Abstract
Developing new antioxidants and using natural examples is of current interest. This study evaluated the antioxidant activities and the ability to inhibit soybean oil oxidation of oat oil obtained with different solvents. Oat oil extract obtained by ethanol extraction gave the highest antioxidant activity with a DPPH radical (1,1-diphenyl-2-picrylhydrazyl) scavenging activity of 88.2 % and a reducing power (A700) of 0.83. Oat oil extracted by ethanol contained the highest polyphenol and α-tocopherol content. Significant correlation was observed between the total polyphenol contents, individual phenolic acid, α-tocopherol, and DPPH radical scavenging activity. Soybean oil with 2 % added oat oil showed low malondialdehyde content (8.35 mmol mL−1), suggesting that the added oat oil inhibited oxidation. Oat oil showed good antioxidant activity, especially when extracted with ethanol which could also retard the oxidation of soybean oil . DPPH radical scavenging activity was the best method to evaluate the antioxidant activity and components of oat oil.
Keywords: Oat oil, Soybean oil, Antioxidant activity, Phenolic acid, Inhibit oxidation
Introduction
Multiple free radicals such as superoxide anion (O2−), alkyl-peroxyl radical (ROO·), or hydroxyl radical (HO·) are natural by-products of human metabolism (Amarowicz et al. 2004). They can be produced by radiation, chemical reactions, and several redox reactions. When present at high levels, radicals may contribute to protein oxidation, DNA damage, and lipid peroxidation in living tissues and cells (Halliwell 1996; Kim et al. 2006; Morrissey and O’Brien 1998). As a result, much work has focused on antioxidants because of their ability to act as free radical scavengers. Antioxidants are important in the prevention of human disease. Compounds with antioxidant activity may function as free radical scavengers, complexing agents of pro-oxidant metals, reducing agents, chain breakers, and quenchers of singlet-oxygen formation (Andlauer and Furst 1998), thereby protecting the body from degenerative diseases such as cancer (Heuvel et al. 2014; Wilding et al. 1993). Although present at low concentrations, antioxidants may prevent oxidation to a remarkable degree (Poljsak and Milisav 2014; Halliwell 1990).
Existing antioxidants can be classified as natural or synthetic antioxidants. Because synthetic antioxidants, such as gallates, 2,6-di-tert-butyl-4-methylphenol (BHT), tert-butyl hydroxyanisole (BHA), and tert-butyl hydroquinone (TBHQ), are unstable at elevated temperature (Frankel et al. 1994; Pokorný 1991; Zhu et al. 2011) and are strictly controlled, preference has shifted to the application of natural antioxidants.
Oat belongs to the Poaceae family and is an annual grass. It was used originally for medical purposes, and afterward became an important food grain. Although oats (annual consumption: 22,619 thousand metric tons in 2014) are consumed in considerably lower quantities worldwide than wheat (annual consumption: 715,89 thousand metric tons) and rice (annual consumption: 484,776 thousand metric tons in 2014) (United States Department of Agricultural, 2015), they have the nutritional advantage of normally being consumed as a whole-grain cereal. Whole grain oat contains abundant nutrients such as proteins (about 15 %, three times of rice), starch, fat (above 5 %, four times of wheat). Oat also contains micronutrients such as vitamin E, folates, zinc, iron, selenium, copper, manganese, carotenoids, betaine, choline, sulphur containing amino acids, phytic acid, lignins, lignane and alkyl resorcinols. (Butt et al. 2008; Flander, et al. 2007; Peterson 2001). Oat is a source of many compounds that are known to exhibit antioxidant activity. Vitamin E, phytic acid, phenolic compounds, and avenanthramides are the most abundant antioxidants in oats, and flavonoids and sterols are also present. These antioxidants are concentrated in the outer layers of the kernel. Several in vitro tests have been used to evaluate the antioxidant activity of oat extracts (Bryngelsson et al. 2002; Kilci and Gocmen 2014; Peterson 2001; Stevenson et al. 2008;). Due to significant price advantage and good nutritional value, soybean oil is widely used in people’s daily life (annual consumption was about 38 million tons). Only in China, the total consumption was 12.5 million tons in 2012 and the total consumption of 2013 was 13.5 million tons (Production, Supply and Distribution Online. http://apps.fas.usda.gov/psdonline/). Furthermore soybean oil is rich in unsaturated fatty acid, including about 23 % monounsaturated fatty acid and 58 % polyunsaturated fatty acid which are highly prone to oxidation (Ivanov et al. 2010). Natural tocopherols in soybean oil is about 100 mg/100 g which can play a role of antioxidant to some extent but it is far enough to protect unsaturated fatty acids from oxidation, so it is necessary to add exogenous antioxidant (Zhang et al. 2007).
The oil content of oats is the highest of the cereal grains (Halima and Slima 2014; Price and Parsons 1975). In a survey of 4000 entries in the World Oat Collection, the oat oil concentration ranged from 3.1 to 11.6 %, with a mean value of 7.0 % (Brown and Craddock 1972; Leonova et al. 2008). Oat oil is a good-quality edible oil because of its high content of monounsaturated fatty acids. Frey and Hammond (1975) postulated that oat could become useful as an oil crop if the oil concentration could be elevated to above 16 % (Peterson and Wood 1997). Frey and associates subsequently increased groat oil concentration through nine rounds of a recurrent selection scheme, and selections with oil contents as high as 18 % were obtained. Seen in this light, oat oil has great potential. In addition, more than 90 % of oat lipids distribute into the endosperm and bran (Zhou et al. 1999),which is usually used as animal feed, or even discarded. From this perspective, development of oat oil has great environmental and economic benefits. Furthermore, through different extraction methods or solvents, oat oil content could contain various antioxidants, such as caffeic acid, ferulic acid, tocopherols, avenanthramides, and sterols (Peterson 2001). To date, many studies have been carried out on the antioxidant activity of virgin olive oil, in which polyphenols play an extremely important role (Pirisi et al. 2000). The presence of different substituents in the phenol backbone structures modulates their antioxidant properties. The functions of different substituents in polyphenols were analyzed by Carrasco Pancorbo et al. (2005). However, oat oil contains so many kinds of antioxidant compounds that the modes of action against free radicals may occur via complex interactions. As a result, an alternative consideration is to develop oat oil as a source of grease antioxidant.
In this study, oil was extracted with different solvents, and evaluated the antioxidants by different methods. According to their antioxidant capacities, we further studied the differences of antioxidant content and their relationship with antioxidant activity. In addition, oat oil was added to soybean oil to observe its ability to inhibit soybean oil oxidation. The detailed experimental design figure is as follows: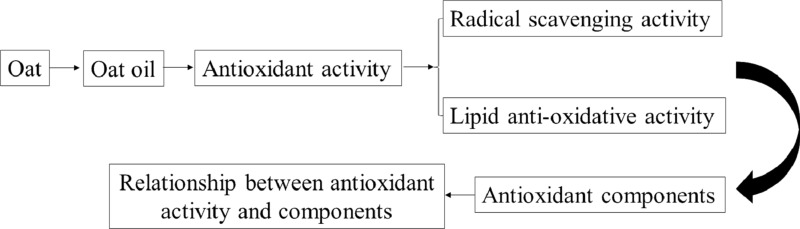
Materials and methods
Chemicals and materials
Folin–Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH radical), and the diammonium salt of 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma (St. Louis, MO, USA). TBHQ, α-tocopherol, caffeic acid, ferulic acid, vanillic acid, and p-coumaric acid standards were obtained from the National Institutes for Food and Drug Control (China). All other reagents and solvents used were of analytical and HPLC grade.
Oats (Bai Yan No.2) harvested in 2014 was supplied by the Baicheng Faculty of Agricultural Science in Jilin Province. Soybeans (Zhonghuang No.1) were purchased from a local market harvested in 2014.
Methods
Determination of the chemical and physical properties of oats and soybeans
The determination of moisture content (GB/T 5497-1985), protein (GB/T 5511-2008), crude fat (GB/T 5512-2008), ash (GB/T 22510-2008), and starch content (GB/T 5514-2008) were carried out according to the National Standard of the People’s Republic of China.
Extraction procedures
Dried hulls of oats and soybean were separately milled using pulverizer and screened through a 40-mesh sieve. The oat hull powder (50 g) was weighed precisely and extracted with 250 mL of hexane, petroleum ether, isopropyl alcohol, ethyl acetate, or ethanol separately at 30 °C with shaking (100 rpm) in a constant temperature oscillation incubator (HZQ-X100, Suzhou Pei Ying Experimental Equipment, Suzhou, China) for 1 day, and then stewed at 30 °C for another 2 days. Soybean powder was extracted with hexane using the same procedure. After filtration on qualitative filter paper, the residues were twice subjected to the extraction procedure described above. The filtrates were combined and evaporated to dryness using a rotary evaporator (RE-52-99, Yarong, Shanghai, China) under reduced pressure at 45 °C. The resultant oil was centrifuged using centrifugal machine (GL-20G-II, Anke, Shanghai, China) for 5 min at 8000 rpm/min to remove suspended solids and then stored at room temperature.
Antioxidant activity evaluation
Assay of DPPH radical scavenging activity
The DPPH radical assay was carried out as described by Fenglin (2004) with some modifications. Samples were diluted 50 times with ethanol. Quantitative measurement of radical scavenging was carried out in a 96-well microplate. The reaction mixture contained 100 μL of test sample (or ethanol in the positive control) and 100 μL of 200 μM solution of DPPH radical in ethanol. A 200-μL aliquot of DPPH radical solution was used for the positive control. A mixture of 100 μL of sample and 100 μL of ethanol was used as a negative control. The 96-well microplate was placed on the microplate mixer (NS-P, ASONE, Japan) for uniform shaking. After an incubation period of 20 min at room temperature in darkness, the absorbance at 520 nm was recorded using a microplate reader (Model 550, BIO-RAD, Japan). Measurements were performed in triplicate. The free radical scavenging activity of each solution was then calculated as percent inhibition according to:
| 1 |
where A is the absorbance at 520 nm.
Reducing power
The reducing power of each test sample was determined by the method of Yen and Duh (1993) with some modification. A 0.01-mL aliquot of extracted oil in 0.5 mL of ethanol was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1 % (w/v) potassium ferricyanide in a 10-mL test tube. The mixture was incubated for 20 min at 50 °C using water bath. At the end of the incubation, 2.5 mL of 10 % (w/v) trichloroacetic acid was added to the mixture, which was then centrifuged at 5000 rpm for 10 min using centrifugal machine. The upper layer (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1 % ferric chloride, and the absorbance was measured at 700 nm by UV-visible absorption spectrometry (UVmini-1240, Shimadzu Corporation, Japan). The reducing power tests were performed in triplicate.
Determination of antioxidant content
Total polyphenol content
Determination of total polyphenols in the crude oil was carried out using the method of Pirisi (2000). Extraction of polyphenols from oil samples was achieved by dissolving 1 g of oil in 2 mL of hexane, followed by liquid–liquid extraction using 3 mL of methanol/water (60:40, v/v). After vigorous mixing, the mixtures were centrifuged for 15 min at 5000 rpm using centrifugal machine. The hydro-alcoholic phase was collected, and the hexane phase was re-extracted twice with 2 mL of methanol/water (60:40, v/v).
The hydro-alcoholic fractions were combined, and 1 mL was transferred to a volumetric flask (10 mL) to which was added 0.5 mL of Folin–Ciocalteu reagent (1:10 diluted with ethanol). The solution was shaken and left to stand for 3 min prior to addition of saturated sodium molybdate solution (1 mL) and diluted with 7.5 mL of deionized water. After incubation for 60 min at room temperature, the absorbance was recorded at 725 nm. A standard curve was prepared based on the same procedure using gallic acid, and the concentration of total phenols was expressed in micrograms of gallic acid equivalent per gram of oil (GAE) (Haiyan et al. 2007; Del et al. 2004).
Determination of β-carotene content
A 1-g portion of oil was weighed accurately, dissolved in isooctane, and taken to a final volume of 10 mL. The carotenoid fractions in the absorption spectrum were determined at 475 nm. Results were given as micrograms of β-carotene per gram of oil (Gutierrez et al. 1999).
Extraction of phenolic compounds from oil
A liquid–liquid extraction system was used to extract the phenolic compounds present in the oil. According to the method of Carrasco-Pancorbo et al.(2005), 1 g of oil was dissolved in 2 mL of hexane, and the solution was extracted successively with three 2-mL portions of methanol/water (60:40, v/v). The extracts were combined, washed twice with 2 mL of hexane, and the hexane fractions discarded. The combined extracts of the hydrophilic layer were evaporated to dryness in a pressure-blowing concentrator (DC12H, ANPEL Scientific Instrument, Shanghai, China). The residue was redissolved in 0.5 mL of methanol/water (50:50, v/v) and filtered through a 0.45-μm filter.
High-performance liquid chromatography (HPLC) analysis of phenolic compounds was performed with a Shimadzu LC-20AT series HPLC instrument equipped with a diode array detector (DAD, SPD-M20A) and a Capcell Pak C18 AG120S5 column (250 × 4.6 mm i.d., 5 μm particle size). The mobile phase was composed of 5 % acetic acid in H2O (solvent A) and methanol (solvent B). It was eluted at a flow rate of 0.9 mL min−1. The gradient elution program was: 0 min, 5 % B; 5 min, 15 % B; 25 min, 30 % B; 39 min, 42 % B; 47 min, 55 % B; 50 min, 70 % B; 56 min, 75 % B; 60 min, 100 % B; 70 min, 5 % B; 73 min, 5 % B. The chromatograms were acquired at 280 and 330 nm. Column temperature and injection volume were 28 °C and 10 μL. Phenolic compounds used as standard references were caffeic acid, vanillic acid, p-coumaric acid, and ferulic acid. Quantification of individual phenolic components was achieved by injecting solutions of each standard (seven standard solutions with concentrations between 5 and 500 μg/mL). Peak identification in HPLC analysis was performed by comparison of retention times with respective reference standards. Quantification of individual phenolic compounds was based on the peak areas of the identified compounds. Peak purity values, which were provided by the instrument facility, were checked in each component analysis.
Squared regression coefficients of calibration curves were calculated using Microsoft Excel within the linear range for each compound. Quantification of individual phenolic components in the fractions was achieved by comparison of peak areas against those of reference compounds. Results were reported as micrograms per milligrams of oil.
Determination of α-tocopherol
α-Tocopherol stock standard solutions were prepared in hexane, and working solutions were prepared by appropriate dilution with the eluent mobile phase. A 0.2-g portion of extracted oil was dissolved in 3 mL of hexane, and 10 μL of the solution was injected into the HPLC (LC-20 AT, Shimadzu, Japan) on a C18 column (50 × 4.6 mm i.d., particle size 5 μm). Ultraviolet (UV) detection was performed at 295 nm. Methanol/acetonitrile (50:50, v/v) was used as eluent at a flow rate of 1 mL min−1. Identification and quantification of chromatographic peaks were conducted by comparison with the response for the α-tocopherol standard. An external calibration curve was prepared from standards to calculate the amount of α-tocopherol present in the oil samples (Carpenter 1979; Sakouhi et al. 2008; Tuberoso et al. 2007).
Inhibition capacity of oat oil on soybean oil oxidation
Sample preparation
Eight 100-g portions of commercially refined soybean oil (contained 0.02 % TBHQ) were added to separate glass tubes. Two tubes were employed as blanks, and 0.02 g of TBHQ was added to two others. Two grams of oat oil was added to two tubes and 4 g of oat oil was added to the remaining two tubes. The samples were heated at 180 °C for 6 h in a tube heater (L-129A, Laiheng Technology, Beijing, China). At the end of the heating, the samples were quickly cooled in the ice-water bath, and stored at room temperature.
Determination of malondialdehyde
Malondialdehyde (MDA) content in soybean oil was determined using a malondialdehyde assay kit (Nanjing Jiancheng Bioengineering Institute) in accordance with the manufacturer’s instructions.
Statistical analysis
All results were determined as the average of three determinations unless otherwise stated. To evaluate significant differences for each of the parameters, we performed a one-way analysis of variance (ANOVA) with the Tukey post-hoc test (SPSS Statistics, SPSS Inc.) with a significance level of 0.05. Pearson’s correlation coefficients were also given to display the correlations between the results obtained from assays.
Results and discussion
Physical properties of oat groats
Analysis of the physical properties of oat groats in their crude form is shown in Table 1. The total fat content was 8.21 %, while the extraction rate by petroleum ether was only 5.3 %. The moisture content was 9.58 %, which is an important factor if the product is to be stored for a long time without the likelihood of contamination by bacteria and fungi that might alter oil quality through decomposition. In addition, a total protein content of 14.04 % (based on N × 6.25 conversion) was noted. Starch content was around 64.7 %.
Table 1.
Main ingredients of oat groat
| Protein (%) | Total fat (%) | Moisture (%) | Ash (%) | Starch (%) |
|---|---|---|---|---|
| 14.04 ± 0.58 | 8.21 ± 0.89 | 9.58 ± 0.79 | 1.63 ± 0.09 | 64.70 ± 0.76 |
All analyses performed on wet basis. Data expressed as mean ± SD, n = 4
Antioxidant activity assay
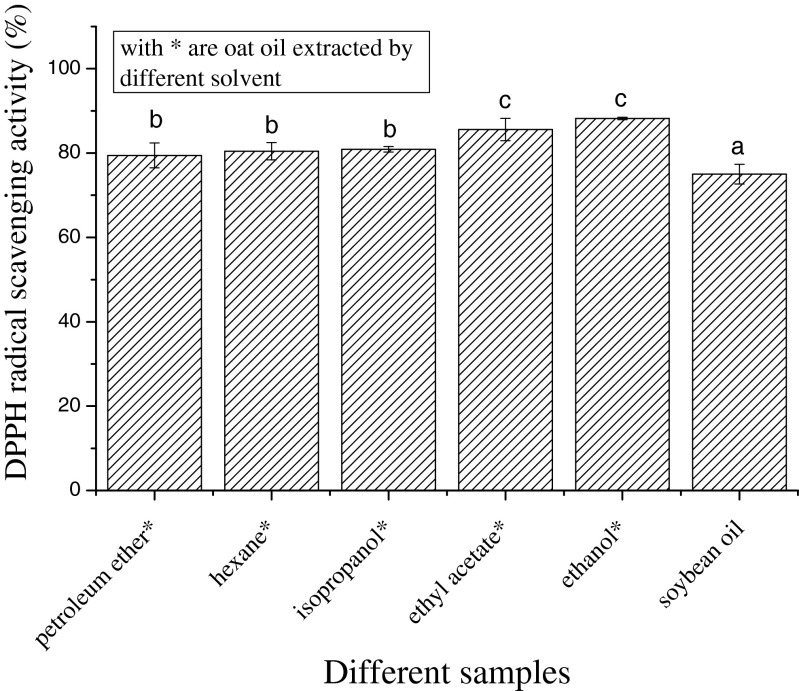
The DPPH radical is very stable. On accepting an electron or combining with another free radical species, its visual absorption spectrum changes noticeably, with the color of the material changing from purple to yellow. The method can accommodate many samples in a short period and is sensitive enough to detect active ingredients at low concentrations (Hseu et al. 2008). In addition, the test is simple to use and occurs at ambient temperature, which avoids thermal degradation of the test substances (Peterson 2001). The DPPH radical scavenging activities of soybean oil and oat oil extracted by different solvents are shown in Fig. 1. The oat oil extract obtained with ethanol exhibited the strongest DPPH radical scavenging activity. With increased polarity of the extraction solvent, the DPPH radical scavenging ability was also gradually enhanced; the increasing order in scavenging ability was for extracts obtained with: petroleum ether < hexane < isopropanol < ethyl acetate < ethanol. The DPPH radical scavenging ability of soybean oil was significantly lower than that of oat oil (P < 0.05).
Fig. 1.
Scavenging activity of oat oil extracts on DPPH radical. Data are given as mean ± SD (n = 3). Different letters on data bars indicate significant differences
The reducing power of the different oils is presented in Fig. 2. In this method, the ferric ferricyanide complex is reduced to the ferrous form depending on the presence of antioxidants (Amarowicz et al. 2004). Oat oil extracts obtained with isopropanol and ethanol had relatively higher reducing powers than the other samples.
Fig. 2.
Reducing power of oat oil extracts. Data are given as mean ± SD (n = 3). Different letters on data bars indicate significant differences
The antioxidant activity of oil tended to improve as the solvent polarity increased. We conclude that volatile components played an important role in the antioxidant activity of oat oil.
Antioxidant compounds
Antioxidants in grains are difficult to extract because of the wide range in solubilities of active compounds. It has been found that polyphenolic compounds are one of the most effective antioxidant constituents in grains (Miller et al. 2000). According to Peterson (2001), α-tocopherol, ferulic acid, vanillic acid, and p-coumaric acids are the main antioxidants in oat. Carotenoids may act as oxygen quenchers and can transfer an electron to the radicals to give rise to a stable carotenoid radical cation (Mortensen and Skibsted 1997). Thus, it is important to assess the quality and contribution of carotenoids to antioxidant activity.
Table 2 shows that among all the phenolics extracted from oat oil with different solvents, caffeic acids were the least plentiful (0.3–14.23 μg/g) while vanillic acids were the most plentiful (1.41–62.99 μg/g). Oat oil extract obtained with ethanol was found to contain all the phenolic acids studied, and these components were present in the highest concentrations among all of the oil extracts. Extracts of oat oil obtained by extraction with ethyl acetate contained the highest β-carotene levels, which is a component that likely affects the color of the oil. Compared with oat oil, soybean oil contained low antioxidant levels. In general, as the polarity of extraction solvents increased, the resulting oil extract contained more antioxidants. This is in accordance with the behavior of antioxidants in oat oil. This implies that the difference in antioxidant activity is related primarily to differences in the antioxidant contents.
Table 2.
Antioxidant contents in extracts of oat oil
| Antioxidant | Ethanol | Ethyl acetate | Isopropanol | Hexane | Petroleum ether | Soybean oil |
|---|---|---|---|---|---|---|
| p-Coumaric acid | 7.86 ± 1.39d | 4.72 ± 0.33c | 1.48 ± 0.37b | 0.44 ± 0.05a | 0.57 ± 0.16a | 0.33 ± 0.07a |
| Ferulic acid | 36.00 ± 0.73b | 41.23 ± 0.76b | 1.71 ± 0.33a | 1.23 ± 0.00a | 0.00a | 0.00a |
| Caffeic acid | 14.23 ± 1.87b | 15.84 ± 0.90b | 0.44a | 0.77 ± 0.05a | 0.35 ± 0.03a | 0.30 ± 0.01a |
| Vanillic acid | 62.99 ± 4.54d | 52.78 ± 2.76c | 37.03 ± 8.03b | 2.97a | 1.23 ± 0.25a | 1.41 ± 0.39a |
| α-Tocopherol | 1724.28 ± 37.91e | 1043.49 ± 112.53c | 1217.51 ± 34.86d | 79.52 ± 11.11b | 2.95a | 0.00a |
| β-Carotene | 0.41e | 0.68f | 0.28c | 0.32d | 0.23a | 0.25b |
| Total polyphenols (GAE) | 529.33 ± 0.42f | 373.33 ± 4.92e | 225.98 ± 2.84d | 35.26 ± 2.84c | 24.03 ± 1.69b | 15.91 ± 0.42a |
Data presented as mean ± SD in μg g−1 (n = 4). Different letters within the same row indicate significant differences. GAE gallic acid equivalent
Table 3 shows the relationship between antioxidant activity and antioxidant components. Individual phenolic acids showed significant correlations with DPPH radical scavenging activity, and the correlation was particularly notable for total polyphenols. Coumaric acid, α-tocopherol, and total polyphenols showed significant correlations with reducing power, while β-carotene showed no significant correlation with DPPH radical scavenging activity or reducing power.
Table 3.
Pearson correlation analysis between antioxidant contents and antioxidant activities
| Antioxidant contents | |||||||
|---|---|---|---|---|---|---|---|
| P-vanillic acid | Ferulic acid | Caffeic acid | Coumaric acid | α-tocopherol | Total polyphenol | β-carotene | |
| DPPH radical scavenging activity | 0.912* | 0.870* | 0.897* | 0.890* | 0.834* | 0.919** | 0.699 |
| Reducing power | 0.790 | 0.638 | 0.688 | 0.913* | 0.963** | 0.892* | 0.427 |
* Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)
Capacity of oat oil to inhibit oxidation of soybean oil
The antioxidant activity results showed that oat oil obtained by ethanol extraction displayed the highest antioxidant activity; thus, this extract was used to measure the inhibition activity of oat oil on soybean oil oxidation.
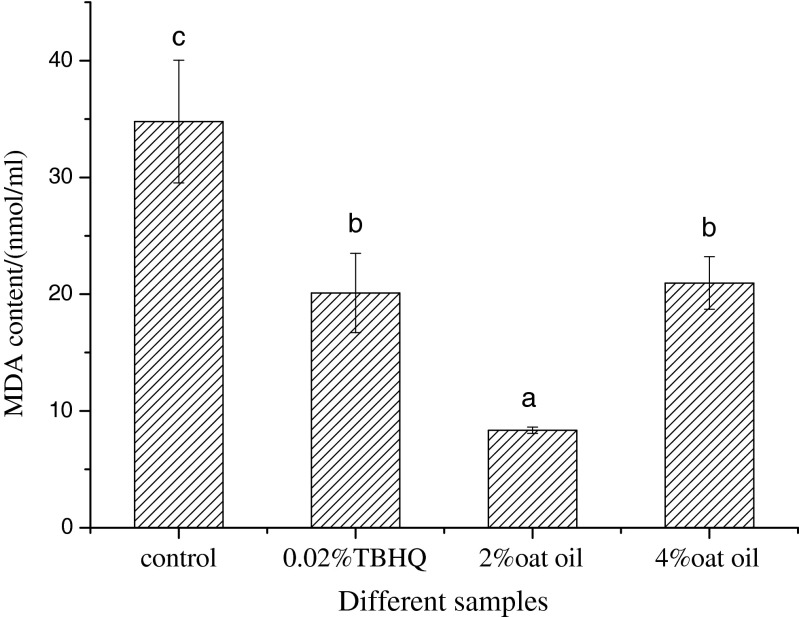
MDA is one end product of lipid peroxidation, so its content is a reflection of the degree of oil oxidation. The MDA content of soybean oil spiked with different antioxidants is shown in Fig. 3. Soybean oil with 2 % added oat oil showed the lowest MDA content, followed by those spiked with 0.02 % TBHQ, 4 % oat oil, and control. Therefore, in terms of inhibiting MDA generation, 2 % oat oil was the most effective. Crude oat oil contains high levels of free fatty acid (pH value all above 8.0), which suggests that the oil must be oxidized during the heating process. Furthermore, the prolonged time and high temperature in the drying process could cause oxygen absorption, lipid oxidation, and peroxide formation (Hernandez et al. 2013). This may be the reason that soybean oil spiked with 4 % oat oil produced more MDA than that spiked with 2 % oat oil.
Fig. 3.
Malondialdehyde (MDA) contents of soybean oil spiked with different antioxidant sources. The control is soybean oil with no additives. Data are given as mean ± SD (n = 4). Different letters on data bars indicate significant differences. TBHQ tert-butyl hydroquinone
Intense and/or prolonged thermal treatment may be responsible for a significant loss of natural antioxidants, considering that most of the compounds are relatively unstable. The results show that oat oil can inhibit oil oxidation even when intense heating is conducted over a long period. After a physical refining process to exclude free fatty acids and preserve the antioxidants, oat oil should provide improved antioxidant activity.
Conclusion
All oat oil extracts displayed high antioxidant activity, particularly that obtained by extraction with ethanol. The same extract also showed good inhibition of soybean oil oxidation. In general, as the polarity of the extraction solvent increased, the oat oil extracts showed better antioxidant activity and contained more antioxidant components. As a result, ethanol was found to be the best solvent for extracting oat oil. This study has also demonstrated that oat oil has the potential to act as an antioxidant agent for the preservation of oil. DPPH radical scavenging activity was the best method to evaluate the antioxidant activity and components of oat oil.
Acknowledgments
This research was carried out with financial support from the Agricultural Industry Technology System Project (nycytx 07–14) and Special Found for Agro−scientific Research in the Public Interest (NO. 201303079). The authors thank the National Oats and Buckwheat Industrial Technology R & D Center for supplying materials.
References
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Andlauer W, Furst P (1998) Antioxidative power of phytochemicals with special reference to cereals. Cereal Foods World 43
- Brown C, Craddock J. Oil content and groat weight of entries in the world oat collection. Crop Sci. 1972;12:514–515. doi: 10.2135/cropsci1972.0011183X001200040038x. [DOI] [Google Scholar]
- Bryngelsson S, Dimberg LH, Kamal-Eldin A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.) J Agric Food Chem. 2002;50:1890–1896. doi: 10.1021/jf011222z. [DOI] [PubMed] [Google Scholar]
- Butt MS, Tahir-Nadeem M, Khan MKI, Shabir R, Butt MS. Oat: unique among the cereals. Eur J Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- Carpenter A. Determination of tocopherols in vegetable oils. J Am Oil Chem Soc. 1979;56:668–671. doi: 10.1007/BF02660070. [DOI] [Google Scholar]
- Carrasco-Pancorbo A, Cerretani L, Bendini A, Segura-Carretero A, Del Carlo M, Gallina-Toschi T, Lercker G, Compagnone D, Fernandez-Gutierrez A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J Agric Food Chem. 2005;53:8918–8925. doi: 10.1021/jf0515680. [DOI] [PubMed] [Google Scholar]
- Del CM, Sacchetti G, Di MC, Compagnone D, Mastrocola D, Liberatore L, Cichelli A. Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil. J Agric Food Chem. 2004;52:4072–4079. doi: 10.1021/jf049806z. [DOI] [PubMed] [Google Scholar]
- Fenglin H, Ruili L, Bao H, Liang M. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia. 2004;75:14–23. doi: 10.1016/j.fitote.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Flander L, Salmenkallio-Marttila M, Suortti T, Autio K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. Lwt-Food Sci Technol. 2007;40:860–870. doi: 10.1016/j.lwt.2006.05.004. [DOI] [Google Scholar]
- Frankel EN, Huang SW, Kanner J, German JB. Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. J Agric Food Chem. 1994;42:1054–1059. doi: 10.1021/jf00041a001. [DOI] [Google Scholar]
- Frey K, Hammond E. Genetics, characteristics, and utilization of oil in caryopses of oat species. J Am Oil Chem Soc. 1975;52:358–362. doi: 10.1007/BF02639196. [DOI] [Google Scholar]
- Gutierrez F, Jimenez B, Ruiz A, Albi M. Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties Picual and Hojiblanca and on the different components involved. J Agric Food Chem. 1999;47:121–127. doi: 10.1021/jf980684i. [DOI] [PubMed] [Google Scholar]
- Haiyan Z, Bedgood DR, Bishop AG, Prenzler PD, Robards K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007;100:1544–1551. doi: 10.1016/j.foodchem.2005.12.039. [DOI] [Google Scholar]
- Halima N, Slima A. Protective effects of oat oil on deltamethrin-induced reprotoxicity in male mice. Food Funct. 2014;5(9):2070–2077. doi: 10.1039/C4FO00190G. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Radic Res. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Commentary oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hernandez B, Luna G, Garcia O, Mendoza MR, Azuara E, Beristain CI, Jimenez M. Extraction and characterization of Oecopetalum mexicanum seed oil. Ind Crop Prod. 2013;43:355–359. doi: 10.1016/j.indcrop.2012.07.022. [DOI] [Google Scholar]
- Heuvel J, Eggels L, Rozen A, Luijendijk M, Fliers E, Kalsbeek A, Adan R, Fleur S. Neuropeptide Y and leptin sensitivity is dependent on diet composition. J Neuroendocrinol. 2014;26(6):377–385. doi: 10.1111/jne.12155. [DOI] [PubMed] [Google Scholar]
- Hseu YC, Chang WH, Chen CS, Liao JW, Huang CJ, Lu FJ, Chia YC, Hsu HK, Wu JJ, Yang HL. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–114. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ivanov DS, Lević JD, Sredanović SA. Fatty acid composition of various soybean products. J Inst Food Technol Novi Sad. 2010;37(2):65–70. [Google Scholar]
- Kilci A, Gocmen D. Phenolic acid composition, antioxidant activity and phenolic content of tarhana supplemented with oat flour. Food Chem. 2014;151:547–553. doi: 10.1016/j.foodchem.2013.11.038. [DOI] [PubMed] [Google Scholar]
- Kim K-H, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95(3):466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- Leonova S, Shelenga T, Hamberg M, Konarev AV, Loskutov I, Carlsson AS. Analysis of oil composition in cultivars and wild species of oat (Avena sp.) J Agric Food Chem. 2008;56(17):7983–7991. doi: 10.1021/jf800761c. [DOI] [PubMed] [Google Scholar]
- Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am Coll Nutr. 2000;19:312–319. doi: 10.1080/07315724.2000.10718966. [DOI] [PubMed] [Google Scholar]
- Morrissey PA, O’Brien NM. Dietary antioxidants in health and disease. Int Dairy J. 1998;8:463–472. doi: 10.1016/S0958-6946(98)00070-3. [DOI] [Google Scholar]
- Mortensen A, Skibsted LH. Importance of carotenoid structure in radical-scavenging reactions. J Agric Food Chem. 1997;45:2970–2977. doi: 10.1021/jf970010s. [DOI] [Google Scholar]
- Peterson DM. Oat antioxidants. J Cereal Sci. 2001;33:115–129. doi: 10.1006/jcrs.2000.0349. [DOI] [Google Scholar]
- Peterson DM, Wood DF. Composition and structure of high-oil oat. J Cereal Sci. 1997;26:121–128. doi: 10.1006/jcrs.1996.0111. [DOI] [Google Scholar]
- Pirisi FM, Cabras P, Cao CF, Migliorini M, Muggelli M. Phenolic compounds in virgin olive oil. 2. Reappraisal of the extraction, HPLC separation, and quantification procedures. J Agric Food Chem. 2000;48:1191–1196. doi: 10.1021/jf991137f. [DOI] [PubMed] [Google Scholar]
- Pokorný J. Natural antioxidants for food use. Trends Food Sci Technol. 1991;2:223–227. doi: 10.1016/0924-2244(91)90695-F. [DOI] [Google Scholar]
- Poljsak B, Milisav I. Oxidized forms of dietary antioxidants: friends or foes? Trends Food Sci Technol. 2014;39(2):156–166. doi: 10.1016/j.tifs.2014.07.011. [DOI] [Google Scholar]
- Price P, Parsons J. Lipids of seven cereal grains. J Am Oil Chem Soc. 1975;52:490–493. doi: 10.1007/BF02640738. [DOI] [PubMed] [Google Scholar]
- Sakouhi F, Harrabi S, Absalon C, Sbei K, Boukhchina S, Kallel H. α-Tocopherol and fatty acids contents of some Tunisian table olives (Olea europea L.): changes in their composition during ripening and processing. Food Chem. 2008;108:833–839. doi: 10.1016/j.foodchem.2007.11.043. [DOI] [PubMed] [Google Scholar]
- Stevenson DG, Inglett GE, Chen D, Biswas A, Eller FJ, Evangelista RL. Phenolic content and antioxidant capacity of supercritical carbon dioxide-treated and air-classified oat bran concentrate microwave-irradiated in water or ethanol at varying temperatures. Food Chem. 2008;108:23–30. doi: 10.1016/j.foodchem.2007.08.060. [DOI] [Google Scholar]
- Tuberoso CI, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501. doi: 10.1016/j.foodchem.2006.08.014. [DOI] [Google Scholar]
- Wilding JP, Gilbey SG, Bailey CJ, Batt RA, Williams G, Ghatei MA, Bloom SR. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132:1939–1944. doi: 10.1210/endo.132.5.7682936. [DOI] [PubMed] [Google Scholar]
- Yen GC, Duh PD. Antioxidative properties of methanolic extracts from peanut hulls. J Am Oil Chem Soc. 1993;70:383–386. doi: 10.1007/BF02552711. [DOI] [Google Scholar]
- Zhang R, Zu LY, Fan T. Oxidation of soybean oil in difference storage conditions. J Chin Cereals Oils Assoc. 2007;122(3):112–114. [Google Scholar]
- Zhou M, Robards K, Glennie-Holmes M, Helliwell S. Oat lipids. J Am Oil Chem Soc. 1999;76:159–169. doi: 10.1007/s11746-999-0213-1. [DOI] [Google Scholar]
- Zhu KX, Lian CX, Guo XN, Peng W, Zhou HM. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. doi: 10.1016/j.foodchem.2010.11.144. [DOI] [Google Scholar]