Abstract
Lack of nutrients in cooking water, high energetic costs, high water consumption and recycling are some drawbacks of vegetable blanching. Those disadvantages could be bypassed using microwave blanching. Three blanching methods (microwave, boiling water and steaming) were compared in this study in order to determine their effects on some functional properties of broccoli. In addition, the thermal damage on broccoli colour was evaluated. The effectiveness of each blanching process was performed measuring the lost of peroxidase activity, that results more rapidly in microwaves and steam treatments (50 and 60 s respectively) than in boiling water treatment (120 s). The colour indexes did not allow to discriminate a significant difference among treatments. The increase of treatment time caused a vitamin C decrease in samples blanched by boiling water and steam; this trend was not observed in microwaved samples. The phenols content did not significantly vary depending both on type and on time of treatment.
Keywords: Blanching, Brassica oleracea, Microwave, Phenolic compounds, Vitamin C, Peroxidase activity
Introduction
Many Brassicaceae crops (e.g., Brussels sprouts, broccoli, cauliflower, kale, etc.) commonly known us “cruciferous vegetables” as they belong to the family of Cruciferae, are widely recognized for their contribution to human nutrition and other health benefits (Salunkhe and Kadam 1998; Roy et al. 2009). Epidemiological studies suggest that high dietary intake of fruit and vegetables protects against tumorigenesis in many tissues (Liu et al. 2000; Knekt et al. 2002). The health beneficial effects seem to be connected to their high content of vitamins, minerals, dietary fibre and other constituents such as floavonoids, carotenoids and glucosinolates (Bengtsson et al. 2006; Monero et al. 2010). It is well known that certain quality attributes like nutrients content, texture, colour and flavour are affected during storage of untreated vegetables (Viña et al. 2007). This is due to the action of enzymes which are usually inactivated by thermal treatment of blanching. Close to traditional treatments that use steam or hot water, the application of microwaves, radiofrequency or infrared radiations are also used (Fellows 1987).
On the one hand, the exposure to heat is effective in reducing the incidence of degrading reaction during storage, on the other, it produces modifications in cellular structure and composition (Philippon 1984) with a consequently significant destruction of nutrients and therefore a decrease of the nutritional quality (Lin and Chang 2005).
Several essays are needed to find the most adequate technology and processing time for each particular product in order to inactivate the enzymes responsible for the main quality damage, minimizing, at the same time, undesirable losses of quality attributes. In that respect, it is important to identify which is the main enzyme that determines the spoilage and its inactivation kinetic in the studied species and variety (Philippon 1984; Williams et al. 1986). Peroxidase are known to be the most heat stable enzymes in vegetables (Arnnok et al. 2010), and their inactivation is usually used to indicate the adequacy of blanching (Akyol et al. 2004; Goncalves et al. 2009).
Nevertheless, cited studies (Philippon 1984; Williams et al. 1986) demonstrate that this enzyme is not generally responsible for the main degradation reactions, but other more sensible enzymes, e.g., lipoxygenase. Therefore, blanching until total peroxidise inactivation generally implies over-processing with consequent unnecessary quality losses. The spherical shape of vegetables often makes blanching difficult since, to reach the effective temperature at the centre and to ensure enzymatic inactivation, the most external part of the vegetable must be processed for long periods at high temperatures. This produces unavoidably a “cooking” process that affects mainly the physical and chemical characteristics of vegetable (Zhang and Hamauzu 2004).
To overcome these limitations, the microwave technology could take the place of conventional thermal treatment in food industry thanks to its greater penetration depth able to provide a more homogeneous heating in a shorter time (Mudgett 1989; Brody 1992; Tong et al. 1994). These elements would considerably reduce blanch time and, consequently, improve product quality characteristics.
Since the determination of temperature profiles during microwave heating is more difficult than that for other traditional heating methods and many commonly methods such as use of thermometers and thermocouples or the insertion of fiber optic probes at various position in the food are limited in this process (Berek and Wickersheim 1988; Yang and Gunasekran 2001), the use of enzyme (peroxidase) as time – temperature indicator could be a fast and easy alternative method to monitoring microwave thermal process (Van Loey et al. 1996; Yingjie et al. 2002).
On the whole, microwave blanching would benefit the industry by decreasing energy costs, water use and clean up costs and would result in a more desirable product (Barrett et al. 2000).
This study is aimed at investigating the influence of three blanching methods (microwave, boiling water and steaming) on some functional properties of broccoli in particular polyphenolics and vitamin C content.
Measurements of peroxidase activity was performed as indicator of blanching process effectiveness.
Materials and methods
Sample preparation
Fresh broccoli (Brassica oleracea L., var. Italica) purchased from local markets in Foggia (Italy) were randomly sampled, deprived of external leaves and ligneous stems. The obtained bunches (a 400 g weight) were washed under running tap water and cut into florets of about 25 g.
Blanching processes
-
Hot water blanching
Samples of 25 g were dipped in boiling water (sample: tap water ratio 1:10 w/w) and blanched for 30, 60, 90, 120 and 180 s respectively (Büchi Bath, mod. R-114, Switzerland). After treatment, they were drained off and placed on blotting paper before the succeeding processes. Blanching experiments were done in duplicate.
-
Steam blanching
Floret samples were leant on a plastic grid placed into a cylindrical foil tube (diameter 14 cm) and crossed by steam (one at a time). Samples were blanched for 30, 60, 90, 120 and 180 s respectively. After the treatment they were placed on blotting paper before the succeeding processes. Blanching experiments were done in duplicate.
-
Microwave blanching
One at a time, samples of florets (25 g) were leant on a plastic grid placed into a 500 mL beaker and microwaved by an oven (Samsung CE 116 KT) working at 2450 MHz–900 W. The beaker was covered with a pyrex plate in order to prevent water loss. Samples were blanched for 40, 50, 60, 70 and 80s respectively. After the heating treatments, samples were placed on blotting paper. Blanching experiments were done in duplicate.
Efficiency of the three blanching methods was tested by determination of peroxidase enzyme (POD) inactivation.
Extraction and enzymatic assays
Twenty g of blanched samples were placed into a chilled blender (Warning Commercial, Torrington, Connecticut, USA) with 2 g of insoluble polyvinylpolypyrrolidone (PVPP) and 40 mL of extracting buffer (0.05 M potassium phosphate/0.05 M citric acid/5 % sodium chloride; pH 6.4). They were blended for 60 s and filtered through cheesecloth (Whatman n.1). The filtrate was centrifuged (ALC mod. 4239R refrigerated centrifuge) at 4 °C and 10000 rpm for 15 min. The supernatant was filtered by a Whatman n.1 filter paper under vacuum. All operations were done in an ice bath. This procedure was doubly and separately repeated for each of the blanching times and processes. Extracts were kept at 4 °C until assays were carried out.
The activity of peroxidase was determined by using guaiacol as hydrogen donors and following for 5 min the tetraguaiacol formation. The reaction mixture contained 1 mL of a 1:10 dilution of broccoli extract in distilled water, 1.35 mL of 5 mM guaiacol, 0.2 mL of 2 mM h hydrogen peroxide and 1.35 mL of 20 mM sodium phosphate buffer, pH 6.5, in a total volume of 3 mL (cuvette capacity). The cuvette was placed in a warm bath for 5 min at 30 °C (in order to activate the enzymes eventually present). After, determination of activity was performed in triplicate using a Beckman spectrophotometer (mod. DU 640 U.S.A.) set at 470 nm and expressed as percentage of activity units/g fresh tissue.
It was assumed that the total initial peroxidise activity was 100 UA/g.
Colour determination
Since the broccoli surface is intrinsically not flat at all, the colorimetric measurements were performed on homogenate samples (broccoli florets:distilled water ratio 1:2 w/w homogenated for 2 min by a blender.
Colour assessments were performed with a Minolta Chroma Meter CR-300 (Minolta, Osaka, Japan) by using CIE Lab system. The instrument was calibrated with a white standard tile (Y = 92.8; x = 0.3132; y = 0.3192). The experimental data of L*, a* and b* were expressed in terms of hue angle or tonality, defined as [(arctang(b*/a*) + 180], and chroma or saturation index defined as (a*2 + b*2)1/2, since these variables give more information related to the spatial colour distribution (Little 1975; Ihl et al. 1994).
Extraction and determination of ascorbic acid
Ten g of samples were homogenized in a chilled blender for 2 min with 100 mL of 0.2 M sodium di-hydrogen phosphate buffer (pH = 2.14) and at firs filtered through Whatman n.1 filter paper followed by a cellulose acetate 0.45 μm Mollipore filter (Millipore Corporate, Billerica, MA, USA).
Twenty μL of clarified sample was injected onto a Symmetry C18 column, 5 μm, 150 × 4.6 mm (Waters s.p.a., Vimodrone, Milan, Italy) and eluted with an isocratic mobile phase of 0.2 M sodium dihydrogen phosphate at a flow rate of 0.5 mL/min. Ascorbic acid was detected by a Waters 2487 Dual λ Absorbance Detector set at 254 nm. For identification and quantification, a standard ascorbic acid solution (Merck s.p.a., Milan, Italy) was employed. Extractions and determinations were carried out in duplicate. Results were expressed as mg of ascorbic acid/100 g of dry weight.
Extraction and determination of phenolics
Blanched samples (3 g) were extracted twice with 15 mL of 80 % methanol for 2 min through a laboratory blender (Warning Commercial, Torrington, CT, USA). The mixture was then centrifuged at 4 °C and 10000 rpm for 15 min, and the supernatant was evaporated under reduced pressure (Rotavapor Laborota 4000-efficient, Heidolph Instruments, Milan, Italy).
The aqueous extract were diluted with 5 mL water (total volume ~10 mL). Total phenolics were determined using the Folin-Ciocalteau reagent. One hundred μL of aqueous extract was added of 3 mL distilled water, 0.5 mL Folin-Ciocalteu reagent. After 3 min, 2 mL of 20 % sodium carbonate was added and the mixture was thoroughly mixed. After 60 min incubation, the absorbance was measured at 650 nm using a UV/Vis spectrophotometer (Beckman DU 640, Fullerton, CA, USA) using gallic acid as standard. The results were expressed as mg gallic acid/g dry weight (Singh et al. 2007).
Statistical analysis
All experimental data were analysed using the one way ANOVA and Fisher’s test by Statsoft ver. 6.0 (Tulsa, OK, USA) software. A p-level < 0.05 was used where values were described as being significant.
Results and discussion
Measurement of blanching efficacy
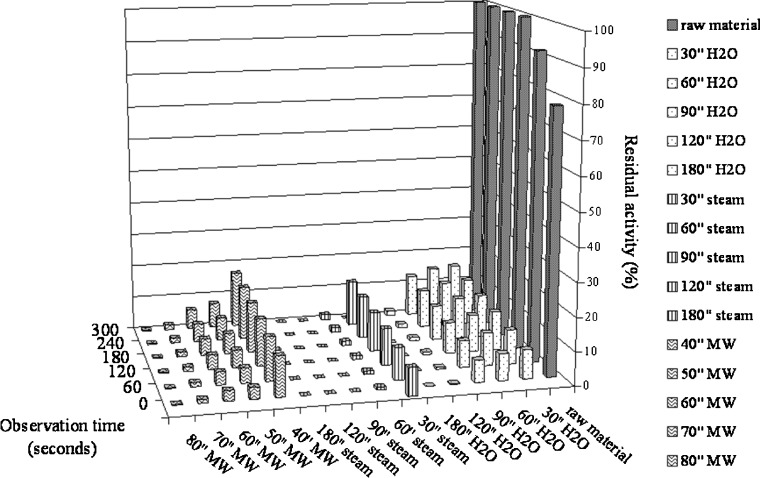
Figure 1 shows the residual activities (%) as a function of time for the three different treatments. As expected, the results show that the inactivation degree increases with the increasing of treatment time both for traditional blanching treatments (hot water and steam) and microwave treatment. 90 % of POD activity was lost within the first 90 s in hot water blanching, within 50 s in microwave blanching and within 30 s in steam blanching. Reduction of 90 % activity of indicator enzyme was aimed for determination of adequate blanching conditions since it was recommended for optimum quality of non-pasteurized vegetable (Bahçeci et al. 2005).
Fig. 1.
Peroxidase inactivation as a function of heating times and type of treatment
The total inactivation of peroxidise was achieved in 80 s using the microwave treatment, while at least 90 and 120 s were necessary if steam or hot water were respectively used. However, these prolonged processing time could cause an unnecessary cooking effect of broccoli.
Effect of blanching on colour of broccoli florets
Results related to luminescence (L*) (Table 1) as affected by different treatments suggested that this index was not able to discriminate any significant difference either among treatments or among blanching times within each treatment, as it resulted from p-level > 0.05.
Table 1.
Luminosity values (L*) of samples submitted to different blanching treatments
| Treatment time (seconds) | |||||||
|---|---|---|---|---|---|---|---|
| MICROWAVE BLANCHING | |||||||
| Observation time (hours) | Raw sample | 30 | 40 | 50 | 60 | 70 | 80 |
| 0 | 45.97 | 46.72 | 47.77 | 46.45 | 48.93 | 48.04 | 50.53 |
| 6 | 45.32 | 46.54 | 46.90 | 48.06 | 48.44 | 47.32 | 50.47 |
| 12 | 44.76 | 47.26 | 44.01 | 50.13 | 51.72 | 51.64 | 51.76 |
| 24 | 46.68 | 48.97 | 47.90 | 51.02 | 52.20 | 51.89 | 51.72 |
| 48 | 46.77 | 49.87 | 48.59 | 51.30 | 52.36 | 51.61 | 51.95 |
| HOT WATER BLANCHING | |||||||
| Observation time (hours) | Raw sample | 30 | 60 | 90 | 120 | 180 | |
| 0 | 45.97 | 51.06 | 49.98 | 52.16 | 46.68 | 47.50 | |
| 6 | 45.32 | 48.92 | 49.72 | 51.42 | 46.46 | 47.13 | |
| 12 | 44.76 | 49.42 | 49.25 | 51.86 | 45.87 | 45.77 | |
| 24 | 46.68 | 51.28 | 53.21 | 56.10 | 50.23 | 51.02 | |
| 48 | 46.77 | 53.57 | 54.17 | 56.99 | 51.19 | 50.93 | |
| STEAM BLANCHING | |||||||
| Observation time (hours) | Raw sample | 30 | 60 | 90 | 120 | 180 | |
| 0 | 45.97 | 47.45 | 47.46 | 49.41 | 49.36 | 52.11 | |
| 6 | 45.32 | 48.37 | 47.29 | 48.18 | 48.11 | 51.59 | |
| 12 | 44.76 | 47.98 | 48.19 | 48.21 | 48.85 | 51.01 | |
| 24 | 46.68 | 53.25 | 51.97 | 53.32 | 52.91 | 54.40 | |
| 48 | 46.77 | 53.65 | 53.71 | 53.26 | 55.45 | 57.33 | |
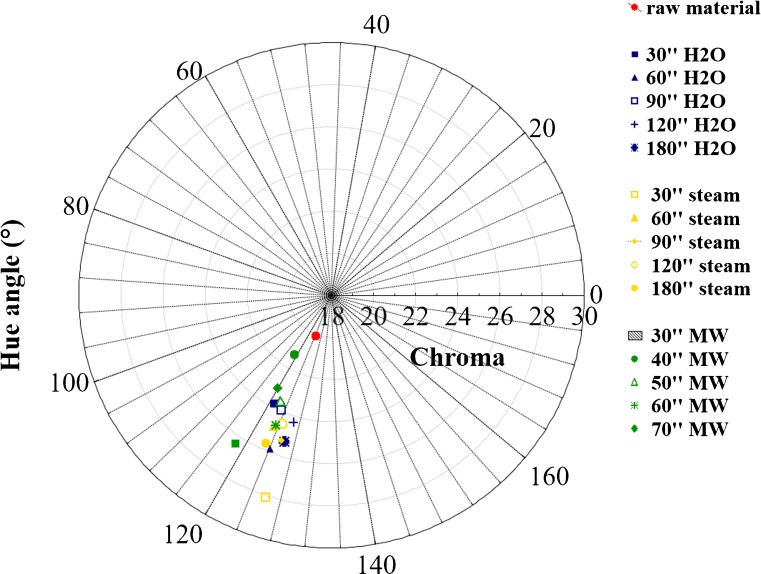
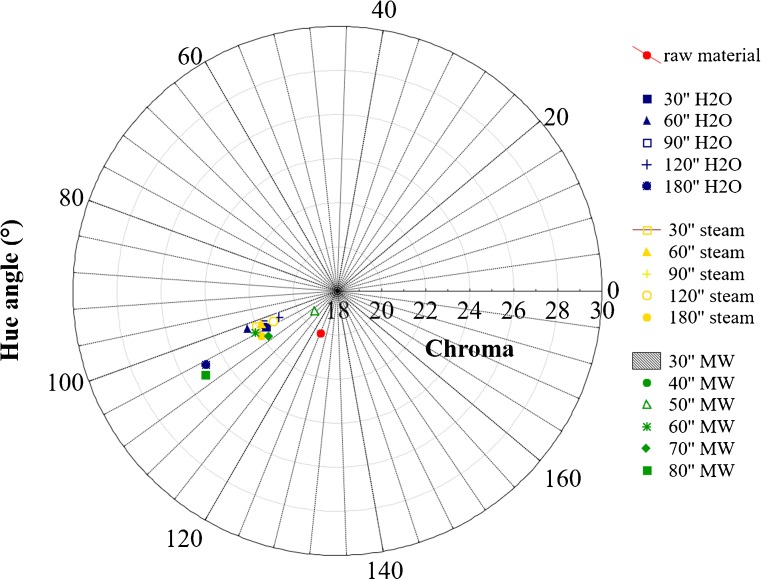
Moreover, none correlation between POD inactivation and L* index was found since a permanence of POD activity presumes a decrease in luminescence, as clearly showed by other indexes chosen in this research such as hue angle and chroma (see Figs. 2 and 3). Probably translucent surfaces like those of blanched vegetables invalidate this index.
Fig. 2.
Hue angle and chroma values of raw broccoli and blanched samples just homogenized
Fig. 3.
Hue angle and chroma values of samples after 48 h from blanching
Figures 2 and 3 show hue angle and chroma values of fresh broccoli, hot water, steamy and microwaved blanched samples. Colour measurements were carried out on samples just homogenized and after 48 h of storage in air.
At the beginning of analysis, raw sample showed the typical green colour of fresh vegetables represented by a 124° angle, in according to Miglio et al. (2008), while microwaved blanched samples showed overlapped values in the yellow zone (hue angle values between 118° for the shortest treatment and 123° of 60 s one). The values of b* (yellow parameter) changed according to hue angle (data not shown). On the contrary, samples blanched by hot water and steam showed an initial increase in green colour.
Data relating to conventional treatments were in agreement with the study conducted by Lin and Brewer (2005) that demonstrated as blanched peas were visually lighter green than unblanched peas immediately after blanching and after 12 weeks of frozen storage whereas they did not observe any significant difference for a* and b* value of blanched frozen green peas.
This change in green colour due to heat treatments has been reported in literature (Tijskens et al. 2001). Attention is focused on prolonged heating at high temperature, e.g., during sterilisation, or else the vegetables were already blanched before colour measures were performed (Gupte et al. 1964; Gunawan and Barringer 2000). Only a few authors mentioned an initial increase upon heating: Lau et al. (2000) noticed an initial increase in green colour of green asparagus at heat treatment between 70 and 98 °C. MacKinney and Weast (1940); Meyer (1960) and Woolfe (1979) attributed this initial increase to air removal around the fine hairs on the surface of the plant and to the expulsion of air between the cells. Both processes were assumed to alter the surface reflecting properties. Changes in hue angle can be observed during time for every treatment: nevertheless, microwaved blanching induced the smaller hue angle variation respect to untreated samples.
Proportion of grey component characterizing colour is given by values of chroma: as this index decreases, colour becomes less intense changing from a vivid to a dull green (Lancaster et al. 1997). In our case a not significant difference in colour saturation (chroma) was noticed between samples differently blanched: in particular, microwaved samples showed a green colour less brilliant than those of traditionally blanched samples, to exception of samples blanched for 80 s, for which a value of 26 is recorded. This was due to a greater loss of water suffered by microwaved samples and also to the thermal degradation of the chlorophyll. Yuan et al. (2009) have showed that treatments as boiling water and microwave cooking led a great loss of chlorophyll in broccoli, while steaming did not cause significant loss of chlorophyll content.
Van Loey et al. (1998) reported that the increase of the temperature in a range between 80 and 120 °C led the increase of the rate of reaction degradation of a and b chlorophylls in aqueous extracts of broccoli. Moreover, many authors (Sweeney and Martin 1958; Buckle and Edwards 1970; Steet and Tong 1996) noticed a smaller stability to the heat of the chlorophyll a, that is most important pigment in green vegetables.
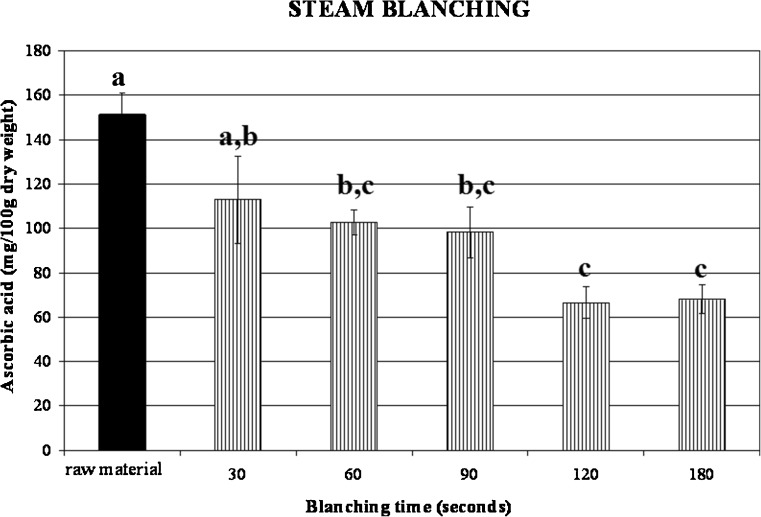
Effect of blanching on ascorbic acid content
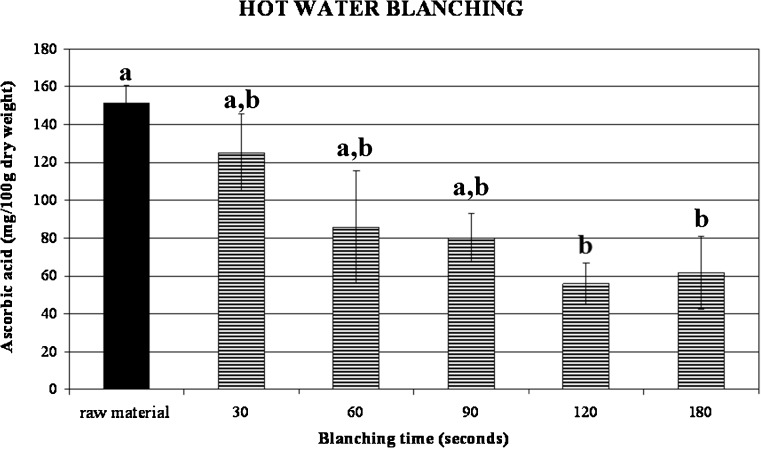
Ascorbic acid content of fresh broccoli was extremely variable and its content depends on different maturity at harvest, condition of growing, soil state and postharvest storage (Podsedek et al. 2006). In this work an initial content of ascorbic acid of about 151.32 mg/100 g dry weigh was found in raw broccoli. Hot water and steam blanching significantly decrease the ascorbic acid content to 80.13 and 112.92 mg/100 g respectively (Figs. 4 and 5). These values were referred to the minimum blanching times that were valued in 90 s for hot water blanching and 30 s for steamy blanching. It can be appreciated that, for both treatments, the longer the blanching time the higher the loss in ascorbic acid. The lower retention of broccoli blanched by hot water could be attributed to the readily lost of nutrients through leaching, since it is known that blanching by immersion in hot water produces higher ascorbic acids losses than blanching with steam (Arroqui et al. 2001; Vallejo et al. 2002). These losses need to be taken into account when calculating the dietary intake of these compounds from cooked broccoli.
Fig. 4.
Ascorbic acid content changes in hot water blanched broccoli as a function of treatment time
Fig. 5.
Ascorbic acid content changes in steam blanched broccoli as a function of treatment time
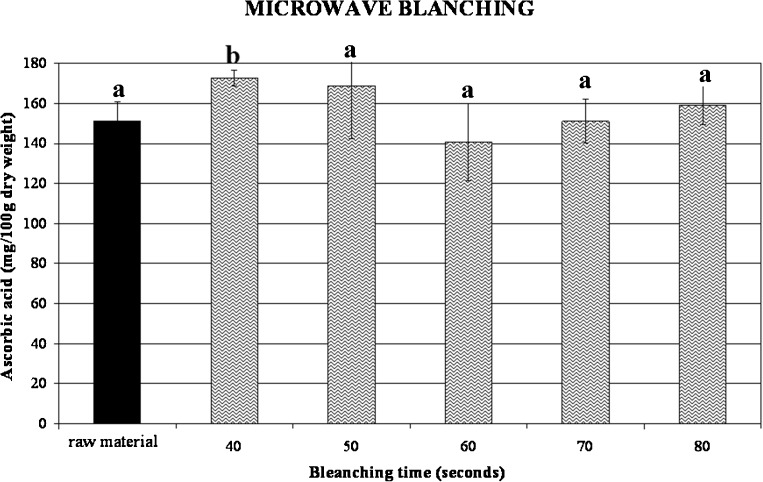
Conversely, microwave treatment induced a considerable increase of ascorbic acid content (Fig. 6). These results are in accordance with many authors who reported the increase of certain organic compounds content such as glucosinolates (Verkerk and Dekker 2004; Oerlemans et al. 2006) in microwaved vegetables tissues. These authors explained this phenomenon by an increase in chemical extractability from plant tissue after heating.
Fig. 6.
Ascorbic acid content changes in microwave blanched broccoli as a function of treatment time
Effect of blanching on total phenolics
References related to phenols content in treated vegetables is scanty and data very contrasting. This is probably due to the extreme variability of raw material as we see before for ascorbic acid content and for all antioxidants in general (Podsẹdek et al. 2006).
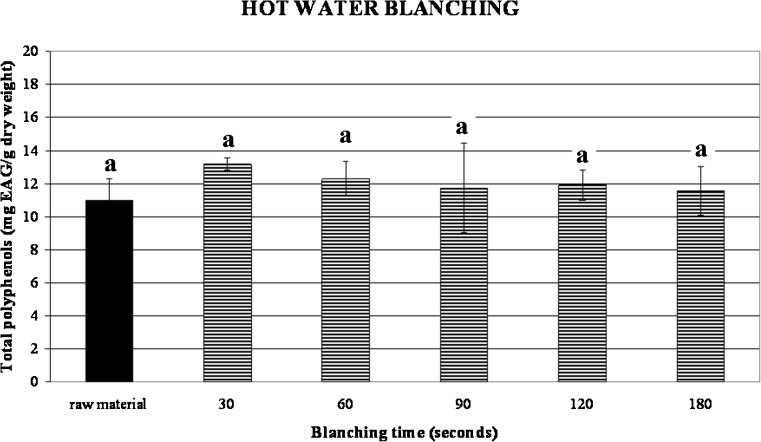
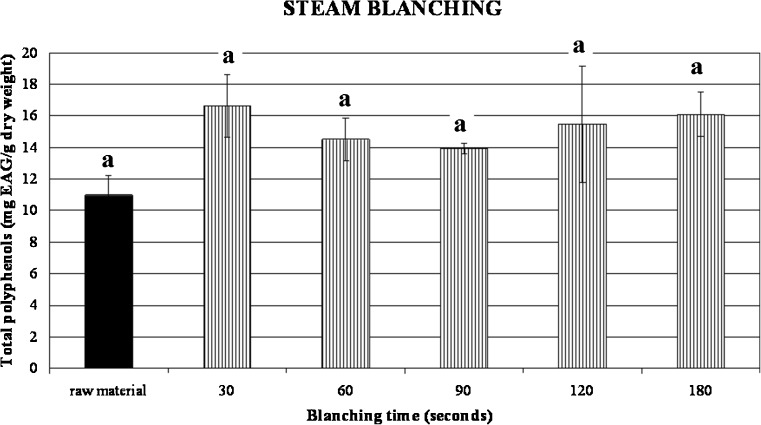
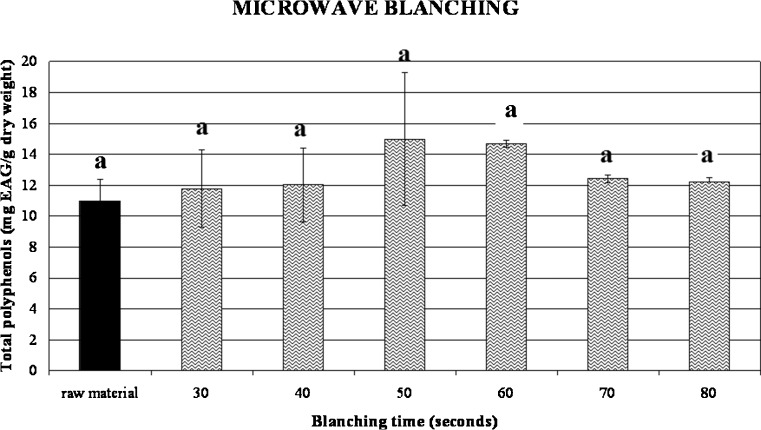
Our samples had in average 10.88 mg EAG/g dry weight and both hot water blanching and steam blanching did not promote any damaging effect (Figs. 7, 8, and 9). In fact the values of phenols did not significantly vary depending on treatment time as it results from p > 0.05. These results are in accordance with Turkmen et al. (2005) that reported a total phenol content of 12.05 mg EAG/g dry weight for the same cultivar of broccoli. Moreover, values related to microwave blanching showed a small increase of phenolics already noticed in previous works (Stewart et al. 2000; Turkmen et al. 2005) for different vegetables such as green beans, peppers and broccoli after steam and microwave blanching treatments. This behaviour was attributed by Stewart et al. (2000) to the increase of free phenols as a consequence of heating treatments.
Fig. 7.
Influence of different hot water blanching times on total phenolics content of broccoli
Fig. 8.
Influence of different steam blanching times on total phenolics content of broccoli
Fig. 9.
Influence of different microwave blanching times on total phenolics content of broccoli
Conclusion
Compared with most food, vegetables contain so many quality aspects. As expected many of them were lost when inadequate blanching times were used. This research allowed to assert that microwave heating did not negatively affect the nutritional characteristics of broccoli. Time necessary to enzymes inactivation by microwave was higher than that by steam but lower than that by hot water.
In conclusion, this technology, if appropriately used as blanching method, could benefit the industry by decreasing water use, clean up costs, energy costs and would result in a more desirable product in terms of retention of nutrients. To complete this study it will be desirable a further examination on mechanisms that allowed the formation of certain organic compounds as a consequence of heating treatments and their interaction.
References
- Akyol C, Bayindirli A, Alpas H (2004) Effect of combined treatment of high hydrostatic pressure and mild heat on peroxidise inactivation in green beans, peas and carrots. Proceedings of IFT Annual Meeting, July 12–16, Las Vegas, NV, USA
- Arnnok P, Ruangviriyachai C, Mahachai R, Techawongstien S, Chanthai S. Optimization and determination of polyphenol oxidase and peroxidase activities in hot pepper (Capsicum annuum L.) pericarb. Int Food Res J. 2010;17:385–392. [Google Scholar]
- Arroqui C, Rumsey TR, Lopez A, Virseda P. Effect of different soluble solids in the water on the ascorbic acid losses during blanching of potato tissue. J Food Eng. 2001;47:123–126. doi: 10.1016/S0260-8774(00)00107-2. [DOI] [Google Scholar]
- Bahçeci KS, Serpen A, Gökmen V, Acar J. Study of lipoxygenase and peroxidise as indicator enzymes in green beans: change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. J Food Eng. 2005;66:187–192. doi: 10.1016/j.jfoodeng.2004.03.004. [DOI] [Google Scholar]
- Barrett DM, Garcia EL, Russel GF, Ramirez E, Shirazi A. Blanch time and cultivar effects on quality of frozen and stored corn and broccoli. J Food Sci. 2000;65:534–540. doi: 10.1111/j.1365-2621.2000.tb16043.x. [DOI] [Google Scholar]
- Bengtsson GB, Schöner R, Lombardo E, Schöner J, Borge GIA, Bilger W. Chlorophyll fluorescence for non-destructive measurement of flavonoids in broccoli. Postharvest Biol Technol. 2006;39:291–298. doi: 10.1016/j.postharvbio.2005.11.003. [DOI] [Google Scholar]
- Berek HE, Wickersheim KA. Measuring temperatures in microwavable packages. J Packag Technol. 1988;2:164–168. [Google Scholar]
- Brody AL (1992) Microwave food pasteurization, sterilization and packaging. Food Technology International Europe, 67–71
- Buckle KA, Edwards RA. Chlorophyll, colour and pH changes in HTST processed green pea puree. J Food Technol. 1970;5:173–186. doi: 10.1111/j.1365-2621.1970.tb01555.x. [DOI] [Google Scholar]
- Fellows P (1987). Blanching. In W.C.H. Publisher, Food Processing Technology, 201–209
- Goncalves EM, Pinheiro J, Alegria C, Abreu M, Brandao TRS, Silva CLM. Degradation kinetics of peroxidase enzyme, phenolic content, and physical and sensorial characteristics in broccoli (Brassica oleracea L. ssp. Italica) during blanching. J Agric Food Chem. 2009;57(5370):5375. doi: 10.1021/jf900314x. [DOI] [PubMed] [Google Scholar]
- Gunawan MI, Barringer SA. Green colour degradation of blanched broccoli (Brassica oleracea) due to acid and microbial growth. J Food Process Preserv. 2000;24:253–263. doi: 10.1111/j.1745-4549.2000.tb00417.x. [DOI] [Google Scholar]
- Gupte SM, El-Bisi HM, Francis FJ. Kinetics of thermal degradation of chlorophyll in spinach puree. J Food Sci. 1964;29:379–382. doi: 10.1111/j.1365-2621.1964.tb01747.x. [DOI] [Google Scholar]
- Ihl M, Shene C, Scheuermann E, Bifani V. Correlation for pigment content through colour determination using tristimulus values in a green leafy vegetable Swiss chard. J Sci Food Agric. 1994;66:527–531. doi: 10.1002/jsfa.2740660416. [DOI] [Google Scholar]
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Halulinen T, Aromaa A. Flavonoids intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- Lancaster JE, Lister CE, Reay PF, Triggs CM. Influence of pigment composition on skin color in a wide range of fruits and vegetables. J Am Soc Hortic Sci. 1997;122(4):594–598. [Google Scholar]
- Lau MH, Tang J, Swanson BG. Kinetics of textural and color changes in green asparagus during thermal treatments. J Food Eng. 2000;45:231–236. doi: 10.1016/S0260-8774(00)00069-8. [DOI] [Google Scholar]
- Lin S, Brewer MS. Effects of blanching method on the quality characteristics of frozen peas. J Food Qual. 2005;28:350–360. doi: 10.1111/j.1745-4557.2005.00038.x. [DOI] [Google Scholar]
- Lin CH, Chang CY. Textural change and antioxidant properties of broccoli under different cooking treatments. Food Chem. 2005;90(1–2):9–15. doi: 10.1016/j.foodchem.2004.02.053. [DOI] [Google Scholar]
- Little AC. Off on a tangent. J Food Sci. 1975;40:410–411. doi: 10.1111/j.1365-2621.1975.tb02213.x. [DOI] [Google Scholar]
- Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Burning J. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr. 2000;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- MacKinney G, Weast CA. Color changes in green vegetables; frozen-pack peas and sting beans. Ind Eng Chem. 1940;32(2):392–395. doi: 10.1021/ie50363a025. [DOI] [Google Scholar]
- Meyer LM. Food chemistry. New York: Reinhold Publ. Corp; 1960. [Google Scholar]
- Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J Agric Food Chem. 2008;56:139–147. doi: 10.1021/jf072304b. [DOI] [PubMed] [Google Scholar]
- Monero DA, Perez-Balibrea S, Ferreres F, et al. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010;123(2):358–363. doi: 10.1016/j.foodchem.2010.04.044. [DOI] [Google Scholar]
- Mudgett RE. Microwave food processing. Food Technol. 1989;43:117–126. [Google Scholar]
- Oerlemans K, Barrett DM, Suades CB, Verkerk R, Dekker M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006;95:19–29. doi: 10.1016/j.foodchem.2004.12.013. [DOI] [Google Scholar]
- Philippon J. Méthodes de blanchiment-refroidissement des legumes destinés a la congélation. Sci Aliment. 1984;4:523–550. [Google Scholar]
- Podsedek A, Sosnowska D, Redzynia M, Anders B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int J Food Sci Technol. 2006;41(1):49–58. doi: 10.1111/j.1365-2621.2006.01260.x. [DOI] [Google Scholar]
- Roy MK, Juneja LR, Isobe S, et al. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009;114(1):263–269. doi: 10.1016/j.foodchem.2008.09.050. [DOI] [Google Scholar]
- Salunkhe DK, Kadam SS (1998) Handbook of vegetable science and technology: production, composition, storage and processing. Marcel Dekker (Ed.), New York
- Singh J, Upadhyay AK, Kundan P, Anant B, Mathura R. Variability of carotene, vitamin C, E and phenolics in Brassica vegetables. J Food Compos Anal. 2007;20:106–112. doi: 10.1016/j.jfca.2006.08.002. [DOI] [Google Scholar]
- Steet JA, Tong CH. Degradation kinetics of green colour and chlorophylls in peas by colorimetry and HPLC. J Food Sci. 1996;61:924–931. doi: 10.1111/j.1365-2621.1996.tb10903.x. [DOI] [Google Scholar]
- Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Michael EJ, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- Sweeney JP, Martin M. Determination of chlorophyll and pheophytin in heated by various procedures. Food Res. 1958;23:635–647. doi: 10.1111/j.1365-2621.1958.tb17615.x. [DOI] [Google Scholar]
- Tijskens LMM, Schijvens EPHM, Biekman ESA. Modelling the change in colour of broccoli and green beans during blanching. Innov Food Sci Emerg Technol. 2001;2:303–313. doi: 10.1016/S1466-8564(01)00045-5. [DOI] [Google Scholar]
- Tong CH, Letz RR, Possen JL. Dielectric properties of pea puree at 915 MHz and 2450 MHz as a function of temperature. J Food Sci. 1994;59(121–122):134. [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- Vallejo F, Tomas-Barberan FA, Garcia-Viguera C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol. 2002;215(4):310–316. doi: 10.1007/s00217-002-0560-8. [DOI] [Google Scholar]
- Van Loey A, Hendrickx M, De Cordt S, Haentijens T, Tobback P. Quantitative evaluation of thermal processes using time-temperature integrators. Trends Food Sci Technol. 1996;71:16–26. doi: 10.1016/0924-2244(96)81353-7. [DOI] [Google Scholar]
- Van Loey A, Ooms V, Weemanes C, Van den Broeck I, Ludikhuyze L, Indrawati DS, Hendrickx M. Thermal and pressure-temperature degradation of chlorophyll in broccoli (Brassica oleracea L., italica) juice: a kinetic study. J Agric Food Chem. 1998;46:5289–5294. doi: 10.1021/jf980505x. [DOI] [Google Scholar]
- Verkerk R, Dekker M. Glucosinolates and myrosinase activity in red cabbage (Brassica oleracea L., var. capitata f. rubra DC) after various microwave treatments. J Agric Food Chem. 2004;52:7318–7323. doi: 10.1021/jf0493268. [DOI] [PubMed] [Google Scholar]
- Viña SZ, Olivera DF, Marani CM, Ferreyra RM, Mugridge A, Chaves AR, Mascheroni RH. Quality of Brussels sprouts (Brassica oleracea L. gemmifera DC) as affected by blanching method. J Food Eng. 2007;80:218–225. doi: 10.1016/j.jfoodeng.2006.02.049. [DOI] [Google Scholar]
- Williams DC, Lim MH, Chen AO, Pangborn RM, Whitaker JR. Blanching of vegetables for freezing: which indicator enzyme to choose. Food Technol. 1986;40(6):130–140. [Google Scholar]
- Woolfe ML. Effect of heating on foodstuffs. In: Priesley JR, editor. Pigments. London: Applied Science Publishers; 1979. pp. 77–119. [Google Scholar]
- Yang HW, Gunasekran S. Temperature profiles in a cylindrical model food during pulsed microwave heating. J Food Sci. 2001;66:998–1004. doi: 10.1111/j.1365-2621.2001.tb08225.x. [DOI] [Google Scholar]
- Yingjie D, Singh RK, Lee JH. Estimation of temperature profiles in microwaved particulates using enzyme and vision system. Lebensm Wiss Technol. 2002;36:331–338. [Google Scholar]
- Yuan G, Sun B, Yuan J, Wang Q. Effects of different cooking methods on health-promoting compounds of broccoli. J Zhejiang Univ SCI B. 2009;10(8):580–588. doi: 10.1631/jzus.B0920051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88(4):503–509. doi: 10.1016/j.foodchem.2004.01.065. [DOI] [Google Scholar]