Abstract
Effect of the mixture of squid ink tyrosinase (SIT) at 300 and 500 U/g protein and tannic acid (TA) at 0.5 and 1 % (based on protein) with different reaction times (90 and 180 min) on gel properties of sardine surimi was investigated. Surimi gel incorporated with mixture of SIT (500 U/g protein) and 1 % TA with a reaction time of 90 min had the highest breaking force and deformation (p < 0.05), in which the increases by 29.3 % and 11.9 % were observed, in comparison with the control. However, gels added with SIT/TA mixture had the lower whiteness, compared to the control (p < 0.05). Gel added with SIT/TA mixture showed more compact and finer network with higher connectivity of strands, compared to the control. This was coincidental with decreased expressible moisture content. Based on sensory evaluation, the highest overall likeness score was found in gel added with the mixture of SIT (500 U/g protein) and 1 % TA (p < 0.05). Therefore the mixture of tyrosinase from squid ink and tannic acid could be used as additives to improve the properties of surimi gel.
Keywords: Sardine, Surimi, Tyrosinase, Tannic acid, Cross-linking, Gelation
Introduction
Surimi is the concentrated myofibrillar proteins, mainly prepared via washing the fish mince in order to remove water soluble sarcoplasmic proteins, lipids and pigments. Gelation is an important property of surimi and determines the quality of surimi. Gelation involves the ordered aggregation of proteins, forming a three dimensional network with water entrapment (Ko et al. 2007). Lean fish have been extensively used for surimi production. Owing to their overexploitation, dark fleshed fish have been used as an alternative raw material for surimi production. Nevertheless, the dark fleshed mince has high contents of lipid and myoglobin, leading to poor gel forming ability (Chaijan et al. 2004). To improve the properties of surimi gel from dark fleshed fish, various food-grade ingredients have been used. Microbial transglutaminase (MTGase) has shown the potential in increasing gel strength of surimi by introducing non-disulphide covalent bond (Benjakul et al. 2008), whereas protein additives have been widely used to alleviate the softening (modori) induced by endogenous thermostable proteases (Benjakul et al. 2004). However, some additives such as bovine plasma protein, porcine plasma protein and egg white have been prohibited due to safety concern. Additionally, the use of cross-linking enzymes, especially MTGase, is still costly for surimi manufacturing. Therefore, the novel and cheap additives capable of improving gel quality of mince or surimi has been paid increasing attention.
Polyphenols are the natural compounds which are abundant in plants. Tannic acid (TA) belongs to the polyphenol consisting of a central carbohydrate (glucose) and 10 galloyl groups (Lopes et al. 1999). Different kinds of foods such as red wine, coffee, chocolate, tea, sorghum, spinach and fruits (Bananas, grapes and persimmons) contain tannic acid (Lopes et al. 1999; Naczk and Shahidi 2004). Depending on the type of food to which it is added, tannic acid can be used as a food additive in the range of 10–400 mg/L (Chen and Chung 2000). TA contains sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with proteins and other macromolecules. Phenols may be oxidised easily to their corresponding quinones (Hurrell and Finot 1984). The quinone, a reactive electrophilic intermediate, can readily undergo attack by nucleophiles such as lysine, methionine, cysteine and tryptophan residues in a protein chain (Hurrell and Finot 1984). The formation of rigid molecular structures of proteins by ortho-quinones has been demonstrated by Strauss and Gibson (2004).
Tyrosinase is a copper containing enzyme, belonging to the group of polyphenol oxidases (PPOs). Tyrosinase catalyses the oxidation of phenolic ring of tyrosine side chain, via inducing the conversion of L-dopa (3,4-dihydroxy-Lphenylalanine) intermediate to diquinone (Kim and Uyama 2005). These diquinones are extremely reactive and can further react with various amino acid side chains such as sulfhydryls, amines, amides, indoles, and other tyrosines commonly present in proteins, resulting in the formation of inter- and intramolecular crosslinks (Bittner 2006). Recently, tyrosinase has been reported in squid ink (Vate et al. 2014). The squid ink is a good source of tyrosinase, responsible for synthesis of melanin in squid ink. The tyrosinase from squid ink can be alternatively used for improving the properties of surimi along with the phenolic compound, which more likely acts as substrate for the enzyme. The quinone formed could serve as the protein cross-linker, thereby improving gel strength of surimi. Hence this study aimed to investigate the effect of tyrosinase from squid ink in combination with tannic acid on the properties of sardine surimi gel.
Materials and methods
Chemicals and surimi
Tannic acid, 2-thiobarbituric acid, β-mercaptoethanol (β-ME) and wide range molecular weight protein markers were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Sodium dodecyl sulphate (SDS), Coomassie Blue R-250, N,N,N,N-tetramethyl ethylene diamine (TEMED) and all chemicals for electrophoresis were procured from Bio-Rad Laboratories (Hercules, CA, USA).
Frozen surimi from sardine (Sardinella albella) with grade AA was obtained from Pacific Fish Processing Co., Ltd. (Songkhla, Thailand) and kept at −20 °C until use, but not longer than two months.
Preparation of squid ink tyrosinase
Preparation of melanin-free ink
Melanin-free ink was prepared according to the method of Vate and Benjakul (2013). Squids were purchased from a local market in Hat Yai, Thailand, stored in ice using a squid/ice ratio 1:2 (w/w), and transported to the Department of Food Technology, Prince of Songkla University, Thailand. Upon arrival, ink sac was separated from the squid by cutting the ink duct and ink was squeezed out from the ink sac. The squid ink was diluted ten-fold using the cold deionised water (2–4 °C). Thereafter, it was subjected to centrifugation at 18,000 × g for 30 min at 4 °C to remove the melanin using a refrigerated centrifuge (Allegra 25 R centrifuge, Beckman Coulter, Palo Alto, CA, USA). The supernatant obtained was used as melanin-free ink (MFI).
Fractionation of tyrosinase
Tyrosinase from MFI was fractionated as per the method of Simpson et al. (1987) with a slight modification. MFI (50 mL) was mixed with 50 mL of the extracting buffer (0.05 M sodium phosphate buffer, pH 7.2, containing 1.0 M NaCl and 0.2 % Brij 35). The mixture was stirred continuously at 4 °C for 30 min. Solid ammonium sulphate was added into the mixture to obtain 60 % saturation. The mixture was allowed to stand at 4 °C for 30 min. The precipitate was collected by centrifugation at 12,500 × g at 4 °C for 30 min. The pellet obtained was dissolved in a minimum volume of 0.05 mM sodium phosphate buffer, pH 7.2 and dialysed with 15 volumes of the same buffer with three changes overnight at 4 °C. The fraction containing tyrosinase referred to as ‘squid ink tyrosinase, SIT’ was kept at −20 °C until used.
Measurement of tyrosinase activity
Tyrosinase activity was assayed using L-DOPA (3,4-Dihydroxy-L-phenylalanine) as a substrate according to the method of Simpson et al. (1987) with a slight modification. Reaction mixtures consisted of 600 μl of 15 mM L-DOPA in deionised water, 400 μl of 0.05 M phosphate buffer (pH 6.0) and 100 μl of deionised water. To initiate the reaction, 100 μl of SIT were added and the reaction was run for 3 min at room temperature. The formation of dopachrome at 475 nm was monitored using a UV-160 spectrophotometer (Shimadzu, Kyoto, Japan). One unit of tyrosinase activity was defined as the amount of enzyme that induced an increase in the absorbance by 0.001 at 475 nm/min.
In vitro oxidation of TA by SIT
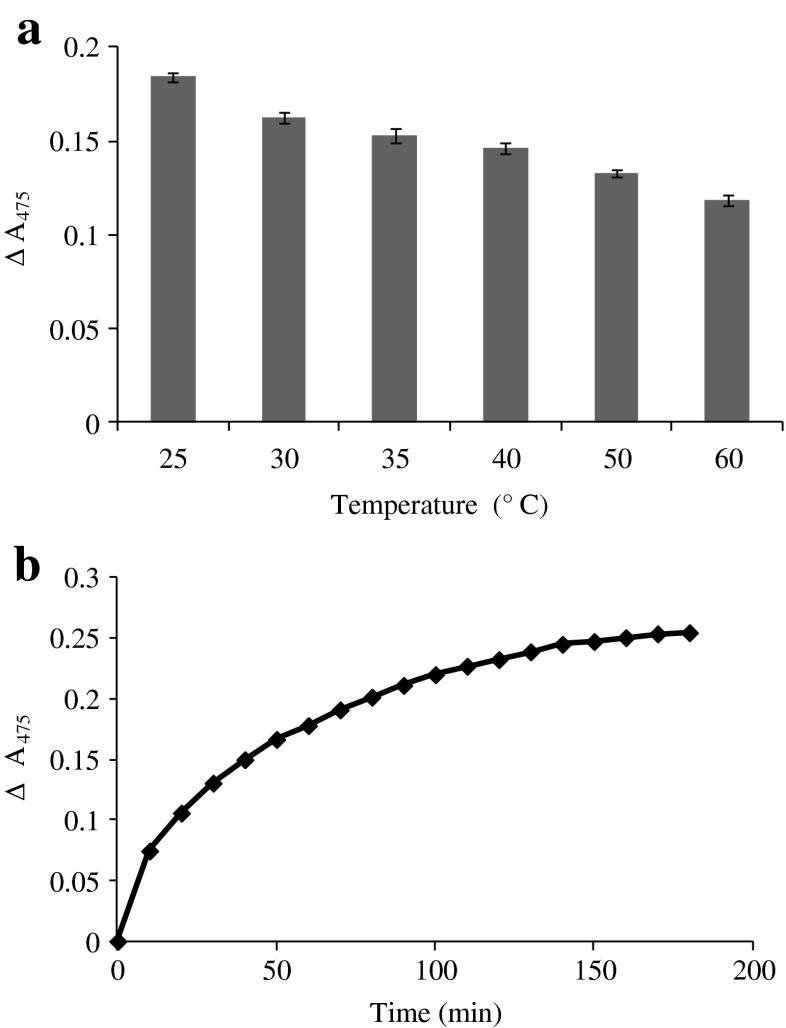
Oxidation of TA by SIT was determined at different temperatures. TA solution was prepared in deionised water and pH was adjusted to 7 using 1 M NaOH. TA solution was mixed with SIT to obtain final concentrations of 0.5 % and 30 U/mL respectively. The mixture (1 mL) was incubated at various temperatures (25, 30, 35, 40, 50 and 60 °C) for 30 min. For blank, distilled water was used instead of SIT. The increase in the absorbance at 475 nm, representing the formation of quinone, was recorded after blank subtraction. The temperature yielding the highest quinone formation was selected.
The formation of quinone in the assay system containing SIT and TA was monitored as the function of time. The assay mixture prepared as previously described was incubated at 25 °C. The increase in absorbance at 475 nm was recorded every 10 min up to 180 min.
The impact of SIT/TA mixtures on properties of surimi gel
Preparation of SIT/TA mixtures
TA solution (2 % w/v) was firstly prepared in deionised water and pH was adjusted to 7 using 1 M NaOH. TA solution was mixed with SIT (2500 U/ mL) to obtain the different TA and SIT working concentrations and incubated at 25 °C for 90 and 180 min. The reaction was terminated by boiling the mixture for 3 min. Thereafter, the obtained mixtures were cooled and used for surimi gel preparation.
Gel preparation
Frozen surimi was partially thawed at 4 °C for 6 h prior to cutting into small pieces. The sample was ground for 2 min using a Moulinex Masterchef 350 mixer (Paris, France). NaCl (2.5 %, w/w) was then added and the mixture was chopped for 1 min. The surimi paste was added with the prepared SIT/TA solutions to obtain various TA (0.5 % and 1 % based on protein) and SIT (300 and 500 U/g protein) levels in the surimi paste. The mixtures were then chopped for 1 min. Final moisture content was adjusted to 80 % using cold distilled water (1–2 °C). All mixtures were chopped for another 4 min at 4 °C to obtain the homogenous paste. The paste was then stuffed into polyvinylidine casing with a diameter of 2.5 cm and both ends of casing were sealed tightly. Two-step heated gels were prepared by setting the paste at 40 °C for 30 min, followed by heating at 90 °C for 20 min in a temperature controlled water bath (Memmert, Schwabach, Germany). The gels were then cooled in iced water and stored for 24 h at 4 °C prior to analyses.
Textural analysis
Gel samples were subjected to textural analysis using a Model TA-XT2i texture analyser (Stable Micro Systems, Surrey, England). Gels were equilibrated and evaluated at room temperature (28–30 °C) for approximately 30 min. Cylinder-shaped samples of 2.5 cm in length were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/deformability) were measured using the texture analyser equipped with a spherical plunger (diameter 5 mm, depression speed of 60 mm/min).
Determination of expressible moisture content
Expressible moisture content was measured according to the method of Benjakul et al. (2001). Expressible moisture content was expressed as percentage of sample weight.
Determination of whiteness
Whiteness of gels was measured using a Hunterlab (ColorFlex, Hunter Associates Laboratory, Reston, VA). Illuminant C was used as the light source of measurement. L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were measured and whiteness was calculated as described by NFI (1991) as follows:
SDS-polyacrylamide gel electrophoresis
Protein patterns of gels were analysed by SDS–PAGE according to the method of Laemmli (1970). Samples solubilised in SDS according to the method described by Benjakul et al. (2008) and were mixed at a 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4 % SDS, 20 % glycerol and 10 % β-ME) and boiled for 3 min. Samples (15 μg protein) were loaded onto polyacrylamide gels comprising a 10 % running gel and a 4 % stacking gel and subjected to electrophoresis at a constant current of 15 mA/gel using a Mini Protein III unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, the gel was stained with 0.02 % (w/v) Coomassie Blue R-250 in 50 % (v/v) methanol and 7.5 % (v/v) acetic acid and destained with 50 % (v/v) methanol and 7.5 % (v/v) acetic acid, followed by 5 % methanol (v/v) and 7.5 % (v/v) acetic acid.
Characterisation of the selected surimi gels added with SIT/TA mixtures
Rheological property
Surimi pastes containing different SIT/TA mixtures were prepared as previously described and were subjected to dynamic rheological measurement following the method of Rawdkuen et al. (2008) with a slight modification. A rheometer (HAAKE RheoStress1, ThermoFisher Scientific, Karlsruhe, Germany) with 35 mm, 4° slope cone and plate geometry was used for monitoring the changes in storage or elastic modulus (G’). An oscillation of 1 Hz with 1 % deformation was used for testing. This condition yielded a linear response in the viscoelastic region. The temperature sweep was recorded during heating up from 10 to 90 °C with heating rate of 1 °C/min. To minimise water evaporation of surimi pastes during measurement, silicon oil was applied to cover the samples.
Scanning electron microscopy
Gels were cut into small pieces (0.25 × 0.25 × 0.25 cm3) and fixed with 2.5 % (v/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 2 h at room temperature. The fixed samples were rinsed twice with distilled water. Fixed specimens were dehydrated in graded ethanol solution with serial concentrations of 50 %, 70 %, 80 %, 90 %, and 100 %. Samples were critical point dried (Balzers mod. CPD 030, Liechtenstein, Switzerland) using CO2 as transition fluid. The prepared samples were mounted on copper specimen holders, sputter-coated with gold (Sputter coater SPI-Module, West Chester, PA, USA) and examined on a JSM 5800 scanning electron microscope (JEOL, Ltd., Tokyo, Japan) at an acceleration voltage of 20 kV.
Sensory evaluation
Gel samples were cut into a bite-size (1 cm thick and 2.5 cm in diameter), equilibrated at room temperature (28–30 °C) for 30 min and coded with 3-digit random numbers. Gel samples were served on the white paper dishes at room temperature under the fluorescent daylight-type illumination. Eighty non-trained panelists (aged between 20 and 45) were the students and staffs at the Department of Food Technology, who were acquainted with surimi products. The panelists were asked to evaluate for colour, taste, texture and overall liking of gel samples using 9-point hedonic scale (Meilgaard et al. 1999). Between samples, the panelists were asked to rinse their mouth with distilled water.
Statistical analysis
All experiments were run in triplicate using three different lots of samples. Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple range tests (Steel and Torrie 1980). Statistical analysis was performed using the Statistical Package for Social Science (SPSS 17.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
In vitro oxidation of TA by SIT
Oxidation of TA by SIT as influenced by temperatures was monitored by the increase in A475 as depicted in Fig. 1a. The highest formation of quinone as indicated by the highest increase in A475 was observed at 25 °C. At higher temperature, tyrosinase might undergo denaturation, thereby losing its activity. Yang and Wu (2006) reported that tyrosinase from fungi Agaricus bisporus showed the optimal temperature at 27 °C. Nevertheless, phenoloxidase purified from heads of shrimp (Penaeus setiferous) had optimum temperature of 45 °C (Simpson et al. 1987). Increase in A475 indicates the formation of dopachrome (Simpson et al. 1987). This was most likely caused by the oxidation of TA by SIT. The formation of quinone decreased with increasing incubation temperatures. Hence the optimum temperature for the oxidation of TA by SIT was 25 °C.
Fig. 1.
Increase in A475 of TA solution induced by SIT as the function of temperature (a) and incubation time (b). Bars represent the standard deviation (n = 3)
Formation of quinone from TA induced by SIT at 25 °C as a function of time was investigated (Fig. 1b). The formation of quinone increased as the incubation time increased and the highest A475 was observed after 180 min. This indicated that TA was oxidised by SIT to a higher extent as the incubation time at the optimal temperature increased. Generally phenolic ring of compound is oxidised by tyrosinase, resulting in the formation of L-DOPA (3,4-dihydroxy-Lphenylalanine), which is further converted to the dopachromes (Buchert et al. 2010). Thus the conversion of TA to quinones by SIT required the sufficient time, in which oxidation took place effectively.
Effect of SIT/TA mixtures on textural properties of sardine surimi gel
Breaking force and deformation
Breaking force and deformation of sardine surimi gels added without and with SIT/TA mixtures at various concentrations with different reaction times, are depicted in Table 1. Surimi gels added with SIT/TA mixtures had higher breaking force and deformation, compared to the control (without SIT and TA) (p < 0.05). In the presence of mixture of 0.5 % TA and 300 U SIT/g protein, breaking force of gels increased as the reaction times increased from 90 to 180 min (p < 0.05). Tyrosinase is able to react on various monophenolic and diphenolic compounds, such as phenol and catechol or phloretic acid and hydrocaffeic acid. Tyrosine in the side chain of proteins can be oxidised by tyrosinase to quinone, which can further crosslink with lysyl, tyrosyl, and cysteinyl residues in proteins (Buchert et al. 2010). The result suggested that quinone was formed to a higher extent when the incubation time increased. Within the reaction time used, the level of quinone was not excessive for self-aggregation. As a consequence, the quinones formed were available for protein cross-linking, as evidenced by the increased breaking force. The quinones are extremely reactive and can react with various amino acid side chains, resulting in the formation of inter- and intra-molecular protein crosslinks (Mattinen et al. 2008). On the other hand, breaking force and deformation of gels decreased with increasing reaction time when added with the mixture of 1 % TA and 500 U SIT/g protein. With higher level of TA, a larger amount of quinone was generated. This might favour the self-aggregation of oxidised TA, quinone. As a result, the cross-linking sites of oxidised TA were reduced. This led to the poorer gel properties, especially when the reaction time increased (180 min). The concentration of substrate, tyrosinase and the reaction times played an important role in formation of quinone, a protein cross-linking agent. Amongst all samples, surimi gel added with the mixture of 1 % TA and 500 U SIT/g protein and reaction time of 90 min had the highest breaking force (p < 0.05). Significant increase in the gel strength of bigeye snapper surimi was found when oxidised phenolic compounds and oxidised kiam wood extract were added (Balange and Benjakul 2009a, 2011). Tyrosinase in combination with caffeic acid increased the cross-linking of whey proteins such as α-lactalbumin and β-lactoglobulin (Thalmann and Lötzbeyer 2002). Amongst all oxidised phenolic compounds, the oxidised TA exhibited the highest gel strengthening effect, compared with oxidised ferulic acid, catechin and caffeic acid. The use of TA in combination with appropriate washing process was shown to markedly improve the gel property of surimi from dark fleshed fish (Balange and Benjakul 2009b). Due to the plenty of hydroxyl group in TA, it could induce the hydrogen bonding with hydrogen acceptor in surimi proteins. Both reduced and oxidised forms of TA were able to improve gel property of surimi via the enhanced protein cross-linking.
Table 1.
Breaking force, deformation, expressible moisture content and whiteness of gels from sardine surimi added without and with different SIT/TA mixtures having different reaction times
| SIT (U/g protein) | TA (%) (based on protein) | Incubation time (min) | Breaking force (g) | Deformation (mm) | Expressible moisture content (%) | Whiteness |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 413.90 ± 3.21f | 10.34 ± 0.03d | 4.84 ± 0.01a | 69.85 ± 0.50a |
| 300 | 0.5 | 90 | 445.25 ± 16.36e | 10.73 ± 0.04c | 4.55 ± 0.06b | 64.56 ± 0.53bc |
| 180 | 469.15 ± 11.39d | 10.80 ± 0.13c | 4.51 ± 0.06bc | 65.18 ± 0.47bc | ||
| 1 | 90 | 506.49 ± 7.66bc | 11.24 ± 0.25b | 4.51 ± 0.01bc | 64.78 ± 1.00 bc | |
| 180 | 489.72 ± 9.34c | 10.84 ± 0.18c | 4.58 ± 0.01b | 65.18 ± 0.52 bc | ||
| 0.5 | 90 | 512.06 ± 3.12b | 11.15 ± 0.13b | 4.49 ± 0.11bc | 65.43 ± 0.37b | |
| 180 | 521.91 ± 2.12ab | 11.34 ± 0.06ab | 4.48 ± 0.06c | 64.51 ± 0.42c | ||
| 1 | 90 | 535.22 ± 19.37a | 11.58 ± 0.17a | 4.50 ± 0.05bc | 64.55 ± 0.52 c | |
| 180 | 466.95 ± 10.79d | 10.75 ± 0.18c | 4.54 ± 0.01b | 64.77 ± 0.37 bc |
Mean±S.D (n = 3). Different superscripts in the same column indicate significant differences (P < 0.05)
Expressible moisture content
Expressible moisture content of sardine surimi gels without and with different mixtures of SIT and TA is given in Table 1. Expressible moisture content of sardine surimi gels with SIT/TA mixtures was lower, compared to that of control (p < 0.05). This indicated that water holding capacity of surimi gels increased when added with the SIT/TA mixture. Lower expressible moisture content of the gels suggests more water retained in the gel network (Niwa 1992). In general, no differences in expressible moisture content were observed amongst all gel added with different SIT/TA mixtures (p > 0.05). The ordered aggregation of finer protein network imbibes more water, as indicated by lowered expressible moisture content. Vate et al. (2014) reported that sardine surimi gels added with melanin free ink at levels of 0.08 and 0.1 g/kg surimi had the improved water holding capacity as evidenced by the lowest expressible moisture content. For gels prepared from mackerel mince washed by conventional process, the lowest expressible moisture content was found with the addition of 0.5 % oxidised TA (Balange and Benjakul 2009b). The results suggested that TA, both reduced and oxidised forms, was able to enhance the formation of ordered gel network, in which water could be more imbibed.
Whiteness
Whiteness of sardine surimi gels decreased slightly when added with different SIT/TA mixtures (Table 1). The dark colour of TA had significant effect on lowering whiteness of sardine surimi gels. When TA was oxidised by SIT, quinones were formed. This resulted in the darker colour of surimi gel. It is also known that the oxidation products of phenolic compounds are colourful, and the shade of these colours is varying from purple to black (Bittner 2006; Monogioudi et al. 2009). Phenolic compounds were responsible for discolouration in cheese products (O’Connell and Fox 2001). Balange and Benjakul (2009b) reported that addition of 0.75 % oxidised TA resulted in the decrease in whiteness of gels prepared from mackerel surimi. Since gel from sardine was quite dark in colour, the slight decrease in whiteness of gel did not result in the obvious decrease in colour perception.
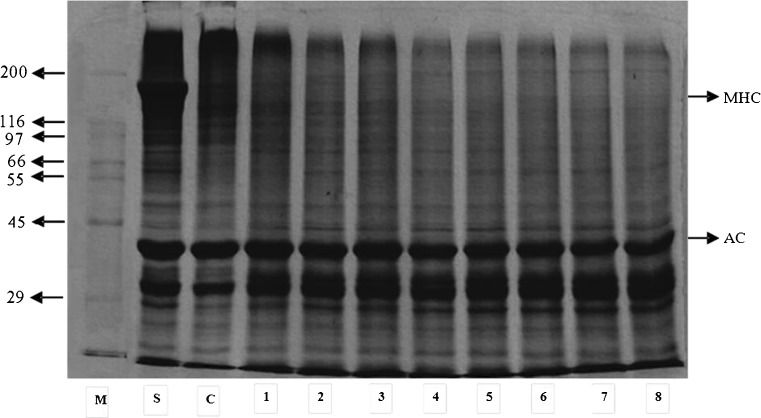
Protein patterns
Protein patterns of sardine surimi gels without and with different SIT/TA mixtures are shown in Fig. 2. Myosin heavy chain (MHC) band was prominent in surimi. No MHC band was observed in gels, regardless of SIT/TA incorporation. It was noted that slight decrease in the intensity of actin band was noticeable in the surimi gels with addition of SIT/TA mixtures. For the control gel (without addition of SIT/TA mixtures), MHC almost completely disappeared. MHC has been known as a preferable substrate for endogenous TGase, which played a major role in protein crosslinking during setting. This result was in agreement with Balange and Benjakul (2009a) who reported that the decrease in MHC band intensity was found in surimi gel from bigeye snapper, regardless of oxidised phenolic addition. Therefore, the complete disappearance of MHC indicated the superior setting phenomenon of sardine surimi gel. However actin was still retained, plausibly due to the structural constraint for cross-linking induced by TGase. In the presence of SIT/TA mixture, quinone formed might induce the polymerisation of actin to some degrees. Ou et al. (2005) reported the polymerisation of protein molecules caused by the reaction of different proteins with phenolic substances. Oxidised phenolic compounds, electrophilic in nature, could induce the formation of non-disulphide covalent bonds between proteins (Benjakul and Visessanguan 2003). Thus, the SIT/TA mixture containing quinones more likely induced the cross-linking of both MHC and actin in sardine surimi. This contributed to the increased gel strength of surimi.
Fig. 2.
Protein pattern of gels from sardine surimi without and with different SIT/TA mixtures having different reaction times. M: Marker; S: Surimi; C: Control (without SIT/TA mixture); 1: 0.5%TA, 300U SIT/g, 90 min; 2: 0.5 % TA, 300U SIT/g, 180 min; 3: 0.5 % TA, 500U SIT/g, 90 min; 4: 0.5 % TA, 500U SIT/g, 180 min; 5: 1 % TA, 300U SIT/g, 90 min; 6: 1 % TA, 300U SIT/g, 180 min; 7: 1 % TA, 500U SIT/g, 90 min; 8: 1 % TA, 500U SIT/g, 180 min; MHC myosin heavy chain, AC actin
Characteristics of the selected surimi gel
Rheological property
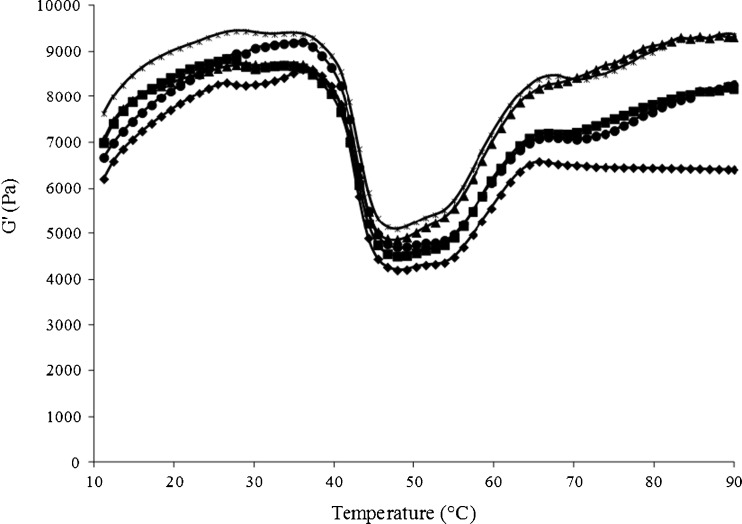
Changes in storage modulus (G’) of surimi paste without and with addition of SIT/TA mixtures during heating from 10 to 90 °C are depicted in Fig. 3. The control had the lowest G’ throughout the heating. The sample with the mixture of 1 % TA and 500U SIT/g protein and the reaction time of 90 min had the highest G’, especially during heating at temperature lower than 60 °C. This was in accordance with the highest gel strength. For all the samples, G’ increased as the temperature increased up to 35 °C. Subsequently, G’ decreased sharply to the lowest value when heated at temperature about 50 °C. This was more likely due to the action of endogenous proteases and dissociation of actomyosin complex. Rawdkuen et al. (2007) reported that G’ of Pacific whiting surimi reached the minimal value at the temperature of 55 °C. The optimum temperature for proteolytic enzymes in surimi was in the range of 50–60 °C (Klomklao et al. 2008). G’ values of surimi added with SIT/TA mixture increased as the temperature increased from 55 to 90 °C, whereas G’ value of the control remained constant when heated from 65 to 90 °C. During heating at higher temperature, the unfolded proteins underwent more aggregation. As a result, the cross-links with higher molecular weight were formed as evidenced by higher G’ values. Higher G’ indicated the higher stiffness or firmness of gel (Gordon 1984). Cross-linking between the dissociated protein molecules mediated by quinones more likely caused the increase in G’. In the presence of 0.5 % TA, the incorporation of 500U SIT/g protein with reaction time of 90 min yielded the lower G’ value than that added with mixture prepared with reaction time of 180 min. This was in agreement with the breaking force (Table 1). Oxidation of TA by SIT resulted in the formation of quinones which might interact with the reactive side chains of unfolded protein molecules. It was noted that G’ values of surimi paste added with SIT/TA mixtures were higher than the control even without heating. This suggested that the quinones formed by the oxidation of TA by SIT in the mixture readily interacted with muscle proteins, in which protein cross-links were formed rapidly. At higher temperatures, TA oxidised by SIT most likely interacted with the unfolded proteins more effectively. This led to the higher polymerisation of proteins as indicated by the higher G’.
Fig. 3.
Storage modulus (G’) of sardine surimi paste without and with different SIT/TA mixtures having different reaction times.  Control (without SIT/TA mixture);
Control (without SIT/TA mixture);  0.5 % TA, 500U SIT/g, 90 min;
0.5 % TA, 500U SIT/g, 90 min;  0.5 % TA, 500 U SIT/g, 180 min;
0.5 % TA, 500 U SIT/g, 180 min;  1 % TA, 300 U SIT/g, 180 min;
1 % TA, 300 U SIT/g, 180 min;  1 % TA, 500 U SIT/g, 90 min
1 % TA, 500 U SIT/g, 90 min
Microstructure of sardine surimi gel
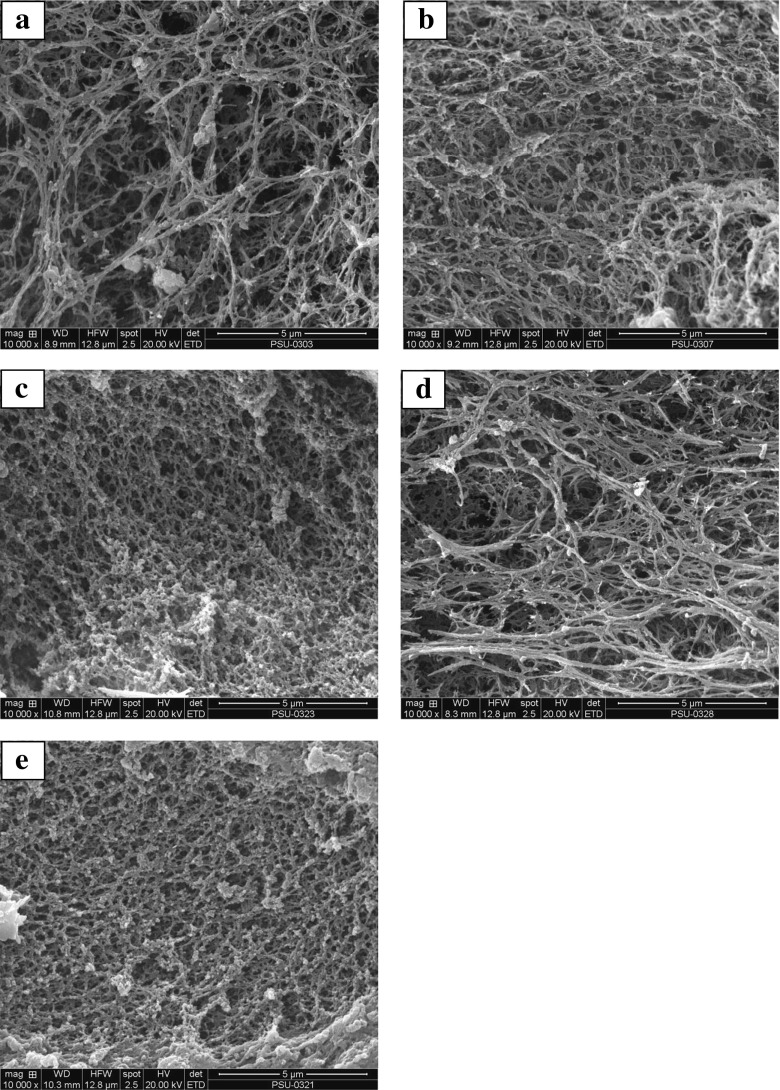
Scanning electron microscopic images of control gel (a), gel with the mixture of 0.5 % TA and 500U SIT/g protein with reaction time of 90 min (b), the mixture of 0.5 % TA and 500 U SIT/g protein with reaction time of 180 min (c), the mixture of 1 % TA and 300 U SIT/g protein with reaction time of 180 min (d) the mixture of 1 % TA and 500 U SIT/g protein with reaction time of 90 min (e) are given in Fig. 4. Surimi gels containing the SIT/TA mixture had a finer and more interconnected matrix with higher density of strands than the control. This suggested that TA oxidised by SIT plausibly induced the cross-linking of proteins, in which the denser network with a larger number of finer strands was formed. Amongst surimi gels, the gel added with the mixture 1 % TA and 500 U SIT/g protein with 90 min incubation time possessed fibrillar structure with more interconnected strands (Fig. 4e). This might be attributed to the cross-linking ability of TA oxidised by SIT, which in turn caused the development of aggregated proteins. The gel added with mixture of 1 % TA and 300 U SIT/g protein (180 min reaction time) had less compact structure with lower density of strands than that containing mixture of 1 % TA and 500 U SIT/g protein having the shorter incubation time (90 min). The result indicated the paramount role of tyrosinase in protein cross-linking. A large amount of quinones might induce the excessive protein cross-linking. As a result, the coarse network with larger voids could be formed. The finer and ordered gel network with smaller voids was observed in gels with the higher gel strength, whilst the looser network with larger voids was formed in the gels with lower gel strength (Balange and Benjakul 2009b). Microstructure of gel confirmed that oxidation of TA by SIT proceeded under the optimum condition could provide the appropriate level of quinone for protein cross-linking at the proper level, in which the matrix with interconnected strands could be formed.
Fig. 4.
Scanning electron microscopic images of gel from sardine surimi without and with different SIT/TA mixtures having different reaction times. a: Control (without SIT/TA mixture); b: 0.5 % TA, 500U SIT/g, 90 min; c: 0.5 % TA, 500 U SIT/g, 180 min; d: 1 % TA, 300 U SIT/g, 180 min; e: 1 % TA, 500 U SIT/g, 90 min. Magnification: ×10,000
Sensory property
Likeness score of sardine surimi gels without and with varying SIT/TA mixtures is shown in Table 2. There was no significant difference in score of appearance likeness amongst all gel samples (p > 0.05). Surimi gels added with SIT/TA mixtures had lower colour likeness score, compared to the control (p < 0.05). This could be attributed to the darker colour of the gels as influenced by oxidised TA induced by SIT. It was noted that gel added with 1 % TA and 500U SIT/g protein with 90 min reaction time showed the higher texture and overall likeness score than others (p < 0.05). This was coincidental with the increased breaking force and deformation and decreased expressible moisture content of the corresponding gel (Table 1). O’Connell and Fox (2001) stated that phenolic compounds play a role in the sensory attributes of many food products. There was no detrimental effect on the acceptability when chestnut and grape seed extracts, containing a high proportion of polyphenols, were added into dry cured sausages. The incorporation of seaweed extract into lesser sardine surimi had no impact on sensory property (Shitole et al. 2014). Thus, the addition of SIT/TA mixture more likely improved sensory property by enhancing gel strength.
Table 2.
Sensory properties of gels from sardine surimi added without and with different SIT/TA mixtures having different reaction times
| Samples | Appearance | Colour | Odor | Texture | Taste | Overall |
|---|---|---|---|---|---|---|
| a | 7.06 ± 0.83a | 7.27 ± 0.76a | 6.94 ± 0.61a | 6.67 ± 0.69c | 7.36 ± 0.49a | 6.91 ± 0.76b |
| b | 6.85 ± 0.56a | 6.64 ± 0.60b | 6.97 ± 0.73a | 7.12 ± 0.60b | 6.97 ± 0.68b | 6.91 ± 0.68b |
| c | 6.91 ± 0.68a | 6.67 ± 0.54b | 6.85 ± 0.62a | 7.00 ± 0.61b | 6.82 ± 0.63b | 7.03 ± 0.58bc |
| d | 7.03 ± 0.58a | 6.70 ± 0.47b | 7.03 ± 0.77a | 6.91 ± 0.52bc | 6.73 ± 0.57b | 6.94 ± 0.66b |
| e | 7.00 ± 0.66a | 6.66 ± 0.48b | 6.97 ± 0.68a | 7.48 ± 0.71a | 6.97 ± 0.73b | 7.33 ± 0.54a |
Mean ± S.D (n = 80). Different superscripts in the same column indicate significant differences (P < 0.05)
a: Control (without SIT/TA mixture); b: 0.5 % TA, 500U SIT/g, 90 min; c: 0.5 % TA, 500 U SIT/g, 180 min; d: 1 % TA, 300 U SIT/g, 180 min; e: 1 % TA, 500 U SIT/g, 90 min
Conclusion
The mixture of SIT and TA improved the breaking force and deformation of sardine surimi gels. Water holding capacity of gels was increased with the addition of mixtures, but the whiteness was slightly decreased. The use of 500U SIT/g protein and 1 % TA mixture with reaction time of 90 min increased the gel strength of sardine surimi gel effectively. The resulting gel with finer and compact structure had the higher texture and overall likeness scores, compared with control. Hence the mixture of tyrosinase from squid ink and tannic acid could be utilised as the additive to improve the gel strength of sardine surimi.
Acknowledgments
The authors would like to express their sincere thanks to Prince of Songkla University and the TRF Distinguished Research Professor grant for the financial support.
References
- Balange A, Benjakul S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009;113:61–70. doi: 10.1016/j.foodchem.2008.07.039. [DOI] [Google Scholar]
- Balange A, Benjakul S. Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009;23:1693–1701. doi: 10.1016/j.foodhyd.2009.01.007. [DOI] [Google Scholar]
- Balange A, Benjakul S. Effect of kiam wood extract as influenced by pH during oxygenation on mackerel surimi gel. J Food Biochem. 2011;35:574–595. doi: 10.1111/j.1745-4514.2010.00403.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W. Transglutaminase-mediated setting in bigeye snapper surimi. Food Res Int. 2003;36:253–266. doi: 10.1016/S0963-9969(02)00167-9. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Srivilai C. Porcine plasma proteins as gel enhancer in bigeye snapper (Priacanthus tayenus) surimi. J Food Biochem. 2001;25:285–305. doi: 10.1111/j.1745-4514.2001.tb00741.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Tueksuban J, Tanaka M. Effect of some protein additives on proteolysis and gel-forming ability of lizardfish (Saurida tumbil) Food Hydrocoll. 2004;18:395–401. doi: 10.1016/S0268-005X(03)00127-9. [DOI] [Google Scholar]
- Benjakul S, Phatcharat S, Tammatinna A, Visessanguan W, Kishimura H. Improvement of gelling properties of lizardfish mince as influenced by microbial transglutaminase and fish freshness. J Food Sci. 2008;73:S239–S246. doi: 10.1111/j.1750-3841.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- Bittner S. When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids. 2006;30:205–224. doi: 10.1007/s00726-005-0298-2. [DOI] [PubMed] [Google Scholar]
- Buchert J, Cura DE, Ma H, Gasparetti C, Monogioudi E, Faccio G, Mattinen M, Boer H, Partanen R, Selinheimo E, Lantto R, Kruus K. Crosslinking food proteins for improved functionality. Annu Rev of Food Sci Technol. 2010;1:113–138. doi: 10.1146/annurev.food.080708.100841. [DOI] [PubMed] [Google Scholar]
- Chaijan M, Benjakul S, Visessanguan W, Faustman C. Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res Int. 2004;37:1021–1030. doi: 10.1016/j.foodres.2004.06.012. [DOI] [Google Scholar]
- Chen SC, Chung KT. Mutagenicity and antimutagenicity of tannic acid and its related compounds. Food Chem Toxicol. 2000;38:1–5. doi: 10.1016/S0278-6915(99)00114-3. [DOI] [PubMed] [Google Scholar]
- Gordon JE. The new science of strong materials or why you don’t fall through the flor. 2. New Jersey: Princeton University Press; 1984. [Google Scholar]
- Hurrell RF, Finot PA (1984) Nutritional consequences of the reactions between proteins and oxidized polyphenols. Adv Exp Med Biol 177:423–435 [DOI] [PubMed]
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomklao S, Kishimura S, Benjakul S. Endogenous proteinases in true sardine (Sardinops melanostictus) Food Chem. 2008;107:213–220. doi: 10.1016/j.foodchem.2007.08.007. [DOI] [Google Scholar]
- Ko WC, Yu CC, Hsu KC. Contribution of hydrophobicity, netcharge, and sulfhydryl groups to thermal properties of ovalbumin. LWT-Food Sci Technol. 2007;40:1316–1320. doi: 10.1016/j.lwt.2006.10.002. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes GKB, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/S0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Mattinen ML, Lantto R, Selinheimo E, Kruus K, Buchert J. Oxidation of peptides and proteins by Trichoderma reesei and Agaricus bisporus tyrosinases. J Biotechnol. 2008;133:395–402. doi: 10.1016/j.jbiotec.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. 3. Florida: CRC Press; 1999. [Google Scholar]
- Monogioudi E, Creusot N, Kruus K, Gruppen H, Buchert J, Mattinen ML. Cross-linking of β-casein by Trichoderma reesei tyrosinase and Streptoverticillium mobaraense transglutaminase followed by SEC–MALLS. Food Hydrocoll. 2009;23:2008–2015. doi: 10.1016/j.foodhyd.2009.03.011. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- NFI . A manual of standard methods for measuring, specifying the properties of surimi. Washington, DC: National Fisheries Institute; 1991. [Google Scholar]
- Niwa E. Chemistry of surimi gelation. In: Lanier TC, Lee CM, editors. Surimi technology. New York: Marcel Dekker; 1992. pp. 389–428. [Google Scholar]
- O’Connell JE, Fox PF. Significance and applications of phenolic compounds in the production and quality of milk and dairy products. Int Dairy J. 2001;11:103–120. doi: 10.1016/S0958-6946(01)00033-4. [DOI] [Google Scholar]
- Ou S, Wang Y, Tang S, Huang C, Jackson MG. Role of ferulic acid in preparing edible films from soy protein isolate. J Food Eng. 2005;70:205–210. doi: 10.1016/j.jfoodeng.2004.09.025. [DOI] [Google Scholar]
- Rawdkuen S, Benjakul S, Visessanguan W, Lanier TC. Effect of chicken plasma protein and some protein additives on proteolysis and gel-forming ability of sardine (Sardinella gibbosa) surimi. J Food Process Preserv. 2007;31:492–516. doi: 10.1111/j.1745-4549.2007.00132.x. [DOI] [Google Scholar]
- Rawdkuen S, Benjakul S, Vissessanguan W, Lanier TC. Effect of cysteine proteinase inhibitor containing fraction from chicken plasma on autolysis and gelation of Pacific whiting surimi. Food Hydrocoll. 2008;21:1209–1216. doi: 10.1016/j.foodhyd.2006.10.002. [DOI] [Google Scholar]
- Shitole SS, Balange AK, Gangan SS. Use of seaweed (Sargassum tenerrimum) extract as a gel enhancer for lesser sardine (Sardinella brachiosoma) surimi. Int Aquat Res. 2014 [Google Scholar]
- Simpson BK, Marshall MR, Otwell WS. Phenoloxidase from shrimp (Penaues setiferus): Purification and some properties. J Agric Food Chem. 1987;35:918–921. doi: 10.1021/jf00078a017. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2. New York: McGraw-Hill; 1980. [Google Scholar]
- Strauss G, Gibson SM. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004;18:81–89. doi: 10.1016/S0268-005X(03)00045-6. [DOI] [Google Scholar]
- Thalmann CR, Lötzbeyer T. Enzymatic cross-linking of proteins with tyrosinase. Eur Food Res Technol. 2002;210:276–281. doi: 10.1007/s00217-001-0455-0. [DOI] [Google Scholar]
- Vate NK, Benjakul S. Antioxidative activity of melanin-free ink from splendid squid (Loligo formosana) Int Aquat Res. 2013 [Google Scholar]
- Vate NK, Benjakul S, Agustini TW. Application of melanin-free ink as a new antioxidative gel enhancer in sardine surimi gel. J Sci Food Agric. 2014;95:2201–2207. doi: 10.1002/jsfa.6934. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wu F. Catalytic properties of tyrosinase from potato and edible fungi. Biotechnol. 2006;5:344–348. doi: 10.3923/biotech.2006.344.348. [DOI] [Google Scholar]