observed between the 2 age groups. However, other studies without differences in sample type have found similar results.16,17 Finally, we limited testing to 96 hours postsymptom onset, as some infants do shed virus for longer, we may have underestimated the true incidence.
Strengths of our study include active surveillance of infants, high study acceptance in the community, high compliance with study procedures and a broad testing definition, which includes not only ILI but also fever without a defined focus and afebrile cases with severe respiratory disease. Importantly, 10% of the influenza virus infections detected in the study occurred in infants presenting with fever without a defined focus, showing a clear benefit of our testing strategy to enable more complete estimates of influenza incidence. However, our testing definition did not include afebrile infants presenting with mild respiratory symptoms.
ACKNOWLEDGMENTS
The authors would like to thank the study staff at the Health HCSFV and the Centro Nacional de Diagnóstico y Referencia for conducting the study, and Jorge Jara for helpful conversations, and the infants and their families for participating in the study.
Supplementary Material
Footnotes
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the CDC or the institutions with which the authors are affiliated. This work was supported by the US CDC (Cooperative Agreement 1U01GH000028-04); and the National Institutes of Health, Fogarty International Center (K02 TW009483 to A.G.). The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118:2409–2417. doi: 10.1542/peds.2006-1475. [DOI] [PubMed] [Google Scholar]
- 2.Chiu SS, Lau YL, Chan KH, et al. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 3.Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009-2012. PLoS One. 2014;9:e105543. doi: 10.1371/journal.pone.0105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heikkinen T, Silvennoinen H, Peltola V, et al. Burden of influenza in children in the community. J Infect Dis. 2004;190:1369–1373. doi: 10.1086/424527. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Mellen BG, Wright PF, et al. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 6.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76. [PubMed] [Google Scholar]
- 8.Ropero-Alvarez AM, Kurtis HJ, Danovaro-Holliday MC, et al. Expansion of seasonal influenza vaccination in the Americas. BMC Public Health. 2009;9:361. doi: 10.1186/1471-2458-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon A, Saborío S, Videa E, et al. Clinical attack rate and presentation of pandemic H1N1 influenza versus seasonal influenza A and B in a pediatric cohort in Nicaragua. Clin Infect Dis. 2010;50:1462–1467. doi: 10.1086/652647. [DOI] [PubMed] [Google Scholar]
- 10.Reyes L, Arvelo W, Estevez A, et al. Population-based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala, 2009. Influenza Other Respir Viruses. 2010;4:129–140. doi: 10.1111/j.1750-2659.2010.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clara W, Armero J, Rodriguez D, et al. Estimated incidence of influenza-virus-associated severe pneumonia in children in El Salvador, 2008-2010. Bull World Health Organ. 2012;90:756–763. doi: 10.2471/BLT.11.098202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PAHO-Centers for Disease Control and Prevention Generic Protocol for Influenza Surveillance. 2006 [cited 2014 Feb 24]. http://new.paho.org/hq/images/stories/AD/HSD/CD/INFLUENZA/flu-snl-gpis.pdf.
- 13.World Health Organization. Handbook: IMCI integrated management of childhood illness. 2005. [Google Scholar]
- 14.Centers for Disease Control and Prevention protocol of realtime RTPCR for swine influenza A(H1N1) 2009 [cited 2014 Feb 24]. http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf.
- 15.Brooks WA, Goswami D, Rahman M, et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010;29:216–221. doi: 10.1097/INF.0b013e3181bc23fd. [DOI] [PubMed] [Google Scholar]
- 16.Homaira N, Luby SP, Petri WA, et al. Incidence of respiratory virus-associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009-2011. PLoS One. 2012;7:e32056. doi: 10.1371/journal.pone.0032056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poehling KA, Edwards KM, Weinberg GA, et al. New Vaccine Surveillance Network. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 18.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 19.Madhi SA, Cutland CL, Kuwanda L, et al. Maternal Flu Trial (Matflu) Team. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371:918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 20.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. Am J Public Health. 1982;72:1008–1016. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigl JA, Puppe W, Schmitt HJ. The incidence of influenza-associated hospitalizations in children in Germany. Epidemiol Infect. 2002;129:525–533. doi: 10.1017/s0950268802007707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 23.Nair H, Simões EA, Rudan I, et al. Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]


