Abstract
The side population (SP) phenotype is associated with the Hoechst dye efflux activity of the Abcg2 transporter and identifies hematopoietic stem cells (HSCs) in the bone marrow. This association suggests the direct use of Abcg2 expression to identify adult stem cells in various other organs. We have generated a lineage tracing mouse model based on an allele that co-expresses both Abcg2 and a CreERT2 expression cassette. By crossing these mice with lox-STOP-lox reporter lines (LacZ or YFP), cells that express Abcg2 and their progeny were identified following treatment with tamoxifen (Tam). In the liver and kidney, in which mature cells express Abcg2, reporter gene expression verified the expected physiologic expression pattern of the recombinant allele. Long term marking of HSCs was seen in multiple peripheral blood lineages from adult mice, demonstrating that Abcg2+ bone marrow HSCs contribute to steady state hematopoiesis. Stem cell tracing patterns were seen in the small intestine and in seminiferous tubules in the testis twenty months after Tam treatment, proving that stem cells from these organs express Abcg2. Interstitial cells from skeletal and cardiac muscle were labeled and some cells co-stained with endothelial markers, raising the question as to whether these may function as tissue-specific muscle stem cells. Altogether, these studies prove that Abcg2 is a stem cell marker for blood, small intestine, and testicular germ cells and provide a new model for studying stem cell activity that does not require transplant-based assays.
Keywords: Abcg2, stem cells, intestine, testis, blood, muscle, Adult haematopoietic stem cells, Adult stem cells, Muscle stem cells, Germline, Leukocytes, Long-term repopulation
Introduction
Many adult tissues contain organ-specific stem cells that continually generate differentiating cells in organs with rapid cellular turnover, such as the hematopoietic system and the gut. Adult stem cells are also present in organs that consist mainly of non-dividing, long-lived mature cells, such as liver and skeletal muscle, where these stem cells support regeneration after injury and mediate the repair response. It is important to identify new markers that are expressed in adult stem cells from various organs in order to isolate these populations for biologic study and to prepare cell populations that can be used in transplant and tissue regeneration protocols. While combinations of various cell surface markers are typically used to isolate stem cells via multiparameter flow cytometry and other enrichment techniques, these protocols may yield impure populations and may not be applicable for clinical protocols. Despite current progress in discovering new stem cell markers, better methods are needed for identifying and isolating adult stem cells.
The side population (SP) assay has been used to identify adult stem cells in various organs and is based on efflux of Hoechst 33342 dye by ABC transporter activity [1, 2]. The Mdr1a/b gene product, P-glycoprotein, and the Abcg2 transporter have been associated with the SP phenotype and stem cell activity[3], however, it appears that the Abcg2 transporter is the most specific marker of stem cells, at least in the adult hematopoietic system[4–6]. Expression of Abcg2 identifies most if not all hematopoietic stem cells (HSCs) in the bone marrow of adult mice[7], as assessed in a standard transplant assay involving reconstitution of lethally irradiated mice. SP cells that express ABCG2/Abcg2 have also been identified in a variety of other organ systems [8, 9], and with various degrees of rigor, have been associated with stem cell activity. However, the stem cell activity of SP cells from adult tissues other than the hematopoietic system has remained unclear in many cases. One reason for this uncertainty is that transplant assays for some adult solid organs are either unavailable or relatively complicated when compared to the hematopoietic transplant model. Another reason is that the SP phenotype is complex and depends on multiple factors in addition to ABC transporter expression. There are few definitive studies of SP stem cells in solid organs prompting an interest in testing whether expression of Abcg2 can be used as a direct stem cell marker.
To address this issue, we have developed a lineage tracing mouse model to better assess the stem cell function of Abcg2-expressing cells. Lineage tracing models are used to produce a stable genetic alteration in stem cells that can then be detected in both the stem cells themselves and in progeny arising from those stem cells. Typically, these systems utilize cell type-specific expression of Cre recombinase to induce a stable DNA rearrangement that activates a reporter gene such as YFP or LacZ[10]. The rearranged reporter gene is stably transmitted and expressed in all stem cell progeny. These systems have allowed the detection of stem cell activity in solid organs in vivo without the need for transplantation assays[11, 12].
In prior work, we have generated an mouse Abcg2 allele that expressed both the endogenous Abcg2 open reading frame along with an ires-GFP gene inserted into a non-coding region of Abcg2[7]. This recombinant allele retained the normal physiologic expression pattern of wildtype Abcg2. We have adapted this model by inserting an ires- CreERT2 gene into the same position to create a lineage tracing model in which tracing can be initiated by administration of tamoxifen (Tam). These mice were crossed with either lox-STOP-lox LacZ[13] or lox-STOP-lox YFP mice so that Abcg2-expressing cells and their progeny could be detected and tracked over time. We now report definitive evidence for Abcg2-expressing stem cells in multiple organs using our novel animal screening model for Abcg2-stem cell activity.
Materials and Methods
Generation of the Abcg2-ires-CreERT2 mouse model
An ires-CreERT2 gene[14] was inserted into the 3’ untranslated region of exon 16 of the Abcg2 allele in order to establish spatiotemporal gene control via Tam-mediated, pulse-chase tracing. The ires-CreERT2 fragment was used to replace the ires-GFP fragment from within our prior targeting construct used to create the Abcg2GFP knock-in mouse model[7]. The resulting construct was electroporated into the murine embryonic stem cell line ES129SVJ-F12 (provided by Dr. Gerard Grosveld, St. Jude Children’s Research Hospital) and selected with ganciclovir and G418 to generate Abcg2-NeoR-ires-CreERT2pA ES cells. One karyotypically normal and correctly targeted ES cell clone was transiently electroporated with a pMC-Cre plasmid[15] to delete the Neomycin resistance gene (NeoR) and subclones were selected. The final Abcg2-ires-CreERT2pA ES cell clone was injected into mouse C57Bl/6J blastocysts and chimeric mice were used to establish germline transmission. All experiments with mice were done according to a protocol approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee.
ES cells and mice were genotyped either by Southern blot or by PCR using primers as show in Figure 1. The sequence of the primers are P1: 5’ TCAGGGCATCGAACTGTC 3’. P2: 5’ CGCCTTTGCAGGTGTATC 3’, P3: 5’ CCAAACAAGCCTCGACCTAC 3’. Primers P1 and P2 amplify a 723bp fragment spanning exon 16 of Abcg2 and the Cre fragment. Primers P1 and P3 amplify a 463bp fragment from the wild type allele. The outside probe for Southern blot was generated by PCR and encompasses a fragment between exon 14 and intron 14.
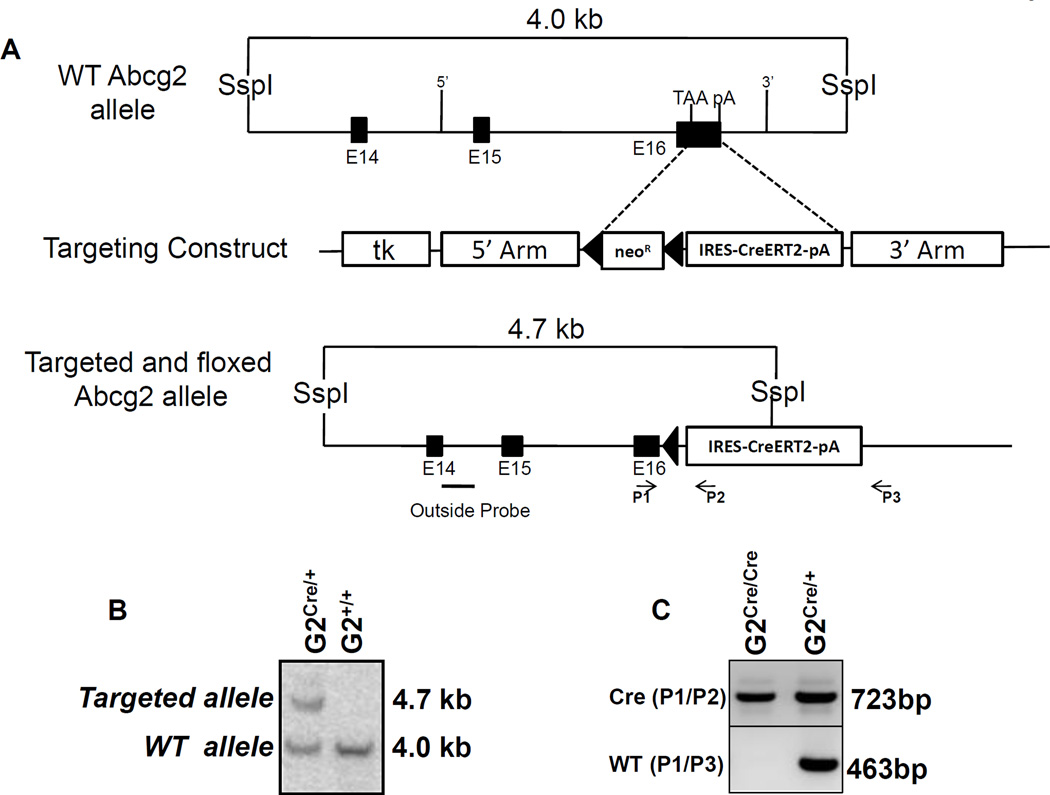
Figure 1. Targeted insertion of the ires-CreERT2 expression cassette into the Abcg2 locus.
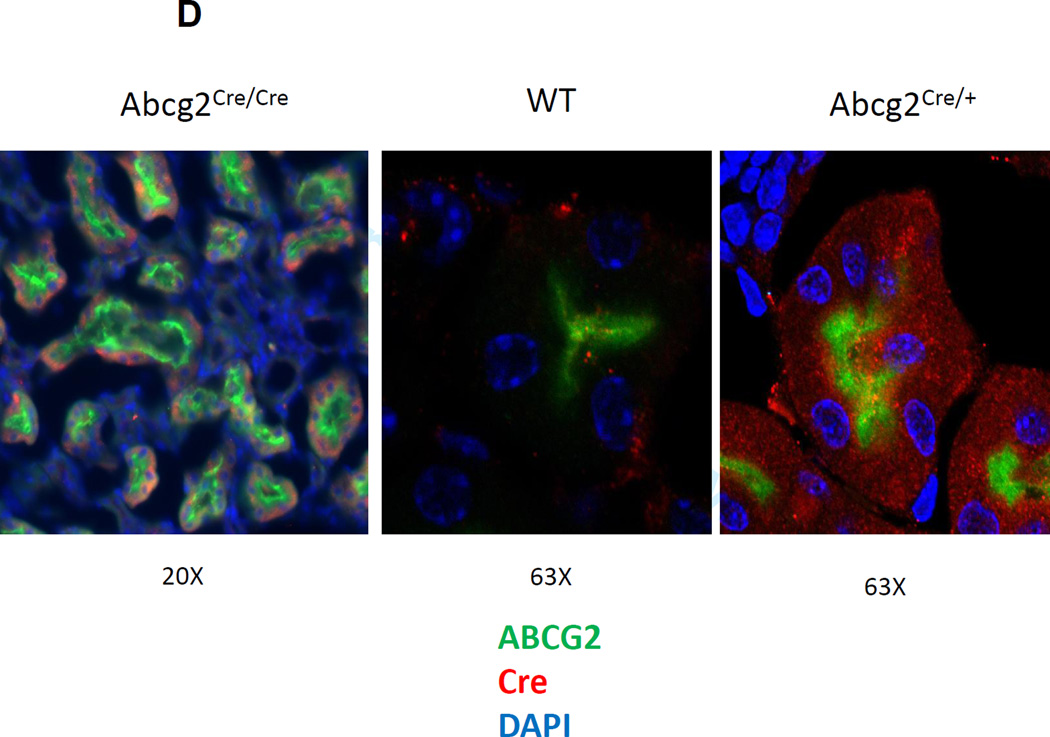
(A) The structure of the wild type allele and the targeted allele are shown. The expression construct was inserted into the 3’UTR as shown. E14, E15 and E16 represent the last three exons of the Abcg2 gene, TAA represents the stop codon of the Abcg2 gene, and pA represents the endogenous polyadenylation signal. The ires-CreERT2 expression cassette was inserted between the TAA and pA sites. NeoR designates the neomycin resistant cassette, TK designated the thymidine kinase gene, and the loxP sites are shown as triangles. The location of the Southern Blot probe, Ssp1 restriction sites, and PCR primer pairs for genotyping is indicated. (B) Southern blot analysis of ES cell clone demonstrating the successful generation of the targeted and floxed Abcg2 allele. (C) Genotyping of homozygous and heterozygous mice by PCR using primer pairs specific to Abcg2CreERT2 allele (P1/P2) and wild type allele (P1/P3). (D) Immunofluorescent confocal microscopy for Abcg2 expression (green), Cre recombinase (red), and nuclear staining (blue) in renal proximal tubular cells from Abcg2CreERT2/CreERT2 mice (left panel), wildtype mice (middle panel), and Abcg2CreERT2/+ mice, right panel.
Tamoxifen treatment of mice
Tamoxifen (Tam) (Sigma-Aldrich, USA) was dissolved in corn oil at a concentration of 20 mg/ml. Adult Abcg2CreERT2/+ RosaLacZ/+ , Abcg2CreERT2/+ RosaEYFP/+, Abcg2CreERT2/ CreERT2RosaLacZ/LacZ and Abcg2CreERT2/CreERT2 RosaEYFP/EYFP mice were treated with intraperitoneal injections of Tam daily for five consecutive days at approximately 2 months of age. On each day, the dose administered was 9 mg of Tam per 40 grams body weight.
Organ collection, processing, and LacZ staining
LacZ expression in tissues from euthanized mice was examined directly by X-gal staining. Mice were intravenously perfused with PBS followed by 2% paraformaldehyde. The organs were dissected and further fixed overnight at 4°C. Organs were cryopreserved with 30% sucrose and embedded in OCT compound (Tissue-Tek, Sakura, USA ). Sixteen µm frozen sections were washed in PBS for 5 min and incubated in the dark in standard β-gal substrate (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml X-gal, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40 in PBS) overnight at 37°C. Photographic images were then obtained using a Nikon E800 light microscope.
Isolation of cells from skeletal muscle and heart
Cells were collected from muscle tissues using a modification of a previously described protocol[16]. Muscle tissue was collected in Hanks Balanced Salt Solution (Gibco, USA) minced into fine slurry and digested with collagenase P (Roche, USA) for 30 min at 37°C. Collagenase was washed away and samples were triturated in DMEM/10% FBS/1% glutamine/1% antibiotic. Triturate was passed through a 100µm filter (Falcon, USA). Cells were stained with CD31-PE (Becton Dickinson, USA) in PBS containing 2% FBS followed by flow analysis.
Antibody Staining for Flow Cytometry Analysis
Expression of YFP in different peripheral blood and bone marrow cells was detected by flow cytometry. Peripheral blood samples were stained for Ter119, Gr1, Mac1, B220 and CD3 using fluorescent conjugated antibodies (Ter119-PE-Cy7, Gr1-APC-Cy7, Mac1-Alexa700, B220-PerCP-Cy5.5, CD3-APC; Becton Dickinson, USA) and DAPI (Molecular Probes, USA) to mark the dead cells. Bone marrow cells were stained for side population with Hoechst (Sigma-Aldrich, USA) and with c–Kit-APC, Sca–1–PE, Lineage–APC-Cy7 (Becton Dickinson, USA) to stain the KSL cells; PI was used to mark the dead cells.
Immunofluorescence Micoscopy
14 µm thick tissue sections prepared on a cryostat were immunostained with antibodies specific for GFP (A11121 Rabbit IgG, Invitrogen, USA), PECAM (BD 553070, Rat IgG, Becton Dickinson, USA), ABCG2 (Clone BXP53 MC-981, Kamiya Biomedical Company, USA), Cre recombinase (polyclonal antibody PRB-1061C, Covance, USA) or laminin α-2 (SC-59854, Santa Cruz Biotechnology, USA). For the PECAM and ABCG2 antibodies, a secondary Alexa488 Donkey anti-Rat antibody was used (A21208, Invitrogen, USA) and for the GFP and Cre recombinase antibodies, a secondary Cy3 Donkey anti-Rabbit antibody was used (AP18C, Millipore, USA). Images were captured with a confocal laser-scanning microscope (Zeiss).
PCR for excision efficiency
The DNeasy Blood & Tissue Detection Kit (69506, Qiagen, USA) was used to isolate DNA from mouse tissues per the manufacturer’s protocol. Cre-mediated excision of the RosaEYFP-lox-stop cassette was detected by PCR using the following primers – Forward: 5’ AAAGTCGCTCTGAGTTGTTAT 3’ Reverse: 5’ AACTTGTTGGCCGTTTACGTC 3’. To determine the amount of DNA loaded, a PCR reaction was carried out using primers to the unrearranged portion of the endogenous Abcg2 gene: – Forward: 5’ GTGCCACCATGTTCAACTTA 3’ Reverse: 5’ CTGCCAGAGTAGTGGAAGATT 3’. For the detection of Cre-mediated excised stop-cassette, the assay was done using Dream Taq Green PCR Master mix 2X (K1081, Fermentas, USA) and 35 cycles of amplification. For detection of the endogenous Abcg2 loading control, HotStar Taq DNA Polymrase (203203, Qiagen, USA) was used with 40 cycles of amplification. PCR products were analyzed by ethidium bromide staining of agarose gels.
Results
Generation and breeding of Abcg2-CreERT2 mice
Homologous recombination was used to generate ES cell clones in which an ires-CreERT2 cassette was inserted into exon 16 of the Abcg2 allele (Fig 1A). This CreERT2 expression cassette was inserted downstream of the Abcg2 stop codon and upstream of the endogenous polyadenylation signal sequence to ultimately generate a bicistronic mRNA that expresses both wildtype Abcg2 and the CreERT2 fusion gene. We have previously used the same recombination arms to express an ires-GFP construct from the Abcg2 locus and showed that tissue specific expression was preserved for both genes [7]. Three ES cell clones were isolated after selection with G418 and gancyclovir. The NeoR gene was deleted by transient transfection of a Cre expression plasmid to yield ES clones containing the final, recombinant Abcg2CreERT2 allele. Germ line transmission of the Abcg2CreERT2 allele was confirmed by Southern blot analysis of tail DNA (Fig 1B). A PCR method for genotyping was used to verify the 3’ structure of the targeted allele and to identify homozygous Abcg2CreERT2/CreERT2 mice (Fig 1C).
These mice were then crossed with either 129S–Gt(ROSA)26Sortm1Sor/J mice (hereafter referred to as RosaLacZ-lox-stop) or the B6.129×1-Gt(ROSA)26Sortm1(EYFP)Cos/J mice (hereafter referred to as RosaEYFP-lox-stop) to generate Abcg2CreERT2/+, RosaLacZ-lox-stop/+ or Abcg2CreERT2/+, RosaEYFP-lox-stop mice. In the absence of Cre activity, the reporter gene is not expressed due to presence of an upstream transcription termination element. With Tam-induced translocation of Cre recombinase into the nucleus, the transcriptional termination element is deleted and the LacZ or the YFP gene is then expressed via the constitutive Rosa promoter. Cells in which this deletion occurs, as well as all progeny arising from these cells, will then express the “activated form” of the reporter gene. Mice were genotyped and treated with Tam, typically given as a daily intraperitoneal injection for 5 days, and then analyzed at various time intervals either by histochemical staining for LacZ expression or by flow cytometry or immunofluorescence for YFP expression. Details of mice examined in this study are presented in Supplemental Tables 1 and 2.
Immunofluorescent confocal microscopy of the kidney confirmed that expression of Cre recombinase colocalized with Abcg2 expression in renal tubular cells (Fig 1D). Proximal tubule cells expressed Abcg2 at the luminal border as demonstrated by staining with the Bxp-53 antibody, while distal collecting tubules, which do not express Abcg2, were negative for staining with Bxp-53. Costaining with a polyclonal Cre antibody showed cytoplasmic expression of CreERT2 in proximal tubules from an Abcg2CreERT2/+ mouse but not in proximal tubules from a wildtype mouse. Similar results were obtained in skeletal muscle, where extralaminar cells showed coexpression of both proteins (Suppl. Fig. 1A). These results confirmed the expected tissue-specific expression pattern of both Abcg2 and CreERT2 and similar results were obtained in Abcg2 expressing cells in the liver and small intestine (data not shown).
Western blot analysis of Abcg2 protein expression in the kidney and liver of Abcg2CreERT2/CreERT2 homozygous mice showed a slight reduction of Abcg2 levels in the kidney and a greater reduction in the liver relative to wildtype mice (Suppl. Fig. 1B), demonstrating that the Abcg2CreERT2 allele is somewhat hypomorphic for Abcg2 protein expression.
Expression of the Abcg2CreERT2 allele in differentiated cells from kidney and liver
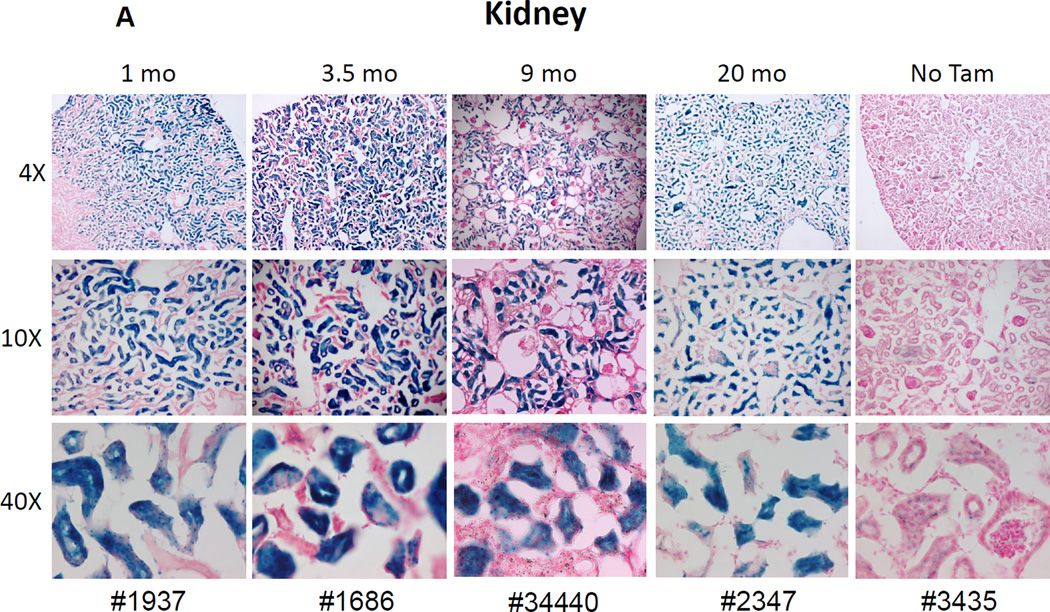
Abcg2 is known to be highly expressed in renal proximal tubules and in mature hepatocytes[17, 18]. To validate that the recombinant allele was expressed in an appropriate, tissue specific manner, and that reporter gene expression was dependent on Tam treatment, we injected Abcg2CreERT2/+, RosaLacZ-lox-stop/+ mice with Tam for 5 days and performed immunohistochemistry at various times after treatment to assay for LacZ expression. There was specific labeling of proximal tubule cells in the kidney at various time points ranging from 1 to 20 months after Tam treatment (Fig. 2A). No staining was seen in distal collecting ducts or in renal glomeruli, showing that LacZ expression was specifically marking Abcg2-expressing cells. No LacZ expression was seen in the kidneys of Abcg2CreERT2/+, RosaLacZ-lox-stop/+ mice that were not treated with Tam, demonstrating that reporter gene activation was Tam-dependent.
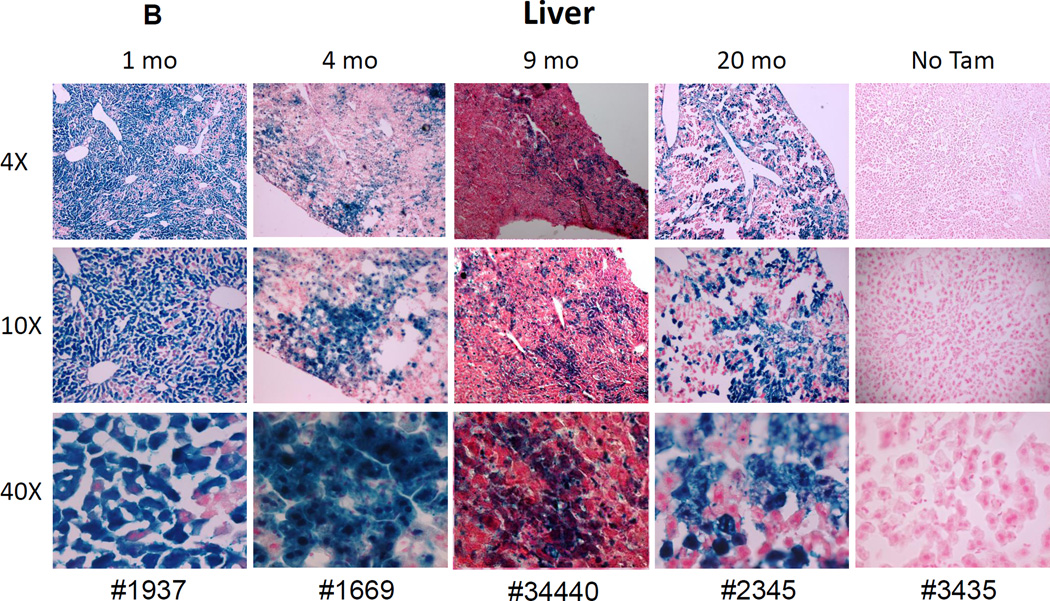
Figure 2. Reporter gene expression in liver and kidney confirms the expected expression pattern of the Abcg2CreERT2 allele.
Abcg2CreERT2/CreERT2 or + RosaLacZ/+ mice were analyzed at various time points ranging from 1 to 20 months after Tam treatment for LacZ expression in the kidney (A) and liver (B). As a negative control, one Abcg2CreERT2/+ RosaLacZ/+ mouse that was not treated with Tam was analyzed at 6 months of age. The magnification is shown on the left and the animal number shown on the bottom of each column corresponds to the genotypes shown in Table 1. LacZ expression in the proximal renal tubules and in hepatocytes corresponds to the known expression pattern of the WT Abcg2 allele.
Similarly, LacZ expression was seen in hepatocytes and bile caniliculi of Tam-treated mice ranging from 1 to 20 months after treatment (Fig. 2B). Analysis of the 5 Tam treated mice shown in Fig. 2, with another 8 mice shown in Supplemental Figures 2–9. Significant levels of Tam-dependent marking was seen in all cases and that there was no correlation between the time of analysis after the Tam pulse (1 to 21 month intervals) and the degree of labeling in the liver. Overall, LacZ staining of the kidney and liver in Tam-treated mice verified the known tissue-specific expression pattern of Abcg2 and Tam-dependent activation of the RosaLacZ-lox-stop reporter gene.
Hematopoietic lineage tracking
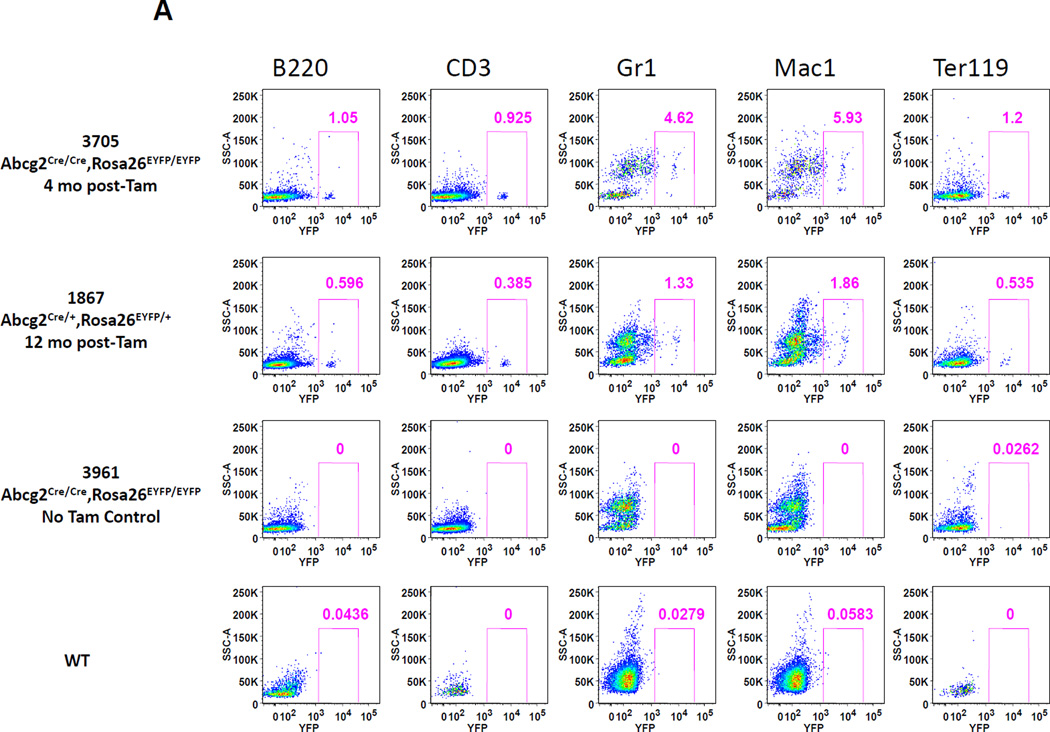
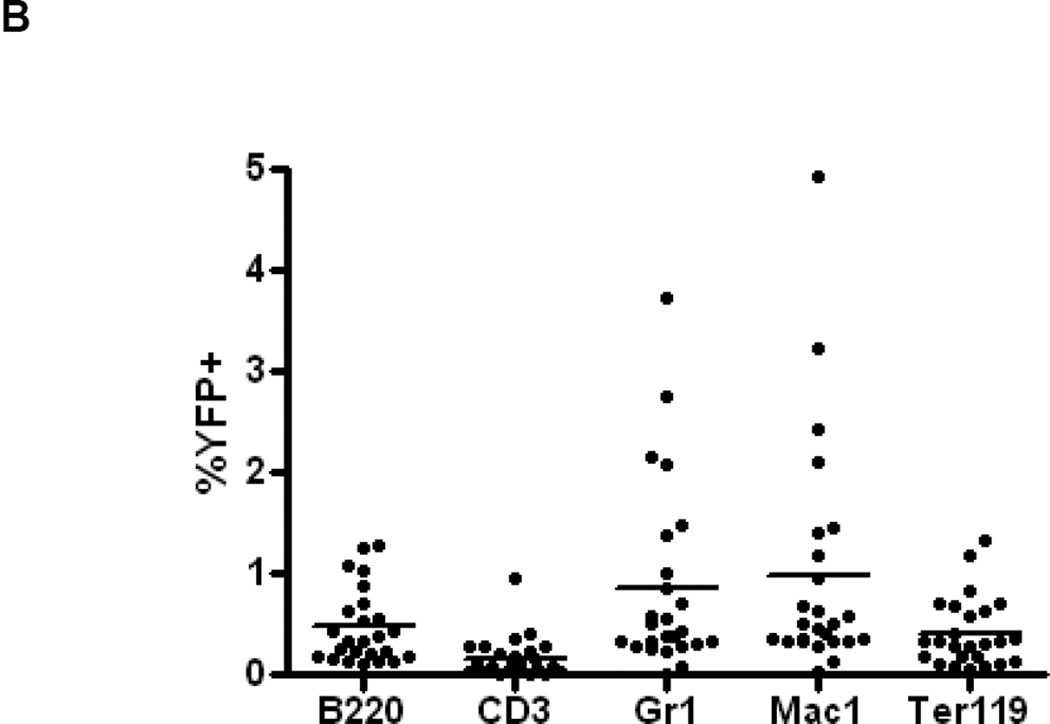
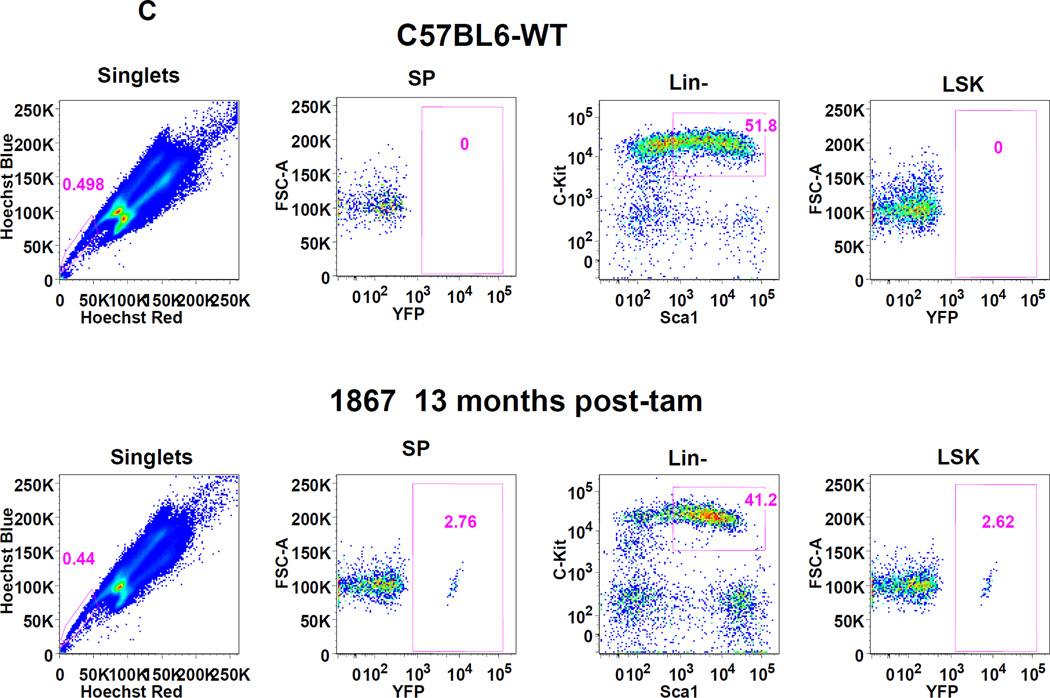
It is known that the majority of murine, adult HSCs in the bone marrow express Abcg2 and appear within the side population[4, 5, 7] predicting that Tam treatment of adult mice should result in long term marking of multiple hematopoietic lineages. Abcg2CreERT2/+, ROSAYFP/+ mice were treated with Tam and peripheral blood lineages were analyzed by flow cytometry for YFP expression at various time points thereafter. Expression of the activated YFP reporter gene could be clearly detected in a small proportion of peripheral blood granulocytes, T-cells, B-cells and erythroid cells 4 to 12 months after Tam labeling (Fig. 3A). Analysis of 26 Tam-treated mice at various intervals after labeling showed consistent multilineage marking, but at an overall low marking efficiency of 1–2 % (Fig. 3B). It is noteworthy that erythroid cells were also labeled in these studies. Given the long time interval between Tam treatment and analysis, it is likely that this labeling is due to HSC marking rather than the more transient labeling that would be expected from Abcg2-expressing erythroid progenitors[7, 19]. To determine the marking level in stem cell-enriched populations, flow analysis was performed on gated SP cells and Lin−, Sca1+, cKit+ (LSK) cells from the bone marrow. A similarly low level of marking was noted in these primitive stem cell subsets (Fig. 3C and Supplemental Figure 10).
Figure 3. Expression of the YFP reporter gene in hematopoietic lineages after Tam treatment confirms activity in Abcg2+ hematopoietic stem cells.
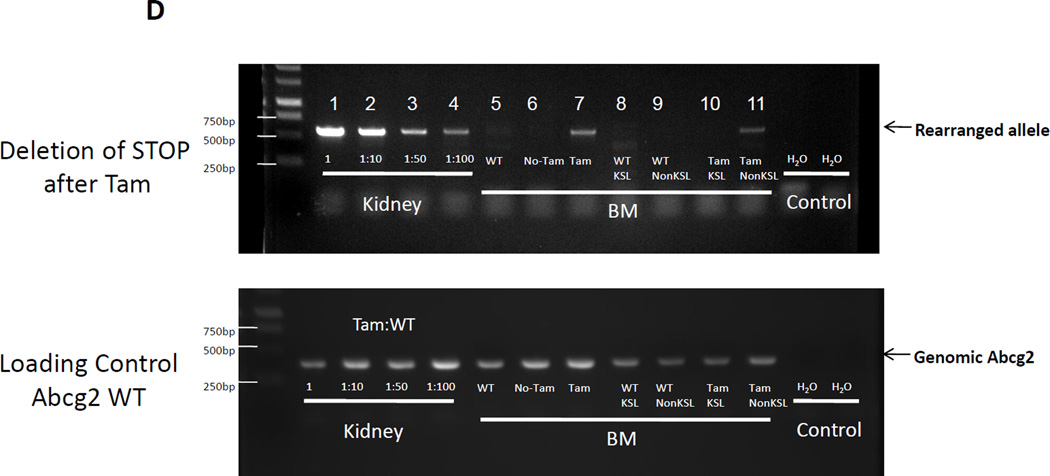
(A) Flow cytometry analysis of peripheral blood cells from Abcg2CreERT2 Rosa26EYFP mice at 4 and 12 months after Tam labeling (first and second rows). As controls, results from Abcg2CreERT2 Rosa26EYFP mice that were not treated with Tam (3rd row) and a wildtype mouse (last row) are shown. Gating was performed on cells labeled with anti- B220, CD3, Gr1, Mac1, and Ter119 antibodies (columns) representing B cells, T cells, granulocytes, monocytes, and erythroid cells respectively. Pink boxes show the percent of cells that express YFP along the X-axis. (B) Total YFP labeling data for peripheral blood lineages in 26 mice treated with Tam at 1.5 to 21 months prior to analyses. Horizontal bars represent mean marking levels. (C) Representative YFP marking data in gated SP and LSK bone marrow cells from a control WT mouse (top row) and a Abcg2CreERT2 Rosa26EYFP mouse 13 months after Tam (bottom row). Panels show SP gates and LSK gates along with YFP expression analyses. (D) PCR analyses of reporter gene excision efficiency. Detection of the rearranged band is shown in the upper row and detection of a genomic control band from an unrearranged portion of the Abcg2 allele shown in the lower row as a loading control. The first 4 lanes show results from kidney DNA from a Tam treated Abcg2CreERT2 Rosa26EYFP mouse that was diluted at the indicated concentrations with DNA from a wildtype mouse. Lane 5 shows results from a bone marrow sample from a wildtype mouse. Lanes 6 and 7 are from Abcg2CreERT2 Rosa26EYFP mice that were not treated or treated with Tam respectively. Lanes 8 and 9 are from fractionated cells from a WT mouse while lanes 10 and 11 are from fractionated cells from a Tam treated Abcg2CreERT2 Rosa26EYFP mouse. Water negative controls are also shown.
To directly measure recombination efficiency, a quantitative gel PCR technique was used to measure the frequency of the excised RosaEYFP-lox-stop allele in bone marrow cells from Tam treated mice. As a standard curve, DNA from the kidney of a Tam-treated mouse was diluted with wildtype DNA. The excision frequency in bone marrow cells was about 1% of the level seen in the undiluted kidney sample (Fig. 3D). This low marking efficiency in HSCs is most likely due to the relatively low level of expression of Abcg2 mRNA in bone marrow HSCs relative to that in renal proximal tubule cells. The relatively low levels of the transcript in HSCs, combined with the hypomorphic expression of the recombinant allele, is likely causing reduced CreERT2 expression due to attenuated expression of the bicistronic transcript. Another possible explanation for the low level of marking in peripheral blood cells is that the active metabolite of Tam, endoxifen, is a substrate for P-glycoprotein[20], another ABC transporter that is highly expressed in hematopoietic stem cells[21, 22]. Nonetheless, the low but significant level of stable marking of multiple hematopoietic lineages over a 12 month time period proves that at least some Abcg2-expressing HSCs contribute to steady state hematopoiesis in adult mice.
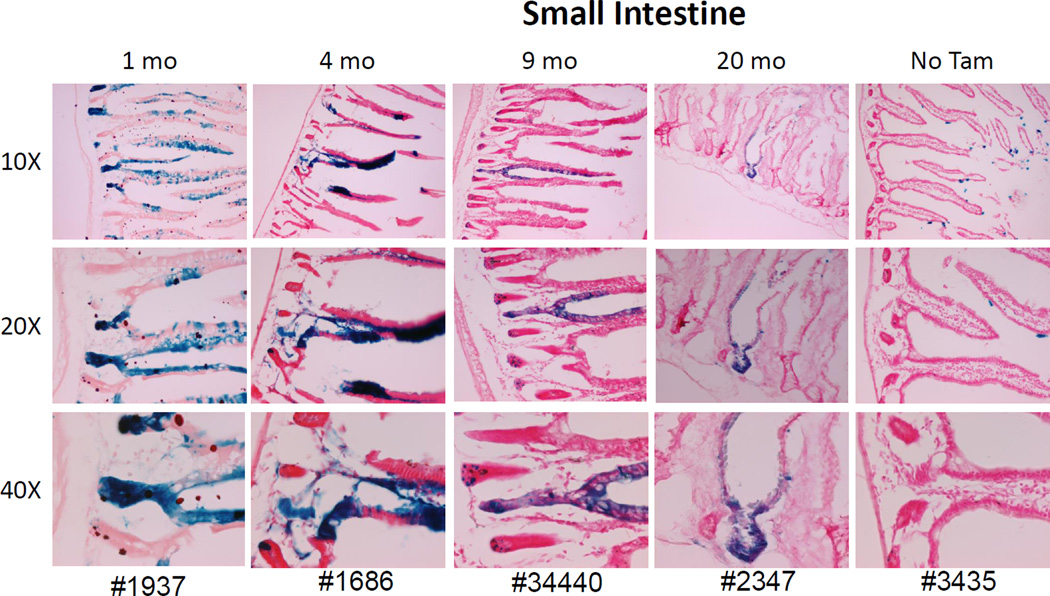
Small intestinal stem cells that express Abcg2 generate mature epithelial cells for at least 21 months
Mature intestinal epithelia cells express relatively high levels of Abcg2, which serves as a barrier against absorption of dietary xenobiotic substrates[23, 24]. In mice, the entire intestinal epithelium is shed into the lumen every 3–5 days and replaced by intestinal stem cells that are located adjacent to Paneth cells at the bottom of the intestinal crypts[25]. We examined labeling patterns in the small intestine at distant points after Tam treatment to determine if Abcg2+ stem cell activity would be observed. One month after Tam treatment, roughly one half of the crypts and the associated villi in the jejunum showed LacZ staining (Fig 4 and Supplemental figures 2–9). The LacZ marking of the villi persisted for up to 21 months after Tam treatment with no evidence of diminution with time after Tam treatment. LacZ labeling was often observed on only one side of the villi, giving a classic intestinal stem cell pattern seen in other lineage tracing studies[12]. The presence of long term persistent marking provides the first definitive proof that intestinal stem cells express Abcg2 and addresses conflicting results from prior SP stem cell studies in the small intestine [26, 27]. The fact that not all villi are labeled is consistent with inefficienct Cre excision, although it is also possible that some but not all intestinal stem cells express Abcg2.
Figure 4. Abcg2 expression marks long term stem cell activity in the small intestine.
Mice were treated with Tam and analyzed at the indicated time points for LacZ expression in small intestine sections from the jejunum. One Abcg2CreERT2/+ RosaLacZ/+ mouse, not treated with Tam, was used as negative control at 6 months of age. Labeling of crypt cells and epithelial cells along the villi were seen at all time points and demonstrated classic progeny tracking patterns consistent with stem cell activity.
Abcg2 expression in spermatogonial stem cells
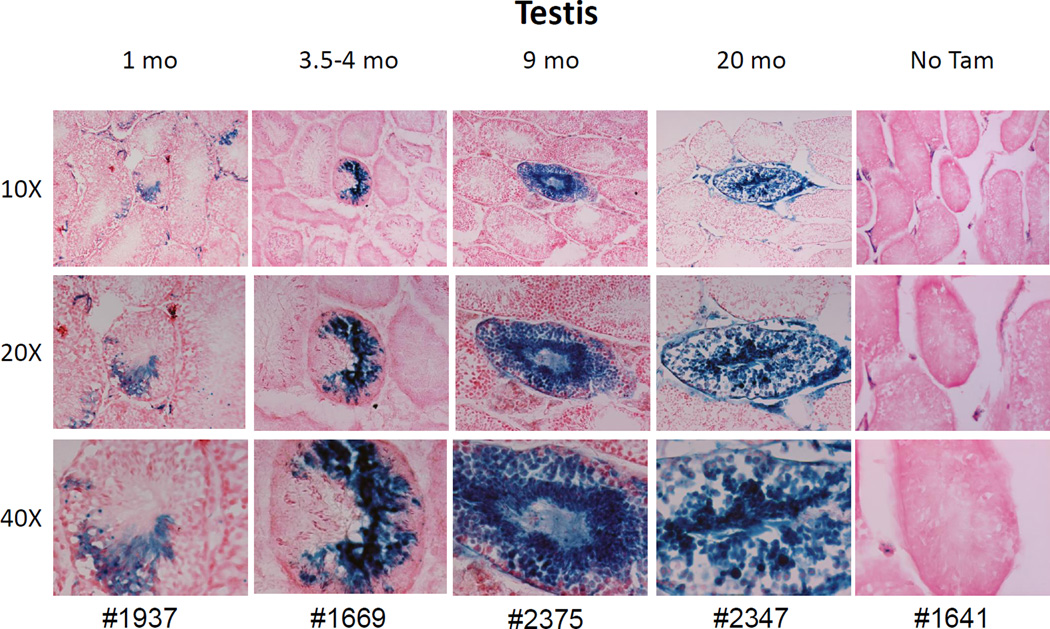
After puberty and throughout adult life, spermatozoa are continuously produced in the seminiferous tubules and originate from germ line stem cells, also called spermatogonial stem cells (SSCs), which exist in very low frequencies in the testis. The SSCs self-renew to maintain the stem cell pool and differentiate/proliferate through several stages including spermatogonia, primary spermatocytes and spermatids to produce mature spermatozoa. A variety of markers have been identified for isolation and enrichment of SSCs[28] and involve multiparameter selection strategies that require transplant assays for verification. In particular, testicular SSCs have been isolated based on a testicular side population (SP) phenotype[29, 30], indirectly implying that Abcg2 may be a SSC marker.
Testicular sections from Abcg2CreERT2/ RosaLacZ mice were examined for LacZ expression at various time points after Tam treatment. One month after Tam treatment, LacZ+ cells were seen in about one quarter of all tubules on a transverse section (Fig 5). Within each tubule, about 5–20% of the cells were labeled and included differentiating spermatocytes, spermatids, and mature sperm. At 9 and 20 months after the Tam pulse, the total number of tubules containing marked cells was reduced, but stable marking of all differentiation stages continued to be detected in some tubule cross-sections (Fig 5 and Supplemental Figures 4–8). At these later stages, the tubule section was sometimes entirely filled with LacZ+ cells demonstrating production of all stages of differentiating spermatogonia by Abcg2-expressing SSC. It is likely that marking of specific sections of individual tubules reflects the fact that some SSCs are dormant and don’t actively generate progeny at a given point in time[31], and that individual germ line stem cells typically support proliferation in only a small region of the seminiferous tubule[32].
Figure 5. Abcg2 expression marks germline stem cells in seminiferous tubules from the testis.
Mice were treated with Tam and LacZ expression in the testis was examined at various time points. LacZ expression was seen in developing spermatogonia and spermatids in some but not all sections of seminiferous tubules. Significant labeling was noted in tubule cross sections as long as 20 months after Tam administration, demonstrating that male germline stem cells express the Abcg2CreERT2 allele. No marking was noted in control mice that were not treated with Tam.
Pulse tracing patterns in the skeletal and cardiac muscle show frequent labeling of interstitial endothelial cells
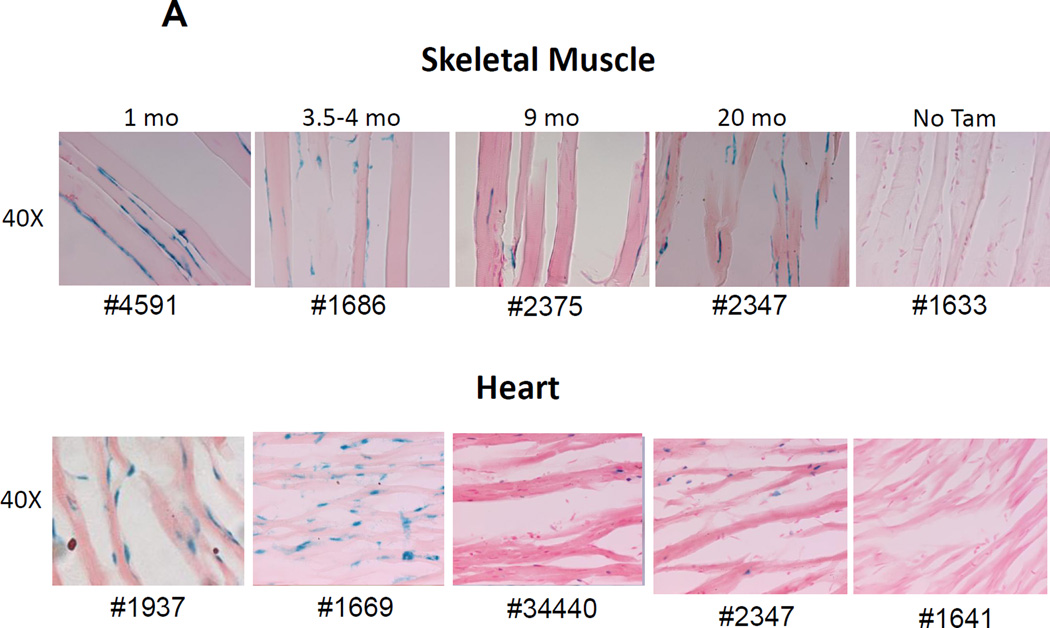
Under normal conditions, adult skeletal and cardiac muscle contain very few actively proliferating myocytes, however, under certain injury conditions, a small subset of muscle stem cells become activated and contribute to muscle regeneration. There is conflicting data regarding the phenotype and origin of these muscle stem cells, but several reports indicate that they can be identified by the SP phenotype[33], may in part be derived from bone marrow hematopoietic cells[34], and that they express Abcg2 and correspond to a satellite cell population that resides along the laminar surface of the myofiber[35]. Cardiac muscle also contains an analogous population of SP cells that may have the capacity to regenerate injured cardiac muscle[36]. In the heart, Abcg2 expression has a direct functional role in promoting cardiac repair activity[37].
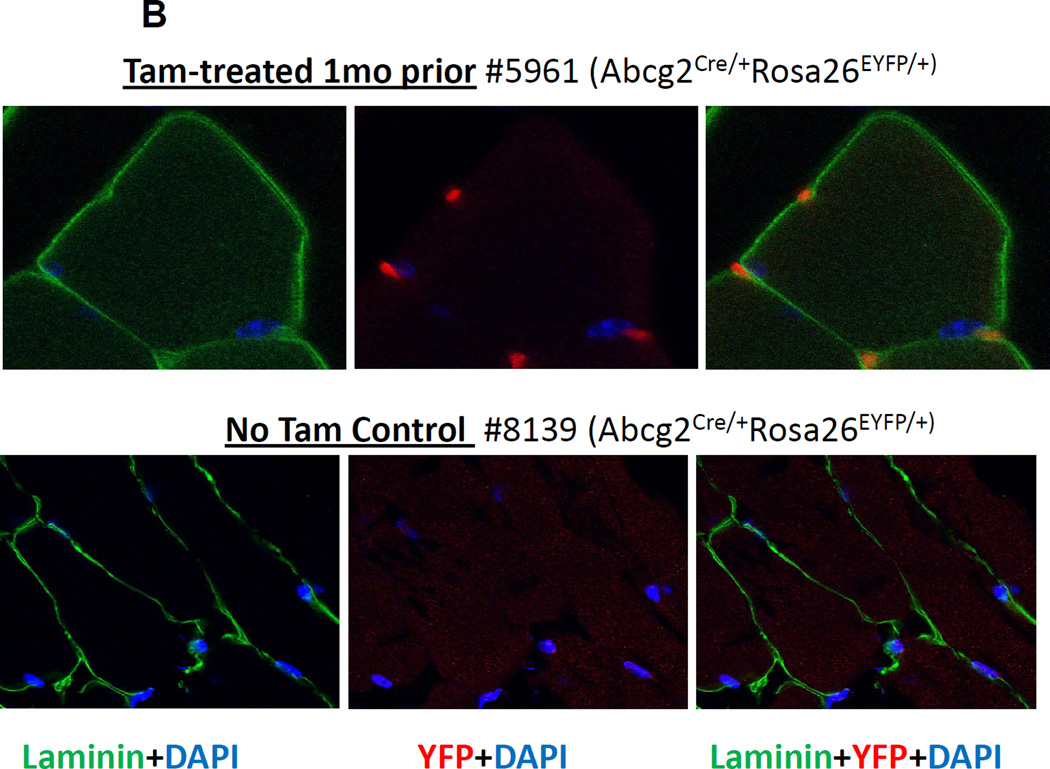
We analyzed skeletal (anterior tibialis) and cardiac muscle samples from Tam-treated, Abcg2CreERT2 RosaLacZ mice and found numerous LacZ+ cells located at the outside periphery of the myofibers in both skeletal and cardiac muscle sections (Fig 6A). The LacZ+ cells have an elongated shape and are located in the position expected for satellite, endothelial, and other non-myogenic cells. Since satellite cells are inside of the muscle basal lamina, we costained sections with an anti-laminin alpha2 chain antibody and used confocal microscopy to determine the orientation of the labeled cells to the basal lamina. These studies clearly showed that the labeled cells were outside of the basal lamina (Fig. 6B). These extralaminar LacZ+ cells were abundant in numerous sections and stably detected for up to 21 months after Tam labeling (Fig. 6A and Supplemental figures 2–9).
Figure 6. LacZ expression in perilaminar endothelial cells in skeletal and cardiac muscle.
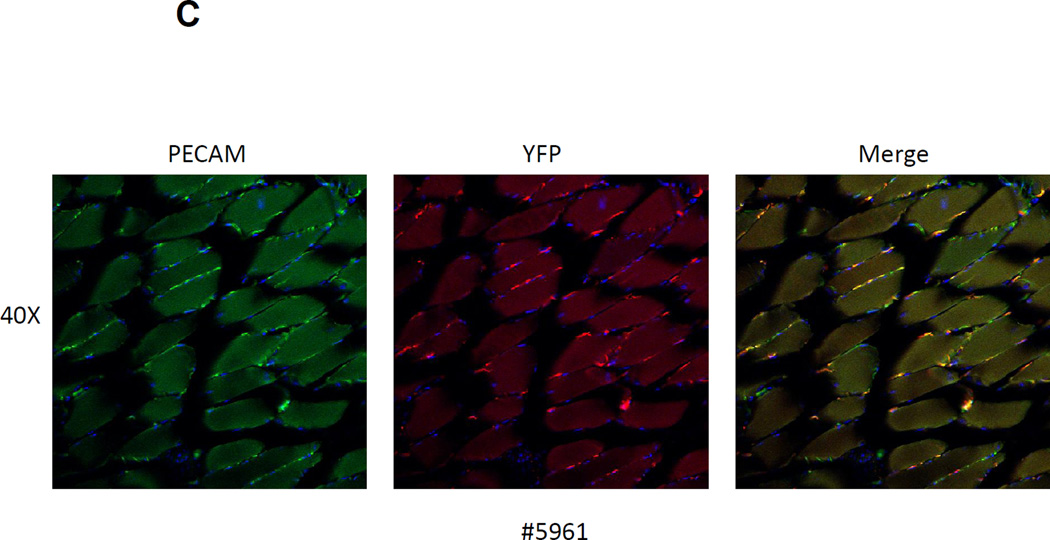
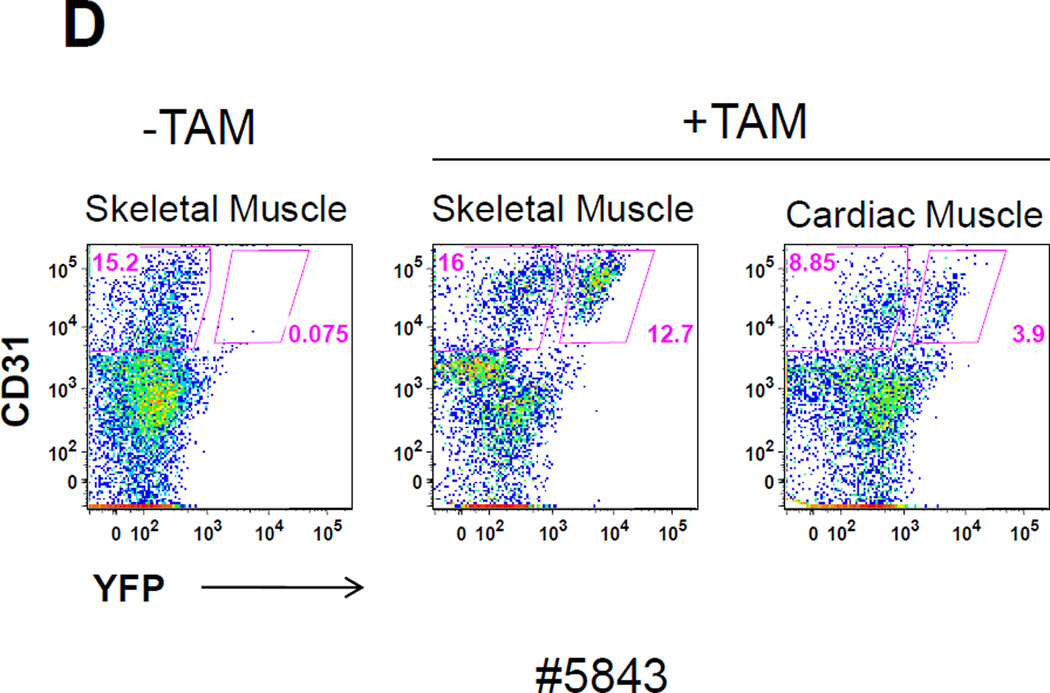
(A) LacZ expression in skeletal muscle from the tibialis anterior and in cardiac muscle was examined at various time points after Tam treatment. Prominent LacZ marking was noted in perilaminar cells at all time points in both skeletal and cardiac muscle. No LacZ staining was detected in myofibers from either skeletal muscle or the heart. (B) Confocal immunofluorescence analysis of the location of YFP-marked cells relative to the basal lamina in skeletal muscle. Sections from a Tam treated Abcg2CreERT2/+ ROSA 26EYFP/+ mouse were stained with an anti-laminin alpha2 chain antibody (green), an anti-YFP antibody (red) and a nuclear stain (blue). Results from no Tam control are shown on the bottom row. It can be seen that the YFP+ cells are located outside of the basal lamina. (C) Immunohistochemical costaining of skeletal muscle sections for YFP+ cells and PECAM, an endothelial cell marker. Confocal microscopy was used to detect antibody staining against YFP (red) and PECAM (green) in muscle sections Abcg2CreERT2 RosaYFP mice were treated with Tam. The panel on the right showed the merged images with yellow cells indicating costaining against both markers. (D) Flow cytometry analysis of single cell suspensions from skeletal muscle and heart. Expression of the CD31 endothelial marker is shown on the Y axis and for the YFP reporter gene on the X axis. The panel on the left shows the results from a mouse that was not treated with Tam. The middle and right panel show skeletal muscle and cardiac cells from a Tam-treated mouse. Note that some but not all endothelial cells are marked two weeks after Tam treatment in skeletal muscle and heart.
It has been shown that capillary and venous endothelial cells express relatively high level of Abcg2[18] and that myogenic-endothelial progenitor cells are present in skeletal muscle[38]. To determine if any of the marked cells were endothelial in origin, we costained muscle sections for expression of the CD31 endothelial marker (also known as PECAM) and analyzed these sections by confocal microscopy. At 1 month after Tam treatment, some but not all labeled cells costained for CD31 (Fig 6C), demonstrating that at least some of the Abcg2CreERT2 –ROSAYFP -labelled cells were endothelial in origin. A parallel analysis was done using flow cytometry to detect cardiac and/or skeletal muscle cells that expressed both the YFP reporter gene and CD31. Single cell suspensions were prepared from heart and skeletal muscle and analyzed by flow cytometry. In this assay, almost all of the YFP-labeled cells in cardiac and skeletal muscle expressed the endothelial CD31 marker (Figure 6D), however; not all endothelial cells were marked after the Tam pulse. These results show that endothelial cells comprise a significant fraction of the Tam-labelled cells in skeletal and cardiac muscle and raise the question as to whether these cells have activity in muscle regeneration assays. We have recently reported a more detailed functional analysis of Abcg2-marked cells in the skeletal muscle and their role in the regeneration process[39].
Discussion
SP cells that express Abcg2 have been detected in many adult organs and have been implicated in stem cell function, however; the functional activity of these putative stem cells has remained unclear in many cases. We have utilized a Tam-inducible, progeny tracking model to definitively address the contribution of Abcg2-expressing cells to adult stem cell activity in several mouse organ systems. In this model, reporter gene expression in the kidney and liver recapitulated the expected tissue specific expression of Abcg2 in mature cells from these organs. Labeling of peripheral blood cells validated the expected stem cell activity of adult hematopoietic stem cells (HSCs), which are known to express Abcg2[4], however; the number of labeled cells was relatively low and averaged about 1–2 % in both mature and immature lineages. There are several potential explanations for this decreased efficiency. It is possible that not all adult HSCs express Abcg2; for instance, fetal liver HSCs do not express the SP phenotype or Abcg2 [40], demonstrating that different HSC subpopulations can be associated with differences in Abcg2 expression. A more likely technical explanation is that the excision frequency is low in HSCs due to the expression level of the Abcg2CreERT2 allele in HSCs. Our data indicate that expression of the recombinant allele is hypomorphic in highly expressing tissues such as liver and kidney, so that the lower levels of expression seen in HSCs may result in insufficient CreERT2 expression levels in some HSCs. Nevertheless, this significant but low level marking in the hematopoietic system should allow useful future experiments with the hematopoietic system, such as defining the embryonic timing of the onset of definitive hematopoiesis.
Mature intestinal epithelial cells in the villi are shed into the lumen constantly, and replaced with newly differentiated cells from intestinal stem cells. Prior studies have shown that intestinal stem cells are localized in the crypts, associated with Paneth cells[41], and express markers such as Bmi-1[42], Lgr5[11], prominin-1[12]. Classic progeny tracking patterns were seen in the small intestine upon Tam induction of the Abcg2CreERT2 allele, demonstrating for the first time the small intestinal stem cells express Abcg2.The persistence of LacZ+ cells up to 21 months after Tam treatment demonstrates that Abcg2 expression marks small intestinal stem cells with extensive self-renewal potential. Recent studies have shown that the normal intestinal stem cells are probably the cell of origin of small intestine cancer[43]. Our results suggest that intestinal carcinoma stem cells could also express Abcg2, at least in some cases, potentially explaining the relatively refractoriness of intestinal epithelial cancer with chemotherapeutic drugs that are Abcg2 substrates.
Spermatogonial stem cells are present at a low frequency in the basal layer of the seminiferous tubules and are present throughout the life time of a male mouse while continuously generating maturing spermatids. An unexpected result in our study was that male testicular germ cells express Abcg2 and gave typical progeny tracking in the seminiferous tubules. LacZ+ seminiferous tubule segments persisted for as long as 20 months after Tam treatment, proving for the first time that spermatogonial stem cells express Abcg2. Our results are consistent with prior findings suggesting that Abcg2-expressing SP cells within testis identify spermatogonial stem cells[30, 44]. Recent progeny tracking experiments have demonstrated that neurogenin 3 (Ngn3)-expressing cells can give rise to spermatids as long as 14 months after Tam administration[31]. Spermatogonial stem cell activity occurred in transient patches within the seminiferous tubules, consistent with our finding that some cross-sections of tubules are strongly positive for marked progeny while many other cross sections are entirely negative. The patchy distribution of marked progeny that we observed is also consistent with the recent demonstration that spermatogonial stem cells have a short half life and are continuously replaced[32]. The function of Abcg2 in male germ line stem cells is unknown. Abcg2-null mouse strains show normal male fertility[5, 17] demonstrating that there is no developmental requirement for Abcg2 in spermatogonial stem cells. It is tempting to speculate that Abcg2 expression may protect the germline genome from naturally occurring, xenobiotic substrates that are genotoxic.
Previous studies have shown that myogenic stem cells from skeletal and cardiac muscle are relatively enriched in the SP cell fractions from these tissues[35, 45]. Our Abcg2 tracking experiments showed labeling of extra-laminar cells in both skeletal and cardiac muscle, however; we did not see any labeling of muscle fibers. The location of the labeled cells was most consistent with endothelial cells. Immunohistochemistry and flow cytometry experiments confirmed the majority of the Tam labeled cells coexpressed endothelial cell markers. We also found Tam-dependent labeling of venous endothelial cells in other organs, including the brain and testis (data not shown), consistent with the known expression pattern of Abcg2 in venous endothelium. These results suggest that the common appearance of SP cells in various tissues may, at least to some degree, reflect the appearance of endothelial cells in tissue preparations. Because the proportion of SP cells in skeletal muscle is typically 1–2% in single cell suspensions, the relatively high degree of labeling of endothelial cells suggests that Tam might be labeling endothelial precursor cells, giving rise to labeled cells that don’t necessarily express Abcg2 themselves. The degree to which these labeled interstitial cells have the capacity to regenerate skeletal muscle in injury models is the subject of another recent study[39].
Several studies have shown the presence of Abcg2+ cells in the heart and have further shown a functional role for Abcg2 in cardiac repair[37, 46]. Cardiac SP cells can differentiate into cardiomyocytes when injected directly into cardiac infarcts[36], although this activity was not associated with the endothelial compartment. Abcg2+ endothelial cells can play a direct functional role in myocardial repair. Abcg2-null mice have increased mortality after myocardial infarction due to impaired survival of microvascular endothelial cells in the heart as the result of increased accumulation of protoporphyrin IX and oxidative stress in these endothelial cells[47]. This loss of function in Abcg2-null mice was associated with ruptured cardiac aneurysms as the cause of accelerated death. It should be interesting to use this tracking model to study ventricular repair following myocardial infarction to determine if the labeled interstitial cells have a direct role post-infarct repair.
Summary
We have generated a Tam-inducible progeny tracking mouse model to detect stem cell activity from Abcg2-expressing cells. Long-term labeling was noted in multiple lymphoid and myeloid hematopoietic lineages showing that some Abcg2-expressing adult hematopoieitic stem cells contribute to steady state hematopoiesis. Endothelial cell marking was noted in multiple tissues, including skeletal and cardiac muscle, showing that SP cell activity in these organs may be partially due to resident endothelial cells. Stem cell-associated progeny marking was noted in the small intestine and testis, demonstrating for the first time that maturing cells in these organs are generated from Abcg2-expressing stem cells. This model should be useful for studying injury related regeneration in skeletal and cardiac muscle, as well studying embryonic stem cell activity via Tam-treatment of pregnant females.
Supplementary Material
Acknowledgments
We thank and acknowledge Sergei Gregorovich for generation of the targeting construct as well as screening and targeting of ES cell clones and Mehrdad Tadjali for additional assistance in ES cell characterization and preparation of cells for blastocyst injection. We also thank the Transgenic Core Lab at St. Jude Children’s Research Hospital for generation of the knockin mouse strain and Xiuling Li for assistance in preparing ES cells for blastocyst injections. We also thank Drs. Jian Zuo and Zhiyong Liu for instruction and support in immunohistochemical assays and tissue preparation.
This work was supported by National Institutes of Health Grant R01 HL67366 (to B.P.S) and by the American Lebanese Syrian Associated Charities. We thank the following Shared Resources at St. Jude for providing outstanding scientific support for these studies: Flow Cytometry and Cell Sorting Core, Transgenic/Gene Knockout Core, Animal Resource Center, and the Veterinary Pathology Core. These Cores are supported by the National Institutes of Health Cancer Center Grant P30 CA 21765.
Footnotes
Soghra Fatima: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Sheng Zhou: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Brian P. Sorrentino: Conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript
Disclosure of Potential Conflicts of Interest: none
References
- 1.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat. Med. 1997;3(12):1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Zong Y, Lu T, et al. Hematopoietic cells from mice that are deficient in both Bcrp1/Abcg2 and Mdr1a/1b develop normally but are sensitized to mitoxantrone. Biotechniques. 2003;35(6):1248–1252. doi: 10.2144/03356ss04. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Morris JJ, Barnes Y, et al. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99(19):12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 7.Tadjali M, Zhou S, Rehg J, et al. Prospective isolation of murine hematopoietic stem cells by expression of an Abcg2/GFP allele. Stem Cells. 2006;24:1556–1563. doi: 10.1634/stemcells.2005-0562. [DOI] [PubMed] [Google Scholar]
- 8.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24(1):3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 9.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011;29(4):725–735. doi: 10.1002/stem.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. 2011;12(2):113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 14.Yajima I, Belloir E, Bourgeois Y, et al. Spatiotemporal gene control by the Cre-ERT2 system in melanocytes. Genesis. 2006;44(1):34–43. doi: 10.1002/gene.20182. [DOI] [PubMed] [Google Scholar]
- 15.Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 16.Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, et al. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev. Biol. 1999;210(2):440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U. S. A. 2002;99(24):15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–3464. [PubMed] [Google Scholar]
- 19.Zhou S, Zong Y, Ney PA, et al. Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood. 2005;105(6):2571–2576. doi: 10.1182/blood-2004-04-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teft WA, Mansell SE, Kim RB. Endoxifen, the active metabolite of tamoxifen, is a substrate of the efflux transporter P-glycoprotein (multidrug resistance 1) Drug Metab Dispos. 2011;39(3):558–562. doi: 10.1124/dmd.110.036160. [DOI] [PubMed] [Google Scholar]
- 21.Sorrentino BP, McDonagh KT, Woods D, et al. Expression of retroviral vectors containing the human multidrug resistance 1 cDNA in hematopoietic cells of transplanted mice. Blood. 1995;86(2):491–501. [PubMed] [Google Scholar]
- 22.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20(1):11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 23.van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 2006;27(1):10–16. doi: 10.1016/j.tips.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.van Herwaarden AE, Jonker JW, Wagenaar E, et al. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63(19):6447–6452. [PubMed] [Google Scholar]
- 25.Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4(2):124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 26.He DN, Qin H, Liao L, et al. Small intestinal organoid-derived SP cells contribute to repair of irradiation-induced skin injury. Stem Cells Dev. 2005;14(3):285–291. doi: 10.1089/scd.2005.14.285. [DOI] [PubMed] [Google Scholar]
- 27.Alison MR, Poulsom R, Brittan M, et al. Isolation of gut SP cells does not automatically enrich for stem cells. Gastroenterology. 2006;130(3):1012–1013. doi: 10.1053/j.gastro.2006.01.075. [DOI] [PubMed] [Google Scholar]
- 28.Aponte PM, van Bragt MP, De Rooij DG, et al. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113(11–12):727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 29.Falciatori I, Borsellino G, Haliassos N, et al. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. FASEB J. 2004;18(2):376–378. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu Y, Motohashi N, Iseki H, et al. A novel subpopulation lacking Oct4 expression in the testicular side population. Int. J. Mol. Med. 2006;17(1):21–28. [PubMed] [Google Scholar]
- 31.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell. 2007;12(2):195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Klein AM, Nakagawa T, Ichikawa R, et al. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7(2):214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002;159(1):123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J. Clin. Invest. 2003;111(1):71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka KK, Hall JK, Troy AA, et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4(3):217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang SX, Tan TY, Gaudry L, et al. Differentiation and migration of Sca1+/CD31-cardiac side population cells in a murine myocardial ischemic model. Int. J. Cardiol. 2010;138(1):40–49. doi: 10.1016/j.ijcard.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 37.Pfister O, Oikonomopoulos A, Sereti KI, et al. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ. Res. 2008;103(8):825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamaki T, Akatsuka A, Ando K, et al. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002;157(4):571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle MJ, Zhou S, Tanaka KK, et al. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J. Cell Biol. 2011;195(1):147–163. doi: 10.1083/jcb.201103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida N, Dykstra B, Lyons K, et al. ABC transporter activities of murine hematopoietic stem cells vary according to their developmental and activation status. Blood. 2004;103(12):4487–4495. doi: 10.1182/blood-2003-11-3989. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 44.Lassalle B, Bastos H, Louis JP, et al. ‘Side Population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131(2):479–487. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- 45.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev. Biol. 2004;265(1):262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Martin CM, Ferdous A, Gallardo T, et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ. Res. 2008;102(9):1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 47.Higashikuni Y, Sainz J, Nakamura K, et al. The ATP-binding cassette transporter BCRP1/ABCG2 plays a pivotal role in cardiac repair after myocardial infarction via modulation of microvascular endothelial cell survival and function. Arterioscler. Thromb. Vasc. Biol. 2010;30(11):2128–2135. doi: 10.1161/ATVBAHA.110.211755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.