Abstract
Moringa oleifera is an affordable and rich source of dietary folate. Quantification of folate by HPLC showed that 5-formyl-5,6,7,8-tetrahydrofolic acid (502.1 μg/100 g DW) and 5,6,7,8-tetrahydrofolic acid (223.9 μg/100 g DW) as the most dominant forms of folate in M. oleifera leaves. The bioavailability of folate and the effects of folate depletion and repletion on biochemical and molecular markers of folate status were investigated in Wistar rats. Folate deficiency was induced by keeping the animals on a folate deficient diet with 1 % succinyl sulfathiazole (w/w). After the depletion period, animals were repleted with different levels of folic acid and M. oleifera leaves as a source of folate. Feeding the animals on a folate deficient diet for 7 weeks caused a significant (3.4-fold) decrease in serum folate content, compared to non-depleted control animals. Relative bioavailability of folate from dehydrated leaves of M. oleifera was 81.9 %. During folate depletion and repletion, no significant changes in liver glycine N-methyl transferase and 5-methyltetrahydrofolate-homocysteine methyltransferase expression were recorded. In RDA calculations, only 50 % of natural folate is assumed to be bioavailable. Therefore, the bioavailability of folate from Moringa is much higher, suggesting that M. oleifera based food can be used as a significant source of folate.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-1828-x) contains supplementary material, which is available to authorized users.
Keywords: Moringa oleifera, Folate bioavailability, Folate repletion, Gene expression, Liver GNMT
Introduction
Folate is the general term for both naturally occurring food folate and folic acid. Folic acid (not found in food) is the fully oxidized monoglutamate form (pteroylmonoglutamate) of the folate vitamin that is used in fortified foods and dietary supplements. Folate plays an essential role in asssociation with B12 and B6 vitamins in nucleotide synthesis, methionine regeneration in DNA methylation, oxidation and reduction of one-carbon units required for normal metabolism and regulation (Scotti et al. 2013). Folate defeciency causes severe abnormalities in one-carbon metabolism, resulting in DNA hypomethylation, which cause certain types of chronic diseases and developmental disorders, including neural tube defects (Williams et al. 2015). Neural tube defects (NTDs) are a group of abnormalities of the brain, cardiac and spinal cord which normally originate during gestation period causing the failure of the neural tube to close during embryogenesis (Williams et al. 2015). Thus, folate sufficient diet is strongly recommended during pregnancy to prevent the NTDs, and other chronic dysfuctions.
A sustainable food based approach using dietary source of folate, in adequate amount, can be effective in controlling folate deficiency and other usual associated nutritional deficiencies (Neeha and Kinth 2012; Kushwaha et al. 2014). Moringa oleifera leaves are one such promising food due to their easy availability (Saini et al. 2012), high nutritional (Saini et al. 2014a, b, c, d) and nutraceutical value (Saini et al. 2014e). Use of fresh leaves, flowers and tender pods (fruits) of this plant as vegetable is confined to African and Asian countries including India (Anwar et al. 2007; Kushwaha et al. 2014; Nadeem et al. 2014; Pawar et al. 2014). Tender pods and seed and seed oil of M. oleifera is well known for medicinal properties (Coppin et al. 2013; Govardhan Singh et al. 2013). However, information on the bioavailability of folate from fresh or dehydrated Moringa leaves is not available.
Folate bioavailability refers to the proportion of consumed folate that is absorbed and becomes available for metabolic processes. Poor bioavailability of natural food folates is the major cause of folate deficiency in developing countries. In general, bioavailability of folate is measured compared to folic acid (relative bioavailability), which shows great variation in human studies, ranging from 10 to 98 % (Aiso and Tamura 1998). The bioavailability of folates from various foods depends on the content of monoglutamyl (fortified food; highly bioavailable) and polyglutamyl folates (vegetables; low bioavailable) and on the presence of enhancers and inhibitors of folate absorption (Aiso and Tamura 1998). Radioisotope labelling techniques are the most preferred and the best method for the assessment of folate bioavailability, with high sensitivity and reproducibility. However, the use of radiolabeled folate is difficult in human studies due to safety and regulatory issues (Gregory et al. 1991).
The present investigation was therefore conducted to study the bioavailability of folate from the leaves of M. oleifera, in folate depleted rats. Over the past decade, major advance has been made in understanding the molecular mechanism of folate one carbon metabolism and this has led to the identification of key genes, including GNMT (glycine N-methyl transferase) and MTR (5-methyltetrahydrofolate-homocysteine methyltransferase). In the present study, we have also evaluated the effects of different level of dietary folate on modulation of the levels of mRNA encoding genes involved in folate one carbon metabolism. Since dietary antioxidants such as ascorbic acid may enhance folate bioavailability by increasing the stability of folates during food processing and during digestion in the gastrointestinal tract (McNulty and Pentieva 2004), effect of ascorbic acid was also evaluated in folate bioavailability.
Methods and materials
Materials
Fresh leaves were collected from 3 year old M. oleifera (c.v. Bhagya) plants grown in Institute’s orchard in December 2012 (average temperature was 23–25 °C). Recommended cultural practices were followed to raise the plants in field (Saini et al. 2013). Leaves were collected early in the morning, mixed thoroughly and dried using cabinet tray dryer (Armstrong Smith, India), with a capacity of 40 trays of 400 × 800 mm at 50 °C (Saini et al. 2014d). Dehydrated leaves were powdered and stored at −80 °C in amber color air tight containers until use. Content of folate in dehydrated leaves was determined using microbiological assays and HPLC.
For folate quantification, Folic Acid Casei Medium, Lactobacillus Agar and Lactobacillus Broth were obtained from HiMedia Mumbai, India. Folate binding protein, α-amylase from Aspergillus oryzae (30 units/mg) and protease from Streptomyces griseus (Type XIV, 3.5 units/mg) were purchased from Sigma-Aldrich (Bangalore, India). Affigel matrix was purchased from BIO-RAD (Gurgaon, India). Folic acid standards were purchased from Schircks Laboratories (Jona, Switzerland).
Refined Groundnut oil, corn starch, and cane sugar were procured from local supermarkets. Minerals, vitamins, cellulose, choline chloride and L-cystine were purchased from Himedia Laboratories (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India). Clinical enzyme kits namely LDL cholesterol, HDL cholesterol, triacylglycerol, total cholesterol, glucose, albumin and total protein were purchased from Agappe Diagnostics, Kerala (India).
Quantification of folate in dehydrated leaves
Trienzyme extraction of folate
Folate was extracted from the dehydrated leaves M. oleifera (under subdued light) according to the method of Aiso and Tamura (Aiso and Tamura 1998), with some modifications. Dehydrated leaves (200 mg) were homogenised with 10 ml 0.1 M sodium phosplate buffer (pH 6.1) containing 1 % ascorbic acid (w/v) and 0.1 % 2-merceptoethanol (v/v). Similarly, a blank was prepared without adding the leaf extract. Loosely capped, 50 ml digestion tubes containing the samples and the blank were placed in a boiling water bath for 5 min and then allowed to cool to room temperature. After cooling, 0.5 ml charcoal treated rat serum (De Brouwer et al. 2010), and 300 units of α-amylase from Aspergillus oryzae were added. Tubes were gently swirled; their caps were secured and they were incubated at 37 °C for 4 h. Then, the tubes were kept in a boiling water bath for 5 min to deactivate the enzymes and allowed to cool at room temperature. After cooling, 35 units of protease from Streptomyces griseus (Type XIV) were added, gently swirled and incubated overnight at 37 °C in a water bath. To deactivate the enzymes after the incubation period, 2 ml extraction buffer was added and the tubes heated for 5 min in a boiling water bath followed by cooled to room temperature. Subsequently, the tubes were centrifuged at 10,000 g for 5 min and 2 ml supernatant was mixed in 18 ml water containing 2 % extraction buffer. This sample was used for the microbiological assay. Undiluted sample was used for folate purification followed by HPLC analysis. All samples were stored at −80 °C in amber colour tubes to avoid the light mediated degradation of folate. Total folate was quantified by the microbiological assay on the same day as extraction.
Preparation of glycerol cryoprotected Lactobacillus casei
To minimize the analysis time and error between the analyses, cryoprotected Lactobacillus casei (ATCC 7469), were prepared according to standard methods (Grossowicz et al. 1981; Pandrangi and LaBorde 2004; Ortiz-Escobar et al. 2010). Briefly, 20 μl L. casei culture from Lactobacillus broth was transferred to 10 ml assay medium (Folic Acid Casei Medium) containing 250 mg/l ascorbic acid and 30 ng/l folic acid, and incubated at 140 rpm at 37 °C. After obtaining a constant optical density (OD: 0.5) at 550 nm at 14 to 20 h after inoculation, the whole culture was inoculated to 20 ml assay medium containing 250 mg/l ascorbic acid, with no added folic acid, and incubated at 140 rpm and 37 °C. After obtaining the constant OD, the culture was centrifuged and pellets were dissolved in assay medium to obtain the OD value 0.2, followed by dilution to equal volumes with sterile glycerol and water (80:20, v/v). Then, the cultures were aliquoted to 2 ml sterile cryo-vials and stored at −80 °C.
Microbiological assay of total folate
Folic acid content was assayed according to the method of AOAC (1990). Sample extracts (0.5, 1.0, 2.0, 4.0 and 5.0 ml) were assayed in triplicate. The volume in each tube was adjusted to 5 ml with deionized water. Five ml single-strength assay medium was added to each tube. Prepared assay tubes, standard curve tubes, blank and enzyme blank tubes were sterilised by autoclaving at 121 °C for 5 min. To prepare inoculum, 1.0 ml glycerol cryoprotected L. casei culture was diluted with 9.0 ml assay medium to obtain a 10 times dilution. The diluted medium was inoculated (50 μl) to each assay tube incubated at 37 °C for 20 to 24 h, and the growth response was measured at 550 nm.
Folate purification and HPLC analysis
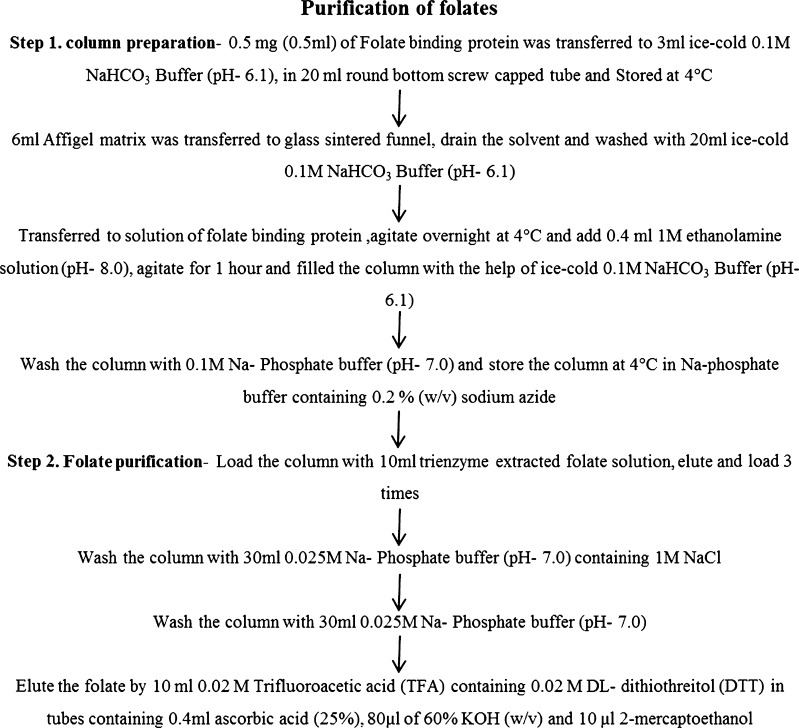
Folate was purified by immunoaffinity chromatography using folate binding protein and quantified by RP-HPLC from dehydrated leaves essentially according to the method of Konings (Konings 1999). The outline of column preparation with affigel matrix and folate binding protein, along with the purification procedure is given in Fig. 1. The binding capacity of the column was analysed by loading an excess amount of 5CH3-H4- folate. The HPLC analysis of purified folate derivatives was performed using a Shimadzu chromatograph (LC 20-AS HPLC), equipped with dual pump, fluorescent detector (RF-20A), YMC-Pack ODS-AQ column (250 × 4.6 mm ID × 5 μ) and YMC ODS-AQ guard column (10 × 5 mm ID × 5 μ). The separation and elution was accomplished by employing a binary gradient mode using solvent A (0.1 % v/v trifluoroacetic acid in water) and solvent B (acetonitrile) with an injection volume of 20 μl sample and at a flow rate of 0.8 ml/min for 25 min. The solvent system was ran as follows (% solvent A/solvent B): 0 min (10/90), 20 min (50/50), and 25 min (10/90). To quantify the folate derivatives, the fluorescent detector was set in dual wavelength mode; H4 folate, 5-CH3-H4 folate and 5-HCO-H4-folate were detected at (Ex/Em) 290/360 nm. Similarly, 10-HCO-folic acid was detected (Ex/Em) at 360/460 nm (Shohag et al. 2011). Folate standards were diluted to a concentration of 1 μg/ml (Patring et al. 2005). The actual concentrations of the folate standards solution were checked spectrometrically using the molar extinction coefficients (Jastrebova et al. 2003). Quantification of folate derivatives was based on an external calibration curve with a linear range of 5–100 ng/ml for H4-folate and 5-CH3-H4-folate, and 100–1000 ng/ml for 5-HCO-H4-folate and 10-HCO-folic acid.
Fig. 1.
Outline of column preparation and folate purification procedure
Dehydrated Moringa leaves were found to be very rich in total folate content as quantified by the microbiological assay (502.1 μg/100 g DW) and HPLC (223.69 μg/100 g DW). 5-HCO-H4-folate (502.1 μg/100 g F\DW) and H4-folate (223.9 μg/100 g FW) were found as the prominent forms of folate in dehydrated leaves of M. oleifera (Fig. 2, Table 1). In the Moringa leaf based diet formulation, dehydrated leaf powder was incorporated at the expense of corn starch to supply the 1.0 and 2.0 mg of total folate per diet, on the basis of folate content data obtained by the microbiological assay and HPLC.
Fig. 2.
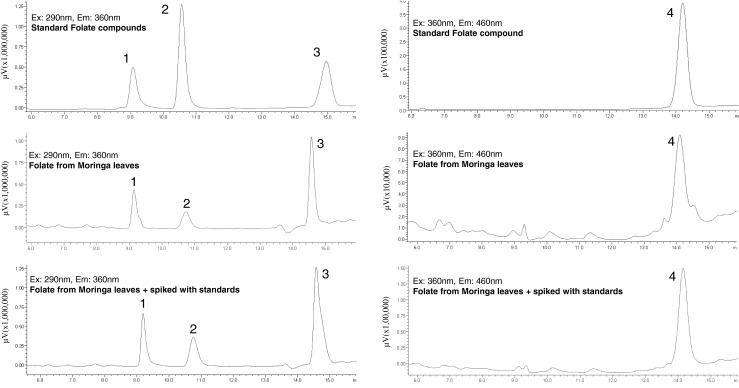
HPLC chromatograms of standards and purified folates form M. oleifera leaves. (1) 5,6,7,8-Tetrahydrofolic acid (H4 folate); (2) 5-Methyl-5,6,7,8-tetrahydrofolic acid (5-CH3-H4 folate); (3) 10-Formylfolic acid (10-HCO folic acid) and (4) 5-Formyl-5,6,7,8-tetrahydrofolic acid (5-HCO-H4 folate)
Table 1.
Folate content of fresh leaves of Moringa oleifera (c.v. Bhagya), purified by immunoaffinity chromatography and analysed by HPLC
| S/No | Folate form | Folate (μg/100 g DW) |
|---|---|---|
| 1 | 5,6,7,8-Tetrahydrofolic acid (H4 folate) | 223.9 ± 15.7 |
| 2 | 5-Methyl-5,6,7,8-tetrahydrofolic acid (5-CH3-H4 folate) | 144.9 ± 16.4 |
| 3 | 10-Formylfolic acid (10-HCO-folic acid) | 29.0 ± 8.2 |
| 4 | 5-Formyl-5,6,7,8-tetrahydrofolic acid (5-HCO-H4 folate) | 502.1 ± 43.2 |
| 5 | Total folate | 899.9 |
Values are mean ± S.D. of three replicates
Rats and diets
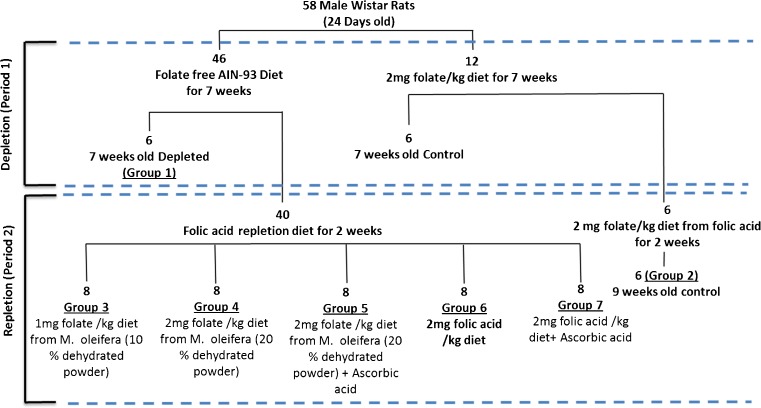
Male Wistar rats (OUTB—Wistar, IND-cft (2c) weighing 60 ± 5 g, were used in this study. The experimental protocol adopted was approved by the Institute’s Animal Ethical Committee. Animals were grouped according to the outline given in Fig. 3, housed in metabolic cages, in groups of four animals, under a 12 h light/dark cycle, at 25 ± 2 °C and 40–60 % relative humidity. Diet plan, duration of treatment and details of folate depletion and repletion schedule is given in Fig. 3. Diets (AIN-93 M) were formulated (Reeves et al. 1993) according to Table 2. In Moringa leaf based diet, 10 and 20 % dehydrated Moringa leaves were incorporated at the expense of corn starch to supply the 1.0 and 2.0 mg folate per kg diet. In folate deficient diet, 1 % succinyl sulfathiazole (w/w) was also added to prevent the growth of folate biosynthetic micro flora in gut (O’Leary and Sheehy 2001). To study the effect of ascorbic acid (AA) supplementation on folate absorption, ascorbic acid was included at 2:1 molar ratio (AA:FA) (Teucher et al. 2004).
Fig. 3.
Experimental outline of the folate depletion and repletion protocol used in the present investigation
Table 2.
Ingredient composition of the folate depleted and repleted diets
| Ingredients | Folate deficient diet | Control (No depletion) diet | 10 % MO diet | 20 % MO diet | 20 % MO + AA diet | 2 mg Folic acid diet | 2 mg folic acid + AA diet |
|---|---|---|---|---|---|---|---|
| Corn starch | 500.0 | 500.0 | 400.0 | 300.0 | 300.0 | 500.0 | 500.0 |
| Casein (85 % protein) | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Groundnut oil | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Fibre (cellulose) | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Mineral mix (AIN-93G-MX) | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix (AIN-93-VX) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| L-cystine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline bitartrate (41.1 % choline) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Moringa dry powder | 0 | 0 | 100 | 200 | 200 | 0 | 0 |
| Ascorbic acid | 0 | 0 | 0 | 0 | 0.217 | 0 | 0.217 |
| Folic acid | 0 | 0.002 | 0 | 0 | 0 | 0.002 | 0.002 |
MO Moringa oleifera, AA Ascorbic acid, #(AIN-93G-VX) deficient in folate. All the Values are in g/kg diet
At the end of experimental depletion and repletion periods, rats were fasted overnight and sacrificed under ether anaesthesia. Blood was drawn by cardiac puncture and serum was separated by centrifugation at 4 °C. Livers were dissected, rinsed with DEPC treated water, approximately 1 g of liver sample was freezed in liquid nitrogen in 15 ml RNase free falcon tubes and immediately stored at −80 °C for RNA extraction. Serum clinical enzymes and metabolites such as LDL, HDL, total cholesterol, triglycerides, glucose, albumin and total protein were analyzed by using Agape diagnostic kits, as per the manufacturer’s guidelines.
RNA extraction and real-time quantitative PCR (qPCR) analysis
Total RNA extraction was carried out using TRIzol reagent (Invitrogen, CA, USA) from 50 mg frozen liver. RNA concentration and purity were determined by spectrophotometry using Nano Drop 1000 (NanoDrop Technologies, inc., Wilmington, DE, USA). After quantification, RNA quality and integrity was analysed in a 1.5 % (w/v) agarose formamide gel. cDNA was synthesized in 40 μl reaction, using 2 μg of total RNA, 0.5 mM dNTP mix, 5 μM random hexamer, 5 μM oligo dT primers, 40 units of RNase inhibitor and 400 units of M-MLV Reverse transcriptase (Sigma Aldrich, Bangalore). cDNA was diluted with 160 μl of water to achieve 5-fold dilution, and used as the template for real-time quantitative PCR. Quantitative RT-PCR was performed in a total volume of 10 μl, including 2 μl of diluted cDNA, 100 μM for each primer, and 5 μl of 2× SsoFast EvaGreen Supermix (Bio-Rad Inc., CA, USA) on an CFX96 Touch Real-Time PCR Detection System (Bio-Rad Inc., CA, USA). The qPCR programme included a preliminary step of 95 °C for 1 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 20 s. Melt Curve was performed from 65.0 to 95.0 °C, with increment of 0.5° at every 5 s. Rattus norvegicus Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the gene expression. Relative gene expression was calculated according to a 2–ΔΔCT method (Livak and Schmittgen 2001). qPCR was performed in triplicates and the fold change in each target gene was compared with non-depleted control (90 days old), which was set to 1. Primer sequences, Tm values, PCR product length and NCBI accession numbers of the genes used in the study is given in Table 3.
Table 3.
List of oligos designed for liver folate metabolism in Rattus norvegicus
| S/No | Primer/Gene Name | Forward Primer (5′-3′) | Reverse primer (5′-3′) | NCBI Accession No. |
|---|---|---|---|---|
| 1 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | AGAACATCATCCCTGCATCC | AGTCACAGGAGACAACCTGG | NM_017008.4 |
| 2 | 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) | GGAGGTTTCAGTGTGCTTGC | CAGGCGGTGGTACCTGTAAG | NM_001039003.1 |
| 3 | Glycine N-methyltransferase (GNMT) | CGTGCTCAAGAAGACAGGCT | TTGTCGACTCCCTGTTTGCC | NM_017084.1 |
Statistical analysis
Statistical analysis was performed using the SPSS statistics 17.0 (SPSS Inc. Chicago, IL, USA). Data were analysed by one-way ANOVA, at 95 % confidence level (P < 0.05). The values are means with standard deviation for all treatments.
Results
Body weight
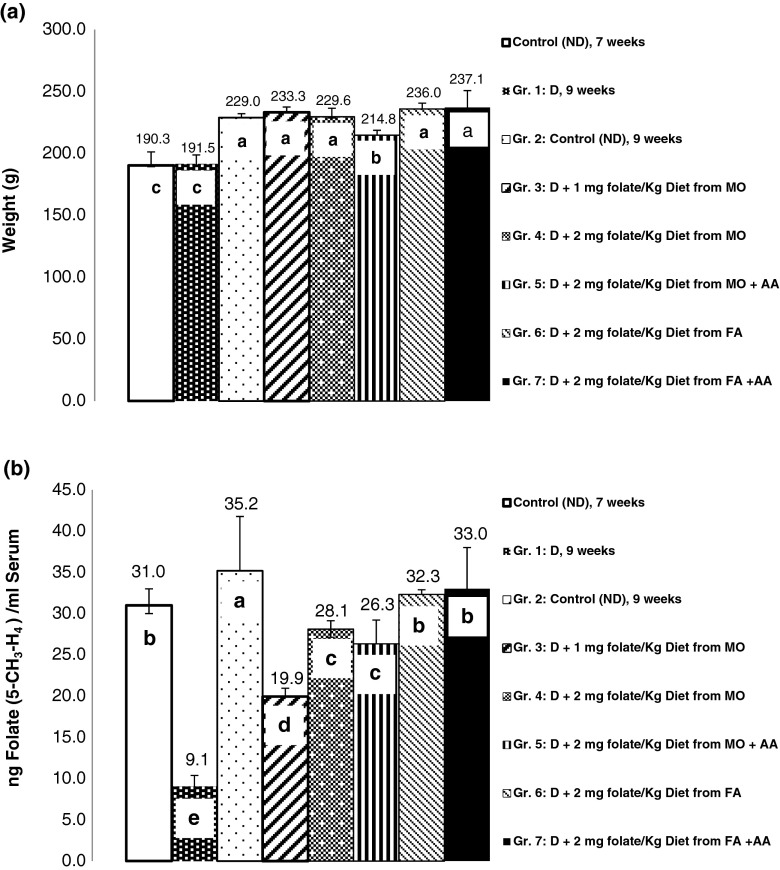
rats fed with folate-deficient diets grew in the same way during depletion period, as control diet (2 mg folate/kg diet) (Fig. 4a). During the repletion period, animals fed with 20 % Moringa leaf alone and with ascorbic acid (Group 5), showed marginally but significant lower body weight (214.8 g), compared to animals fed with folate and 10 % Moringa leaf diet. Whereas, rats fed with control diet (Group 2), 10 % Moringa leaf (Group 3), 20 % Moringa leaf (Group 4), 2.0 mg folic acid (Group 6), and 2.0 mg folic acid with ascorbic acid (Group 7), did not show any significant difference (p < 0.05) in final body weight (229.0–237.1 g).
Fig. 4.
Final body weight (a) and serum folate (b) of depleted and repleted rats. Values are mean ± S.D. of 6 (control) and 8 (treated) animals. Different letters indicate statistically significant differences between the means (P < 0.05)
Serum folate profile
Serum folate concentrations (Fig. 4b) showed a complex pattern in rats fed with different diets. Serum folate level decreased to 9.1 ng/ml in rats fed with folate depleted diet for 7 weeks compared to rats fed with non-depleted diet (31.0 ng/ml), folate content was further increased in rats fed with non-depleted (control) diet for 9 weeks (35.2 ng/ml). During folate repletion period (2 weeks), final serum folate content was absolutely highest (33.0 and 32.3 ng/ml) in rats fed with 2 mg folate and 2 mg folate with ascorbic acid, respectively. Addition of ascorbic acid in 2:1 molar ratio (ascorbic acid:Fe), did not show any significant improvement (p < 0.05) in serum folate content (Fig. 4b).
Other serum parameters
Significantly (p < 0.05) higher amount of serum triglycerides (134.0–165.6 mg/dL) was recorded in folate repleted rats (group 3–7), compared to non-depleted and depleted rats (Table 4). Similarly, content of serum LDL and total cholesterol was recorded higher in non-depleted rats (9 weeks) compared to depleted rats. Content of serum glucose, albumin and total protein was not significantly influenced (p < 0.05) with different treatments.
Table 4.
Influence of dietary Moringa dehydrated powder on serum lipid, glucose and protein profile
| Triacylglycerol (mg/dl) | Cholesterol (mg/dL) | Glucose (mg/dL) | Total protein (g/dl) | Albumin (g/dl) | |||
|---|---|---|---|---|---|---|---|
| HDL (mg/dl) | LDL (mg/dl) | Total (mg/dl) | |||||
| Non-depleted, 52 days | 109.2 ± 12.4c | 16.8 ± 2.2c | 7.0 ± 1.3bc | 49.8 ± 8.2 | 72.5 ± 11.3c | 8.81 ± 0.7c | 2.89 ± 0.23b |
| Depleted, 52 days | 109.2 ± 18.6c | 17.1 ± 3.9c | 7.3 ± 2.2cd | 34.9 ± 4.0d | 87.1 ± 9.2b | 9.20 ± 0.45c | 3.03 ± 0.071ab |
| Non-depleted, 66 days | 106.3 ± 18.3c | 21.6 ± 1.1ab | 13.2 ± 1.8a | 59.9 ± 2.7a | 84.2 ± 10.1b | 9.95 ± 0.24ab | 2.94 ± 0.09ab |
| Depleted + 10 % MO | 158.9 ± 18.4a | 19.0 ± 1.5bc | 9.3 ± 2.6bc | 44.7 ± 4.2bc | 84.9 ± 13.9b | 9.84 ± 0.58ab | 3.01 ± 0.11ab |
| Depleted + 20 % MO | 134.0 ± 18.0b | 19.6 ± 1.8a | 10.6 ± 1.8ab | 51.2 ± 4.5bc | 80.5 ± 12.3b | 10.33 ± 0.47a | 3.04 ± 0.05ab |
| Depleted + 20 % MO + AA | 165.6 ± 15.5a | 23.8 ± 7.1a | 8.7 ± 2.8bc | 51.9 ± 5.7b | 94.2 ± 13.0a | 10.16 ± 0.71b | 3.04 ± 0.17ab |
| Depleted + 2 mg Folic acid | 160.9 ± 17.9a | 19.2 ± 2.6bc | 7.9 ± 2.7bc | 45.8 ± 10.0bc | 89.1 ± 22.9a | 9.82 ± 0.94ab | 2.91 ± 0.20b |
| Depleted + 2 mg folic acid + AA | 161.0 ± 25.3a | 19.09 ± 2.3bc | 4.8 ± 1.3d | 43.4 ± 8.5c | 98.3 ± 17.8a | 9.85 ± 0.58b | 3.09 ± 0.08a |
Values are mean ± S.D. of 6 (control) and 8 (treated) animals. Different letters indicate statistically significant differences between the means (P < 0.05)
MO Moringa oleifera, AA Ascorbic acid
Relative folate bioavailability
Relative bioavailability was calculated as response of Moringa folate relative to the response of folic acid (Hannon-Fletcher et al. 2004).
X = (Serum folate in rats fed with 2 mg folate/kg diet from Moringa for 2 weeks-serum folate in 7 weeks deficient rats), Y = (Serum folate in rats fed with 2 mg folate/kg diet from folic acid for 2 weeks- serum folate in 7 weeks deficient rats)
Relative folate bioavailability from Moringa leaves was found to be ≈82 % when compared to equivalent amount of folic acid.
Expression of genes relevant to folate metabolism
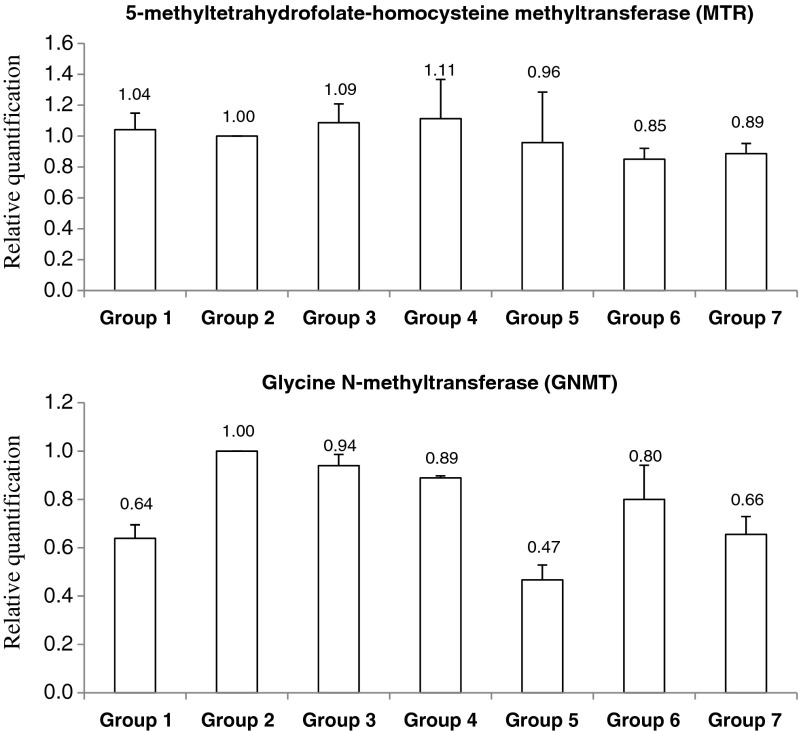
The relative expression pattern of genes relevant to folate metabolism; glycine N-methyl transferase (GNMT) and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) is given in Fig. 5. Both the studied genes did not show any significant changes in expression pattern in folate depleted as well as repleted rats (p < 0.05).
Fig. 5.
Effect of folate deficient and repletion diets on the expression of liver folate metabolite genes, determined by qRT-PCR. Relative transcript abundances of each gene were normalised to the housekeeping gene GAPDH. Values are means ± S.D. Group 1: Depleted (7 weeks), Group 2: Control (non-depleted, 9 weeks), Group 3: Depleted + 1.0 mg folate/kg diet from M. oleifera leaves (MO), Group 4: Depleted + 2.0 mg folate/kg diet from MO, Group 5: Depleted + 2.0 mg folate/kg diet from MO + ascorbic acid (AA), Group 6: Depleted + 2.0 mg folate/kg diet from folic acid (FA), Group 7: Depleted + 2.0 mg folate/kg diet from FA + AA. *Significant (P < 0.05) compared to non-depleted control (Group 2)
Discussion
The present study was designed to investigate the effect of dietary folate from Moringa leaves on body folate status and modulation of folate responsive genes in rat model by using well established folate depleted and repleted approach (Clifford et al. 1989; Saini et al. 2014a). In the present study, M. oleifera in particular was selected to establish the readily available and folate rich underutilized leafy vegetable as a source of dietary folate to prevent the folate deficiency. Folic acid was used as the source of folate in control diet. In our previous studies, dietary iron supplements from Moringa leaves were more beneficial compared to ferric citrate in overcoming the effects of iron deficiency (Saini et al. 2014a).
The different forms of folate in M. oleifera leaves have not been studied earlier. We found, for the first time that 5-CHO-H4-folate was the major form of folate in Moringa leaves, accounting for 55.8 % of the total folate, followed by H4-folate (24.9 %), 5-CH3-H4-folate (16.1 %) and 10-HCO-folic acid. The total folate content, quantified by HPLC (899.9 μg/100 g DW) was 10.2 % lower than shown by the microbiological assay. This could be due to lack of folate standards for all folate forms and loss of folate during purification. These results are in agreement with previous studies, which reported folate contents determined by HPLC to be 20–52 % lower when determined by the microbiological assay (Ruggeri et al. 1999; Konings 1999). Recently, in a comparative study among Fijian vegetables, including, Moringa oleifera leaves, taro leaves, bele leaves, amaranth leaves, okra and French bean, maximum total folate was observed in M. oleifera leaves (Maharaj et al. 2015).
The diets differed in the source of dietary folate viz. folic acid and Moringa leaf, with and without ascorbic acid supplements was well accepted by the rats. Feeding of folate deficient diet for 7 weeks caused significant decrease in serum folate content, compared to non-depleted control (7 weeks). At the end of 7 weeks depletion period, significant difference in body weight was not observed among folate depleted and control rats. However, very small, but significant difference was recorded at the end of 2 weeks repletion period. Significant decrease in body weight of folate deficient anaemic rats has been recorded in previous studies (Endoh et al. 2013).
Liver plays a crucial role in folate metabolism and expresses almost all the genes related to folate metabolism and homeostasis. GNMT is the most abundant methyltransferase in mammalian liver, comprising nearly 1 % of the soluble protein in rat liver (Takusagawa et al. 1999). An outline of one carbon metabolism and specific role of glycine N-methyl transferase (GNMT) and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) is shown in Fig. 6 (Supplement material). Among the different forms of folates, the 5-methyl tetrahydrofolate (5-CH3-H4 folate or 5-methyl-THF) plays the major role in transfer of methyl group to homocysteine by the action of methionine synthase (MTR; 5-methyltetrahydrofolate-homocysteine methyltransferase), and generate methionine (Zetterberg 2004). Glycine N-methyltransferase (GNMT) is a folate-binding protein, express in liver that converts S-adenosylmethionine (AdoMet) to S-adenosylhomocysteine (AdoHcy) while donating the methyl group. Suppression or deletion of GNMT imparts, lower cellular folate, AdoMet to AdoHcy balance, leads to cancer and other metabolic syndromes (Martínez-Chantar et al. 2008). Overexpression of GNMT, reduces the 5-methyl-THFdependent homocysteine remethylation due to higher affinity binding of GNMT with 5-methyl-THF. Overdose of retinoid compounds (Vitamin A) and methionine is reported to markedly elevate the activity and abundance of hepatic GNMT (Rowling et al. 2002). Whereas, folate derivatives inhibit the activity of GNMT (Wagner et al. 1985). In the present study, significant changes in GNMT and MTR expression were not observed during folate deficient and normal conditions. This may be because, we have supplied the sufficient amount of methionine (30 g/kg diet) and B12 vitamin, which is also known to regulate the activity of GNMT in rats (Rowling et al. 2002). Similarly, with folate, other metabolites might influence the regulation of MTR expression in liver. Supplementation of ascorbic acid, which is known to prevent the oxidation of folates, was not effective in modulating the expression of folate one carbon metabolism related genes, and folate absorption. This indicates, oxidation of folate will not influence its absorption in gastrointestinal tract of the rats.
In human and other mammals, majority of dietary folates (polyglutamates) are absorbed in the intestine by proton coupled folate transporter (PCFT) and reduced folate carrier (RFC1) after hydrolysing to monoglutamates by the action of brush-border membrane γ-glutamyl hydrolase (GCPII). Deconjugation of polyglutamates by GCPII strongly depends on pH, with optimum pH of 6.5 (Chandler et al. 1986), thus folate bioavailability is generally influenced by the pH of intestinal lumen. Tamura et al. (1976) recorded the significant decrease in the bioavailability of heptaglutamyl folate ingested with orange juice at a pH of 3.7, compared to monoglutamyl folate. Thus, alterations in the pH of the intestinal lumen during gastrointestinal diseases (atrophic gastritis) have the potential to impair the hydrolysis of polyglutamyl folates, resulting in decrease of their bioavailability.
The bioavailability of folates from various foods is depends on the content of 16 monoglutamyl (fortified food; highly bioavailable) and polyglutamyl folates (vegetables; low bioavailable) and on the presence of enhancer and inhibitors of folate absorption (Aiso and Tamura 1998). Folate intake directly influences the plasma folate concentrations, whereas tissue folate levels saturate at high folate intakes (Clifford et al. 1990). To elevate the tissue folate levels, dose of 5-formyl-THF are more beneficial than folic acid or 5- methyl-THF. Intestinal micro-biota, can synthesize the folate and it may yield inaccuracy in the estimation of available folate. So, in the present study, 1 % succinyl sulfathiazole (w/w) was also added to prevent the growth of folate biosynthetic micro-flora in gut (O’Leary and Sheehy 2001). In general, bioavailability of folate is compared to folic acid (relative bioavailability), which shows great variation in human studies, ranging from 10 to 98 % (Aiso and Tamura 1998). The bioavailability of folate form dried cabbage is reported to be 68 % in rat bioassays (Abad and Gregory 1987). In another study from green leafy vegetables, bioavailability of folate from spinach was 84 % compared with folic acid, based on folate repletion of folate-depleted rats (Babu and Lakshmaiah 1987). In the present study, relative bioavailability of folate from dehydrated leaves of Moringa was observed 81.9 %. In RDA calculations, only 50 % of natural folate is assumed to be bioavailable (DRI, Institute of Medicine 1998). Result shows that bioavailability of M. oleifera leaf folate is much higher and it can be used as a significant source of folate in diet.
In conclusion, Moringa oleifera leaf based food can be used as a significant source of folate due to equal or higher bioavailability compared to other vegetables, which are known as significant source of folate. Easy availability of Moringa leaves in rural areas can also make it a promising food for rural communities with high nutritional and nutraceutical value.
Electronic supplementary material
Overview of folate and B12 dependent homocysteine metabolism reactions involved in cellular one-carbon metabolism, with two major functions of DNA synthesis and DNA methylation (Zetterberg 2004). (DOCX 92 kb)
Acknowledgments
The authors are thankful to Council of Scientific and Industrial Research., New Delhi (India), for financial assistance.
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Abad AR, Gregory JF., 3rd Determination of folate bioavailability with a rat bioassay. J Nutr. 1987;117:866. doi: 10.1093/jn/117.5.866. [DOI] [PubMed] [Google Scholar]
- Aiso K, Tamura T. Trienzyme treatment for food folate analysis: optimal pH and incubation time for alpha-amylase and protease treatment. J Nutr Sci Vitaminol (Tokyo) 1998;44:361–370. doi: 10.3177/jnsv.44.361. [DOI] [PubMed] [Google Scholar]
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical (1990) Official methods of analysis. Vol 15th Ed AOAC Arlingt. VA
- Babu S, Lakshmaiah N. Availability of food folate by liver folate repletion in rats. Nutr Rep Int. 1987;35:831–836. [Google Scholar]
- Chandler CJ, Wang TT, Halsted CH. Pteroylpolyglutamate hydrolase from human jejunal brush borders- Purification and characterization. J Biol Chem. 1986;261:928–933. [PubMed] [Google Scholar]
- Clifford AJ, Heid MK, Müller HG, Bills ND. Tissue distribution and prediction of total body folate of rats. J Nutr. 1990;120:1633–1639. doi: 10.1093/jn/120.12.1633. [DOI] [PubMed] [Google Scholar]
- Clifford AJ, Wilson DS, Bills ND. Repletion of folate-depleted rats with an amino acid-based diet supplemented with folic acid. J Nutr. 1989;119:1956–1961. doi: 10.1093/jn/119.12.1956. [DOI] [PubMed] [Google Scholar]
- Coppin JP, Xu Y, Chen H, et al. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Funct Foods. 2013;5:1892–1899. doi: 10.1016/j.jff.2013.09.010. [DOI] [Google Scholar]
- De Brouwer V, Storozhenko S, Stove CP, et al. Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for the sensitive determination of folates in rice. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:509–513. doi: 10.1016/j.jchromb.2009.12.032. [DOI] [PubMed] [Google Scholar]
- DRI, Institute of Medicine . Dietary reference intakes for thiamin, riboflavin, niacin, vitamin b6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press (US); 1998. [PubMed] [Google Scholar]
- Endoh K, Fenech M, Umegaki K. Green tea is a poor contributor to tissue folate in a Folate Depletion-Repletion Rat Model. Food Nutr (Roma) 2013;4:136–143. doi: 10.4236/fns.2013.42019. [DOI] [Google Scholar]
- Govardhan Singh RS, Negi PS, Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods. 2013;5:1883–1891. doi: 10.1016/j.jff.2013.09.009. [DOI] [Google Scholar]
- Gregory JF, Bhandari SD, Bailey LB, et al. Relative bioavailability of deuterium-labeled monoglutamyl and hexaglutamyl folates in human subjects. Am J Clin Nutr. 1991;53:736–740. doi: 10.1093/ajcn/53.3.736. [DOI] [PubMed] [Google Scholar]
- Grossowicz N, Waxman S, Schreiber C. Cryoprotected Lactobacillus casei: an approach to standardization of microbiological assay of folic acid in serum. Clin Chem. 1981;27:745–747. [PubMed] [Google Scholar]
- Hannon-Fletcher MP, Armstrong NC, Scott JM, et al. Determining bioavailability of food folates in a controlled intervention study. Am J Clin Nutr. 2004;80:911–918. doi: 10.1093/ajcn/80.4.911. [DOI] [PubMed] [Google Scholar]
- Jastrebova J, Witthöft C, Grahn A, et al. HPLC determination of folates in raw and processed beetroots. Food Chem. 2003;80:579–588. doi: 10.1016/S0308-8146(02)00506-X. [DOI] [Google Scholar]
- Konings EJ. A validated liquid chromatographic method for determining folates in vegetables, milk powder, liver, and flour. J AOAC Int. 1999;82:119–127. [PubMed] [Google Scholar]
- Kushwaha S, Chawla P, Kochhar A. Effect of supplementation of drumstick (Moringa oleifera) and amaranth (Amaranthus tricolor) leaves powder on antioxidant profile and oxidative status among postmenopausal women. J Food Sci Technol. 2014;51:3464–3469. doi: 10.1007/s13197-012-0859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maharaj PPP, Prasad S, Devi R, Gopalan R. Folate content and retention in commonly consumed vegetables in the South Pacific. Food Chem. 2015 doi: 10.1016/j.foodchem.2015.02.096. [DOI] [PubMed] [Google Scholar]
- Martínez-Chantar ML, Vázquez-Chantada M, Ariz U, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63:529–536. doi: 10.1079/PNS2004383. [DOI] [PubMed] [Google Scholar]
- Nadeem M, Azeem MW, Rahman F (2014) Assessment of transesterified palm olein and Moringa oleifera oil blends as vanaspati substitutes. J Food Sci Technol 1–7. doi:10.1007/s13197-014-1271-4 [DOI] [PMC free article] [PubMed]
- Neeha VS, Kinth P. Nutrigenomics research: a review. J Food Sci Technol. 2012;50:415–428. doi: 10.1007/s13197-012-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary K, Sheehy PJA. Influence of folic acid-fortified foods on folate status in a folate depletion-repletion rat model. Br J Nutr. 2001;85:441–446. doi: 10.1079/BJN2000288. [DOI] [PubMed] [Google Scholar]
- Ortiz-Escobar TB, Valverde-González ME, Paredes-López O. Determination of the folate content in cladodes of nopal (Opuntia ficus indica) by microbiological assay utilizing Lactobacillus casei (ATCC 7469) and enzyme-linked immunosorbent assay. J Agric Food Chem. 2010;58:6472–6475. doi: 10.1021/jf100503v. [DOI] [PubMed] [Google Scholar]
- Pandrangi S, LaBorde LF. Optimization of microbiological assay of folic acid and determination of folate content in spinach. Int J Food Sci Technol. 2004;39:525–532. doi: 10.1111/j.1365-2621.2004.00812.x. [DOI] [Google Scholar]
- Patring JDM, Jastrebova JA, Hjortmo SB, et al. Development of a simplified method for the determination of folates in baker’s yeast by HPLC with ultraviolet and fluorescence detection. J Agric Food Chem. 2005;53:2406–2411. doi: 10.1021/jf048083g. [DOI] [PubMed] [Google Scholar]
- Pawar N, Gandhi K, Purohit A, et al. Effect of added herb extracts on oxidative stability of ghee (butter oil) during accelerated oxidation condition. J Food Sci Technol. 2014;51:2727–2733. doi: 10.1007/s13197-012-0781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rowling MJ, McMullen MH, Chipman DC, Schalinske KL. Hepatic glycine n-methyltransferase is up-regulated by excess dietary methionine in rats. J Nutr. 2002;132:2545–2550. doi: 10.1093/jn/132.9.2545. [DOI] [PubMed] [Google Scholar]
- Ruggeri S, Vahteristo LT, Aguzzi A, et al. Determination of folate vitamers in food and in Italian reference diet by high-performance liquid chromatography. J Chromatogr A. 1999;855:237–245. doi: 10.1016/S0021-9673(99)00674-3. [DOI] [PubMed] [Google Scholar]
- Saini RK, Manoj P, Shetty NP, et al. Dietary iron supplements and Moringa oleifera leaves influence the liver hepcidin messenger RNA expression and biochemical indices of iron status in rats. Nutr Res. 2014;34:630–638. doi: 10.1016/j.nutres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Saini RK, Prashanth KVH, Shetty NP, Giridhar P. Elicitors, SA and MJ enhance carotenoids and tocopherol biosynthesis and expression of antioxidant related genes in Moringa oleifera Lam. Leaves. Acta Physiol Plant. 2014;36:2695–2704. doi: 10.1007/s11738-014-1640-7. [DOI] [Google Scholar]
- Saini RK, Saad KR, Ravishankar GA, et al. Genetic diversity of commercially grown Moringa oleifera Lam. cultivars from India by RAPD, ISSR and cytochrome P450-based markers. Plant Syst Evol. 2013;299:1205–1213. doi: 10.1007/s00606-013-0789-7. [DOI] [Google Scholar]
- Saini RK, Shetty NP, Giridhar P. Carotenoid content in vegetative and reproductive parts of commercially grown Moringa oleifera Lam. cultivars from India by LC–APCI–MS. Eur Food Res Technol. 2014;238:971–978. doi: 10.1007/s00217-014-2174-3. [DOI] [Google Scholar]
- Saini RK, Shetty NP, Giridhar P. GC-FID/MS analysis of fatty acids in Indian cultivars of Moringa oleifera: potential sources of PUFA. J Am Oil Chem Soc. 2014;91:1029–1034. doi: 10.1007/s11746-014-2439-9. [DOI] [Google Scholar]
- Saini RK, Shetty NP, Giridhar P, Ravishankar GA. Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3. Biotech. 2012;2:187–192. [Google Scholar]
- Saini RK, Shetty NP, Prakash M, Giridhar P. Effect of dehydration methods on retention of carotenoids, tocopherols, ascorbic acid and antioxidant activity in Moringa oleifera leaves and preparation of a RTE product. J Food Sci Technol. 2014;51:2176–2182. doi: 10.1007/s13197-014-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti M, Stella L, Shearer EJ, Stover PJ. Modeling cellular compartmentation in one-carbon metabolism. WIREs Syst Biol Med. 2013 doi: 10.1002/wsbm.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohag MJI, Wei Y, Yu N et al (2011) Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. J Agric Food Chem 59:12520–12526 [DOI] [PubMed]
- Takusagawa F, Ogawa H, Fujioka M (1999) Glycine N-methyltransferase, a tetrameric enzyme. In: Cheng X, Blumenthal RM (eds). Adenosylmethionine-Depend. Methyltransferases Struct. Funct. World Scientific publishing, Singapore, pp 93–122
- Tamura T, Shin YS, Buehring KU, Stokstad ELR. The availability of folates in man: effect of orange juice supplement on intestinal conjugase. Br J Haematol. 1976;32:123–134. doi: 10.1111/j.1365-2141.1976.tb01882.x. [DOI] [PubMed] [Google Scholar]
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985;127:746–752. doi: 10.1016/S0006-291X(85)80006-1. [DOI] [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep. 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H. Methylenetetrahydrofolate reductase and transcobalamin genetic polymorphisms in human spontaneous abortion: biological and clinical implications. Reprod Biol Endocrinol RBE. 2004;2:7. doi: 10.1186/1477-7827-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of folate and B12 dependent homocysteine metabolism reactions involved in cellular one-carbon metabolism, with two major functions of DNA synthesis and DNA methylation (Zetterberg 2004). (DOCX 92 kb)