Abstract
Antibody-mediated depletion of neutrophils is commonly used to study neutropenia. However, the mechanisms by which antibodies deplete neutrophils have not been well defined. We noticed that mice deficient in complement and macrophages had blunted neutrophil depletion in response to anti-Ly6G monoclonal antibody (MAb) treatment. In vitro, exposure of murine neutrophils to anti-Ly6G MAb in the presence of plasma did not result in significant depletion of cells, either in the presence or absence of complement. In vivo, anti-Ly6G-mediated neutrophil depletion was abrogated following macrophage depletion, but not complement depletion, indicating a requirement for macrophages to induce neutropenia by this method. These results inform the use and limitations of anti-Ly6G antibody as an experimental tool for depleting neutrophils in various immunological settings.
Abbreviations: MAb, monoclonal antibody; CVF, cobra venom factor; LC, liposomal clodronate
Keywords: Neutrophils, Depletion, Ly6G, Antibody, Macrophages
Highlights
-
•
In vivo depletion of mouse neutrophils by anti-Ly6G antibody requires macrophages.
-
•
Plasma is not sufficient for anti-Ly6G-mediated neutrophil depletion in vitro.
-
•
Anti-Ly6G depletes neutrophils in mice without active complement.
1. Introduction
Murine models of neutropenia are essential to dissect the role of neutrophils in pathogenesis of infections in immunocompromised hosts, as well as in various auto-immune and inflammatory disorders. The primary means of inducing neutropenia for in vivo models include administration of cytotoxic chemotherapy agents, such as cyclophosphamide, or depletion of neutrophils by antibody. Cytotoxic chemotherapy is more physiological and representative of clinical neutropenia. However, such chemotherapy is non-specific for neutrophils, and is cytotoxic to other granulocytes as well as other cells types that undergo rapid turn-over, such as gastrointestinal mucosa. Hence, antibody-mediated depletion is more useful to specifically dissect the role of neutrophils in disease, without the off-target effects on other tissues mediated by myeloablative chemotherapy.
The most commonly used, specific antibody for neutrophil depletion in rodent models is anti-Ly6G, with numerous publications describing its use in the last decade [1], [11], [12], [5], [7], [9], [10]. These studies have predominantly focused on antibody clone 1A8, since this antibody specifically target neutrophils, as compared to clone RB6-8C5, which additionally cross-reacts with Ly6C, a receptor also present on other cell types including dendritic cells, macrophages, monocytes, and lymphocytes [6]. Recently, Bucher et al. reported that the interaction of anti-Ly6G antibodies with neutrophils in vitro and in vivo could be impacted by distinct fluorophores coupled to the antibody [3]. Their work suggested that macrophage phagocytosis of neutrophils was a central mechanism in anti-Ly6G-induced neutrophil depletion. We recently studied depletion of innate immune effectors during a murine model of bloodstream infection caused by the highly antibiotic-resistant Gram-negative pathogen, Acinetobacter baumannii [2]. When combination depletion experiments were performed, we found evidence of antagonism of the anti-Ly6G antibody at depleting neutrophils. We therefore sought to elucidate the mechanisms by which the 1A8 clone anti-Ly6G antibody specifically depletes neutrophils in vivo.
2. Materials and methods
2.1. Mouse strains
Wild type C3HeB/FeJ or C57BL/6J mice (Jackson Laboratories) between the ages of 8–11 weeks were used for in vivo experiments. All animal experiments were approved by the Institutional Committee on the Use and Care of Animals at the University of Southern California, Keck School of Medicine, following the National Institutes of Health guidelines for animal housing and care.
2.2. Depletion reagents
Mice were injected intraperitoneally with 20 µg of Cobra Venom Factor (Complement Technology, Inc.) in a volume of 100 µL PBS to functionally deplete complement activity [4], or intraperitoneally with 200 µL of a 5 mg/mL stock of Liposomal Clodronate (purchased from ClodronateLiposomes.org, Amsterdam, The Netherlands) to deplete macrophages [8]. Macrophage depletion was confirmed in a small number of control LC-treated animals by harvesting their spleens two days following LC treatment, and evaluating the number of adherent macrophages compared to control mice. To deplete neutrophils, the rat anti-mouse Ly6G monoclonal antibody, clone 1A8 (BioXCell), was injected intraperitoneally at a dose of 170 µg per mouse (in 300 µL), either immediately or two days after the macrophage/complement depletions. For mice that did not receive all 3 reagents, an equivalent amount of PBS was administered in lieu of the reagents not given.
2.3. Isolating neutrophils for in vitro analysis
Neutrophils for in vitro assays were isolated from the bone marrow of mice. Mice were euthanized, femurs and tibias were carefully removed and flushed using a 25-gauge needle filled with RPMI+2mM EDTA, and cells were passed through a 70 µm strainer to remove tissue. Red blood cells were hypotonically lysed by adding 20 mL of 0.2% NaCl for 20 s, followed by the immediate addition of 20 mL of 1.6% NaCl to stop lysis, followed by rinsing. Neutrophils were then isolated by density gradient centrifugation using 3 mL of Histopaque 1119 (Sigma Aldrich) overlaid with 3 mL of Histopaque 1077 (Sigma Aldrich). The bone marrow cells were gently laid over the Histopaque gradient and centrifuged at 2000 RPM at 25 °C for 30 min at low brake setting. Neutrophils were collected at the interface between the two histopaque layers and washed twice with cold RPMI.
2.4. In vitro neutrophil depletion assay
Prior to the assay, isolated neutrophils were stained with DAPI for 30 min at 37 °C in the dark, and subsequently washed twice with RPMI. Neutrophils were resuspended in RPMI containing 15% freshly isolated mouse plasma (previously heat-inactivated or not), and 106 cells were added to each well, in the presence or absence of anti-Ly6G antibody (final concentration of 25 µg/mL). After 2 h incubation at 37 °C, media in each well was gently removed and transferred to Eppendorf tubes. Removed cells were centrifuged at 1000g for 5 min and washed 1x in PBS. Cells were resuspended in 500 µL PBS and 50 µL of BD Liquid Counting Beads were added to each suspension, before transferring to FACS tubes for quantitation by flow cytometry.
2.5. Statistical analysis
Results were compared with the student׳s T test. P values<0.05 were considered significant.
3. Results and discussion
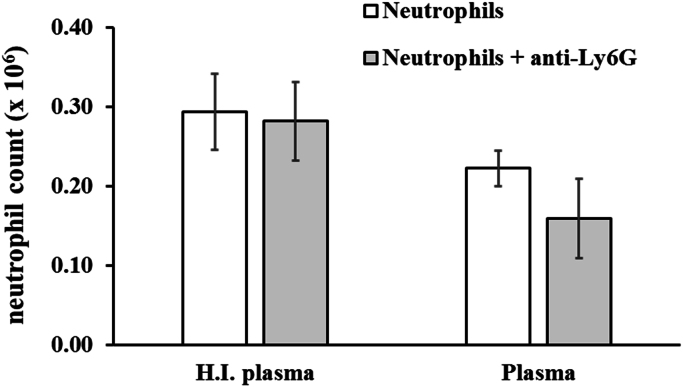
To determine how anti-Ly6G antibody mediates the depletion of neutrophils, mouse neutrophils were freshly isolated from bone marrow and stained with DAPI for detection by flow cytometry. Neutrophils were incubated for 2 h at 37 °C with mouse plasma, either heat-inactivated or not, and anti-Ly6G MAb, to assess the role of complement in neutrophil depletion. Addition of anti-Ly6G MAb did not cause a significant reduction in the numbers of neutrophils recovered from each well, irrespective of whether functional complement was present (Fig. 1). These results suggested that factors in addition to serum complement are necessary for antibody-mediated depletion of neutrophils.
Fig. 1.
Addition of anti-Ly6G to neutrophils in vitro in the presence of plasma does not result in significant cell depletion. Mouse neutrophils purified from mouse bone marrow were stained with DAPI, and incubated with anti-Ly6G in the presence of heat-inactivated (H.I.) or complement-active mouse plasma for 2 h in 12-well plates. Supernatants from each well were gently removed and neutrophils quantitated by flow cytometry. Results shown are mean number of neutrophils recovered in three independent experiments; error bars denote standard deviations.
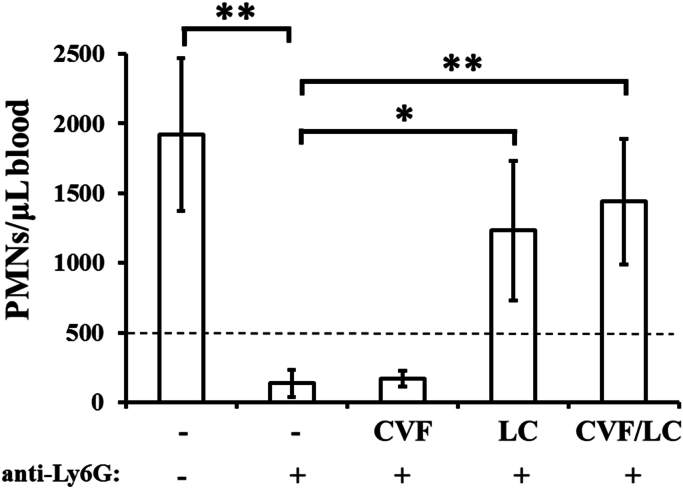
We next sought to identify the in vivo requirements for effective depletion of neutrophils by anti-Ly6G MAb administration. We employed two reagents to deplete specific effector components in vivo: liposomal clodronate (LC) for depleting macrophages, and cobra venom factor (CVF) for depleting complement. We individually depleted mice of macrophages or complement, or both, or neither, and followed by treating with anti-Ly6G MAb. Mice that received anti-Ly6G Ab alone underwent >95% depletion of neutrophils in the blood at 48 h following depletion (Fig. 2). However, neutrophil depletion was significantly abrogated in mice that received anti-Ly6G following depletion of both macrophages and complement (p<0.01). Functional depletion of complement alone by CVF did not significantly impact anti-Ly6G-mediated neutrophil depletion. However, when macrophages alone were depleted by LC, increased numbers of neutrophils (similar to CVF/LC depletion) were recovered from anti-Ly6G-treated mice, compared to non-depleted controls (p<0.05) (Fig. 2).
Fig. 2.
Depletion of macrophages with liposomal clodronate abrogates neutrophil depletion by anti-Ly6G. Mice were depleted of either complement (by Cobra Venom Factor, CVF), or macrophages (by Liposomal Clodronate, LC), or both (CVF/LC), before i.p. injection with Ly6G to deplete neutrophils. Experiment was repeated 3 times, with N=3–5 mice/group. Results shown are the 3 experiments combined; error bars represent standard deviations. *p<0.05, **p<0.01. The dotted line represents an approximation of the clinical definition of severe neutropenia (500 neutrophils per µL of blood).
In summary, these results indicated that macrophage effectors, but not complement, are necessary for efficient anti-Ly6G-mediated systemic neutrophil depletion from the blood. In vitro studies confirm that other factors in addition to complement are necessary for anti-Ly6G-mediated depletion of neutrophils in the presence of plasma. Our in vivo experiments demonstrate that macrophages are required for effective anti-Ly6G antibody-mediated depletion of neutrophils. Thus, anti-Ly6G MAb is not a reliable method of inducing neutropenia in mice with disrupted macrophage function.
Conflict of interest
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by National Institute of Allergy and Infectious Diseases Grants R01 AI081719, R21 AI101750, R56 AI104756, and R41 AI106375 to BS.
Contributor Information
Kevin W. Bruhn, Email: kbruhn@usc.edu.
Ken Dekitani, Email: dekitani@usc.edu.
Travis B. Nielsen, Email: travis.nielsen@usc.edu.
Paul Pantapalangkoor, Email: paul.pantapalangkoor@usc.edu.
Brad Spellberg, Email: brad.spellberg@usc.edu.
References
- 1.Abbitt K.B., Cotter M.J., Ridger V.C., Crossman D.C., Hellewell P.G., Norman K.E. Antibody ligation of murine Ly-6G induces neutropenia, blood flow cessation, and death via complement-dependent and independent mechanisms. J. Leukoc. Biol. 2009;85:55–63. doi: 10.1189/jlb.0507305. [DOI] [PubMed] [Google Scholar]
- 2.Bruhn K.W., Pantapalangkoor P., Nielsen T., Tan B., Junus J., Hujer K.M., Wright M.S., Bonomo R.A., Adams M.D., Chen W., Spellberg B. Host Fate is Rapidly Determined by Innate Effector-Microbial Interactions During Acinetobacter baumannii Bacteremia. J. Infect. Dis. 2015;211:1296–1305. doi: 10.1093/infdis/jiu593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucher K., Schmitt F., Autenrieth S.E., Dillmann I., Nürnberg B., Schenke-Layland K., Beer-Hammer S. Fluorescent Ly6G antibodies determine macrophage phagocytosis of neutrophils and alter the retrieval of neutrophils in mice. J. Leukoc. Biol. 2015;98:365–372. doi: 10.1189/jlb.1AB1014-488RR. [DOI] [PubMed] [Google Scholar]
- 4.Cavinato R.A., Bastos K.R.B., Sardinha L.R., Elias R.M., Alvarez J.M., D’Império Lima M.R. Susceptibility of the different developmental stages of the asexual (schizogonic) erythrocyte cycle of Plasmodium chabaudi chabaudi to hyperimmune serum, immunoglobulin (Ig)G1, IgG2a and F(ab′)2 fragments. Parasite Immunol. 2001;23:587–597. doi: 10.1046/j.1365-3024.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., Albina J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 6.Fleming T.J., Fleming M.L., Malek T.R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 7.Huppler A.R., Conti H.R., Hernández-Santos N., Darville T., Biswas P.S., Gaffen S.L. Role of neutrophils in IL-17–dependent immunity to mucosal candidiasis. J. Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan S.A., Huang X., Barrett R.P., Rooijen N., van, Hazlett L.D. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 2003;170:5219–5227. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 9.Queen J., Satchell K.J.F. Neutrophils are essential for containment of Vibrio cholerae to the intestine during the proinflammatory phase of infection. Infect. Immun. 2012;80:2905–2913. doi: 10.1128/IAI.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C., Hohl T.M., Leiner I., Equinda M.J., Fan X., Pamer E.G. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J. Immunol. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate M.D., Ioannidis L.J., Croker B., Brown L.E., Brooks A.G., Reading P.C. The role of neutrophils during mild and severe Influenza Virus infections of mice. PLoS One. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J.-X., Bair A.M., King S.L., Shnayder R., Huang Y.-F., Shieh C.-C., Soberman R.J., Fuhlbrigge R.C., Nigrovic P.A. Ly6G ligation blocks recruitment of neutrophils via a β2-integrin–dependent mechanism. Blood. 2012;120:1489–1498. doi: 10.1182/blood-2012-01-404046. [DOI] [PMC free article] [PubMed] [Google Scholar]