Abstract
Vitamin B12 is one of nature’s complex metabolite which is industrially produced using certain bacteria. Algae could be an alternative source of vitamin B12 and in this study, vitamin B12 from a halotolerant green alga, Dunaliella salina V-101 was purified and characterized. The extract of Dunaliella was purified by passing through Amberlite XAD-2 and EASI-extract vitamin B12 immunoaffinity column. The total vitamin B12 content in purified sample fractions was 42 ± 2 μg/100 g dry weight as determined by the chemiluminescence method which was almost close to 49 ± 2 μg/100 g dry weight as estimated by microbiological method. Further quantification of total vitamin B12 using gold nanoparticle (AUNPs) based aptamer showed 40 ± 0.8/100 g dry weight. There was a good correlation among all the methods of quantification. Adenosylcobalamin, a form of vitamin B12 which is a cofactor for methylmalonyl CoA mutase was identified by HPLC. Upon quantification, Dunaliella was found to contain 34 ± 4 μg of adenosylcobalamin for 100 g dry biomass. Authenticity of adenosylcobalmin was confirmed by tandem mass spectrometry (MS/MS), selected ion recording (SIR) and multiple reaction monitoring (MRM) studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-2005-y) contains supplementary material, which is available to authorized users.
Keywords: Dunaliella salina; Adenosylcobalamin; Chemiluminescence; Vitamin B12 immunoaffinity column, gold nanoparticle based aptamer
Introduction
Vitamin B12 is an essential nutrient for the maintenance of myelin sheath surrounding nerve cells in humans (Herbert 1996) that promotes growth, cell development, fat and carbohydrate metabolism. It is also essential for the rapid synthesis of DNA during cell division, particularly the bone marrow tissue responsible for red blood cell formation (Stabler 1999). The biologically active forms of vitamin B12 are methylcobalamin and adenosylcobalamin but it is cyanocobalamin that is used in vitamin supplements, pharmaceuticals and fortification of food. Industrially vitamin B12 is produced through certain bacteria only. Plants are generally believed neither to synthesize nor utilize cobalamin for growth hence strict vegetarians have a greater risk of developing vitamin B12 deficiency. However some freshwater green algae, Chlamydomonas reinhardtii (Watanabe et al. 1991) and Chlorella vulgaris (Watanabe et al. 1997) contain vitamin B12. When dried purple lavers were fed to vitamin B12 deficient rat, it was observed that the algal B12 is bioavailable to rats (Takenaka et al. 2001). While in halotolerant green algae there is little information available on the B12 content.
Dunaliella salina a halophilic green microalga is known for its β-carotene content. It produces large amounts of carotenoids and it is being used in dietary supplements and cosmetics. Carotenoids, glycerol, vitamin C, vitamin E are the important intracellular components in Dunaliella (Baz et al. 2002). It has a significant effect on decreasing the risk of lung, esophagus, pancreas, stomach, breast, skin, colon and ovary cancers (Poppel and Goldbohm 1995; Baz et al. 2002; Murthy et al. 2005; Raja et al. 2007). Prevention of tumors, extending the immunology response and neoplastic transformations are the other benefits reported (Wald et al. 1988; Challem 1997; Murthy et al. 2005; Raja et al. 2007). However Dunaliella has not been studied much for the presence of vitamin B12 and its forms.
The aim of the present study was to evaluate the total vitamin B12 and the form of vitamin B12 in the Dunaliella culture.
Materials and methods
Chemicals and instruments
Cyanocobalamin (CN-Cbl), Adenosylcobalamin (Adl-Cbl), methylcobalamin (Me-Cbl), hydroxocobalamin (OH-Cbl), luminol, urea hydrogen peroxide diethylpyrocarbonate (DEPC), silver nitrate, gold (III) chloride and trisodium citrate were procured from Sigma Aldrich chemicals Co. (St. Louis, MO, USA). Amberlite XAD-2 was obtained from Supelco (Sigma Aldrich Co. Bangalore, India). Vitamin B12 assay medium was obtained from Himedia, Bangalore, India. Vitamin B12 RNA aptamer sequence (5′ GGA ACC GGU GCG CAU AAC CAC CUC AGU GCG AGC AA 3′) was adapted from Lorsch and Szostak (1994) report. All pyrimidines were 2′ fluoro modified and obtained from Trilink Biotechnologies (San Diego CA, USA). Stock solutions were prepared in DEPC treated water. Methanol was of HPLC grade. HPLC (SCL-10-AVP) was from Shimadzu Kyoto, Japan. Reverse-phase HPLC Column (4.6 × 300 mm, μ bondpack, particle size 10 μm) was from Waters Corp. (Milford MA, USA). Luminometer from Luminoskan TL plus, thermolab systems (Helsinkin, Finland). Data acquisition was performed with decimal HyperTerminal TL plus software. EASI-EXTRACT vitamin B12 immunoaffinity column was procured from R-Biopharma, Scotland. Column used for ESI-MS was Acquity UPLC HSS T3 2.1 × 50 mm,1.8 μm. Positive ion MS/MS experiments was performed in product mode on a triple quadrupole Xevo TQD mass spectrometer (Waters Corp., Milford USA). Triple Distilled water was used for the preparation of solvent system for HPLC analysis. All other reagents used were of the highest purity commercially available.
Organism and culture
D. salina strain V-101, was obtained from Centre for Advanced Studies in Botany, Madras University, Chennai. Liquid culture of D. salina was maintained in modified AS100 medium (MAS100) (Vonshak 1986). The stock culture was maintained under 16 h light intensity of 1.5 ± 0.2 Klux at 25 ± 1 °C. The outdoor culture was grown in a circular pond at ambient temperature ranging from 24 to 28 °C. The culture growth was monitored by measuring optical density at 560 nm. The biomass was harvested by centrifugation and washed twice with distilled water, lyophilized and stored at −80 °C until further use.
Extraction
The lyophilized Dunaliella cells of 100 g were added to 0.1 mol/L acetate buffer with pH 4.8. Total vitamin B12 was extracted from the cell suspension by boiling with 20 mg of KCN. The solution containing algal cells was boiled for 30 min at 98 °C and centrifuged at 10,000 g. The extraction was repeated twice and the supernatant was pooled and used for the assay. The extraction was carried out in dark. The combined supernatant fractions were loaded on to a column of Amberlite XAD-2 resin which had been washed with methanol and then equilibrated with distilled water. The sample was eluted with 80 % (v/v) methanol solution in the dark. The eluate containing a corrinoid compound was evaporated to dryness using rotavapor (Buchi B 490) and dissolved in distilled water.
To evaluate the forms of B12 the biomass was suspended in triple distilled water and autoclaved at 121 °C for 10 min. The homogenate was centrifuged at 10,000 g for 10 min. The cooled supernatant was used for B12 analysis. The whole extraction process was carried out in dark. Partial purification was done by passing through Amberlite XAD-2 resin column.
Purification using immunoaffinity column
The partially purified sample obtained was passed through the EASI-EXTRACT vitamin B12 immunoaffinity column which contained monoclonal antibody with high affinity and specificity to vitamin B12. The column was washed with 20 ml of 2 % Tween 20 in water followed by 10 ml water. Elution of the vitamin was carried out with 100 % HPLC grade methanol. The eluted sample was evaporated to dryness under reduced pressure and analyzed using HPLC.
High performance liquid chromatography
The concentrated sample was analyzed on a reverse phase μBondapack HPLC column (4.6 × 300 mm, particle size 10 μm). Elution of the vitamin B12 compound was carried out with a linear gradient of methanol [from 0 to 90 % of a 50 % (v/v) methanol solution containing 0.1 % (v/v) acetic acid] for 40 min by measuring the absorbance at 361 nm and 546 nm. The flow rate was 1 ml/min (Watanabe et al.2000). The retention times of authentic standards and sample were recorded.
Microbiological assay of vitamin B12
Lactobacillus delbrueckii MTCC 911 strain obtained from Microbial Type Culture Collection, CSIR-IMTECH, Chandigarh, India was used for microbiological assay. The standard vitamin B12 was prepared in the range of 0.01–0.2 μg/ml for the analysis. Purified fractions from HPLC were collected, pooled, concentrated and used for the assay. The turbidity (%T) of Lactobacillus delbrueckii test culture was measured at 600 nm (Watanabe et al.1998).
Chemiluminescence assay of vitamin B12
Purified fractions from HPLC were concentrated and subjected to chemiluminescence (CL) assay. Chemiluminescence reactions were carried out using a luminometer and in a polystyrene cuvette. The CL signals were plotted at 10-s intervals for a period of 10 min. The sample was prepared according to Kumar et al. (2009). An optimized concentration of luminol and vitamin B12 was added to the cuvette followed by urea– H2O2. This results in CL signals. The generation of signals measured in terms of chemiluminescence units (CLU) was caused due to the reaction between luminol and urea hydrogen peroxide with vitamin B12 (Kumar et al. 2009). An increase in CLU can be observed in the presence of vitamin B12.
Gold nanoparticle based RNA aptamer detection
For the detection of vitamin B12, RNA aptamer-based colorimetric sensor using gold nanoparticles were employed as described in the earlier report (Kumudha et al. 2015). AUNPs were prepared and the concentration and the size of AUNPs were determined using an ultraviolet-visible (UV-vis) spectrophotometer. The mixture containing aptamer and optimized concentration of AUNPs was shaken gently for 10 min at room temperature and incubated for 10 min along with the purified sample. The change in the color and spectra of the sample after addition of NaCl were measured using UV-vis spectrophotometer in a wavelength range from 400 nm to 700 nm.
UPLC-ESI-MS
UPLC-ESI-MS was carried out in a UPLC/Xevo TQD Tandem quadrupole with nominal mass measurement in positive mode. The cone and desolvation gas were set to 28 and 1000 L/H, respectively. Sample source conditions were as follows: capillary voltage, 3.00 kV; sample cone voltage, 10 V; extraction cone voltage, 2 V; source temperature, 135 °C; and desolvation temperature, 450 °C; cone gas flow (L/H) 10; collision gas flow (ml/min) 0.21; LM 1 resolution 15.00; HM 1 resolution 15.00; Ion energy 1, 0.50; MS Mode entrance, 50.00; MS Mode collision energy, 1.00; MS Mode exit 50.00; MSMS Mode entrance, 1.00; MSMS mode collision energy, 1.00; MSMS Mode exit, 0.5; LM 2, resolution, 15.00; HM 2, resolution, 15.00; Ion energy 2, 1.0; Gain, 1.00 and multiplier −522.98. Samples were introduced into the mass spectrometer through a direct-flow injection UPLC system for solvent delivery at the flow rate of 0.6 ml/min. Column used was Acquity UPLC HSS T3 2.1 × 50 mm, 1.8 μm. A linear gradient of 10 mM ammonium formate and 0.1 % formic acid in water (A) and 10 mM ammonium formate and 0.1 % formic acid in methanol (B) was used. Column temperature was set at 35 °C (Kumudha et al. 2010).
MS/MS experiments
Positive ion MS/MS experiments were performed in product mode on a triple quadrupole TQD mass spectrometer (Waters corporation Milford USA). The instrument was operated with the following instrumental conditions: Calibration dynamic 1, capillary (kV) 3.00; cone (V) 10.00; extractor (V) 2.00; RF (V) 0.10; source temperature (°C) 135; desolvation temperature (°C) 450; cone gas flow (L/H) 10; desolvation gas flow (L/H) 1100; collision gas flow (ml/min) 0.21; LM 1, resolution 15.00; HM 1, resolution 15.00; Ion energy 1, 0.50; MS mode entrance 50.00; MS mode collision energy, 1.00; MS mode exit, 50.00; MSMS mode entrance,1.00; MSMS mode collision energy 1.00; MSMS mode exit 0.50; LM 2, resolution 15.00; HM 2, resolution 15.00; Ion energy 2, 1.00; Gain, 1.00; multiplier −522.98.
Results and discussion
Vitamin B12 in Dunaliella salina
In the present study, Dunaliella cells were grown in outdoor circular cement ponds and the fresh biomass was lyophilized and used for the estimation of total B12. In order to quantify the total vitamin B12, cyanide was added in the extraction protocol so as to convert all the natural forms such as OH-Cbl, Adl-Cbl and Me-Cbl to cyanocobalamin.
Purification of Dunaliella extracts on Amberlite XAD −2 ensures efficient binding of the vitamin B12 from the complex matrix (Fenton and Rosenberg 1978). Further purification on EASI-EXTRACT vitamin B12 immunoaffinity column enables concentration of the analyte and removal of interfering compounds allowing accurate quantification of vitamin B12 (Marley et al. 2009). Immunoaffinity columns have also been reported to be highly efficient for the purifiction of vitamin B12 (Heudi et al. 2006). The eluant was analysed by HPLC and the profile observed was similar to that of the standard cyanocobalamin. The purity of the peak was confirmed with spiked standard cyanocobalamin. The fraction was collected and subjected to further confirmation and quantification by chemiluminescence and microbiological assay. It is reported that Co2+ enhances the photon production during luminol and H2O2 reaction (Kumar et al. 2009). In a chemiluminescence reaction, photons are produced and are directly proportional to the vitamin B12 concentration. The sample was quantified by chemiluminescence assay and it was found to contain 42 ± 2 μg of cyanocobalamin for 100 g dry biomass of Dunaliella.
Microbiological assay of vitamin B12 is one of the most frequently applied methods for routine analysis of vitamin B12 (Kralova et al. 1982). The intensity of turbidity caused by cell growth is proportional to vitamin B12 concentration. The quantification of cyanocobalamin in the eluted sample from HPLC was carried out by microbiological assay using Lactobacillus delbrueckii MTCC 911. The sample was quantified and was found to contain 49 ± 2 μg of cyanocobalamin for 100 g dry biomass of Dunaliella.
Total cyanocobalamin in the sample was quantified using gold nanoparticle based RNA aptamer. Aptamer using AUNPs have been considered as an on-site detection method with high specificity and sensitivity (Cho et al. 2008). These aptamers are short DNA or RNA sequences which have high affinity towards its analyte. The AUNPs were treated with vitamin B12 aptamer along with the sample. The addition of salt to the solution creates electrostatic repulsion between negatively charged AUNPs and results in aggregation of AUNPs leading to a red-to-purple colour shift in the presence of vitamin B12. UV-vis studies provided quantitative results and based on the spectral data, the vitamin B12 in the sample was estimated at 40 ± 0.8/100 g dry weight.
Identification of form of vitamin B12
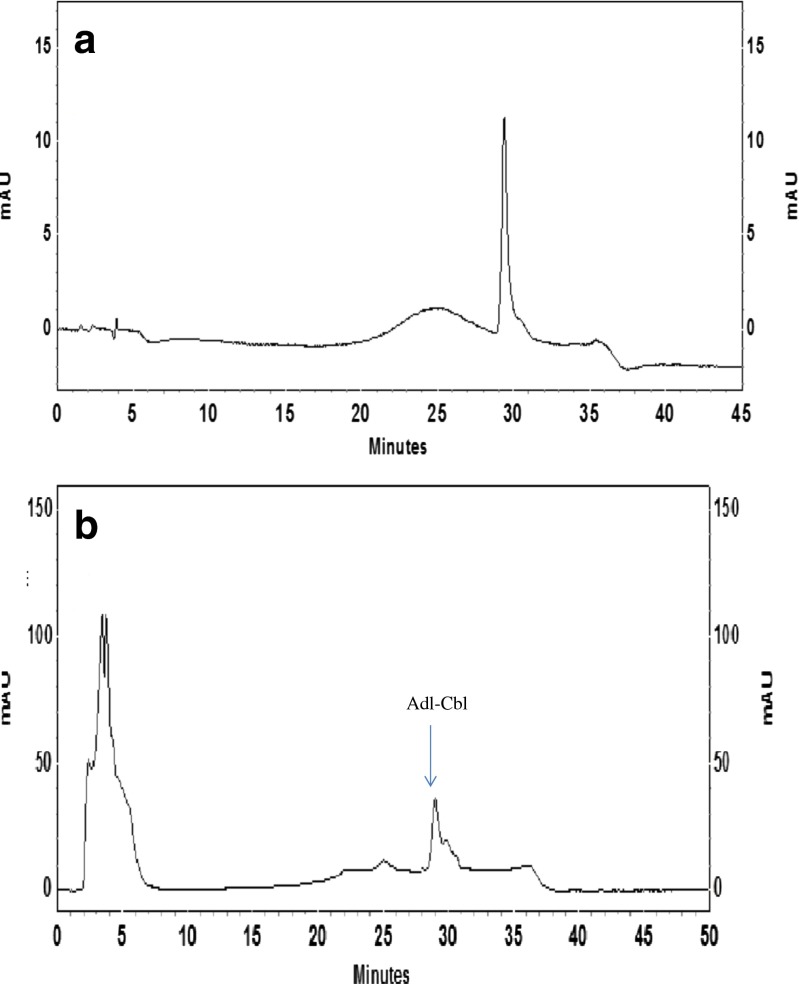
To evaluate the forms of vitamin B12 found in Dunaliella, the extraction was carried out in aqueous solution without cyanide. Lyophilized sample was used for extraction since it exhibited higher stability for biologically active vitamin B12 (Takenaka et al. 2001). Forms of vitamin B12 in lyophilized Dunaliella cells were determined after extracting the lyophilized algal cells in aqueous condition and purifying vitamin B12 by passing the extract through Amberlite XAD-2 column and immunoaffinity column. HPLC analysis of eluant for vitamin B12 was carried out using a suitable solvent system which separated all forms of vitamin B12. Retention time (RT) of standard OH-Cbl, Adl-Cbl and Me-Cbl were found to be 20.1, 29.2 and 35.2 mins respectively. Purified samples were injected into HPLC to identify forms of vitamin B12 based on RT of standards. It was observed that the retention time of the sample was identical to that of standard Adl-Cbl. The form of vitamin B12 was detected at 546 nm along with 361 nm wavelengths. A peak at 29.2 min having absorbance at 546 nm similar to Adl-Cbl was observed (Fig. 1a and b). Further, the purity of the peak was confirmed with spiked standard Adl-Cbl. The Adl-Cbl fraction was collected and subjected to further confirmation and quantification by chemiluminescence and microbiological assay. In chemiluminesence method 27 ± 4 μg of adenosylcobalamin was observed for 100 g dry biomass of Dunaliella while microbiological assay showed 34 ± 4 μg of adenosylcobalamin for 100 g dry biomass of Dunaliella. Similar correlation between microbiological and chemiluminesence assay was observed for methylcobalamin in Spirulina sample (Kumudha et al. 2010).
Fig. 1.
a HPLC chromatogram of the standard Adenosylcobalamin. b HPLC chromatogram of the Dunaliella extract
LC-MS and MS/MS of the sample
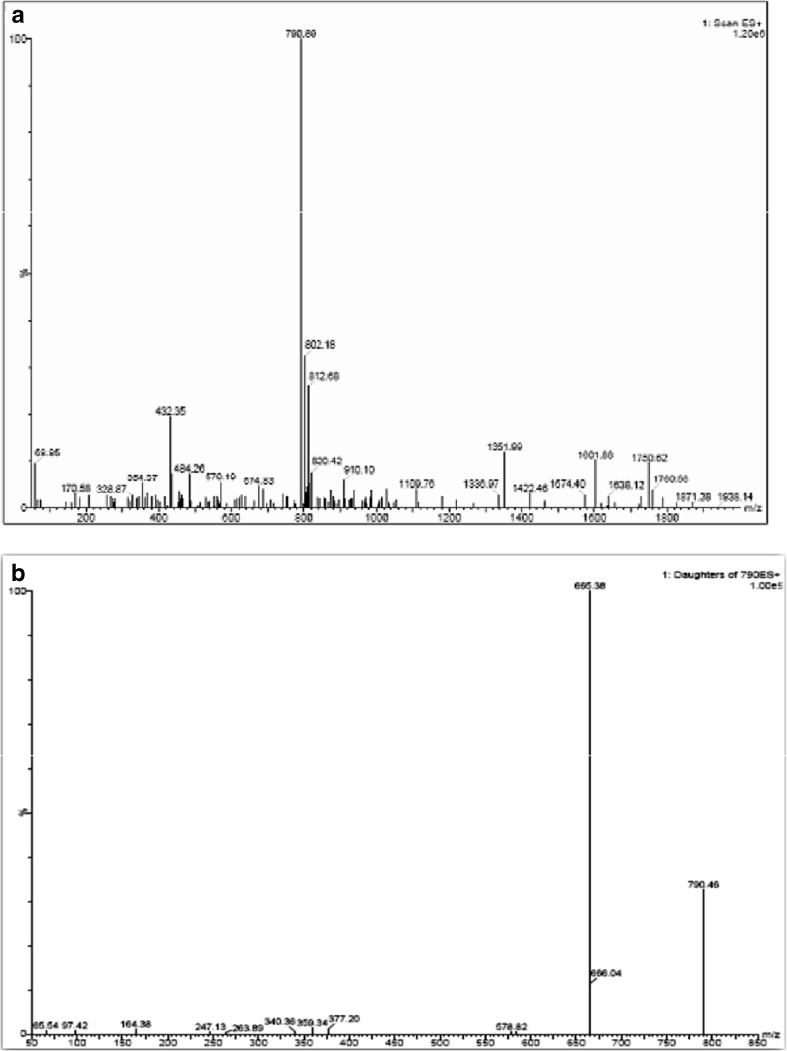
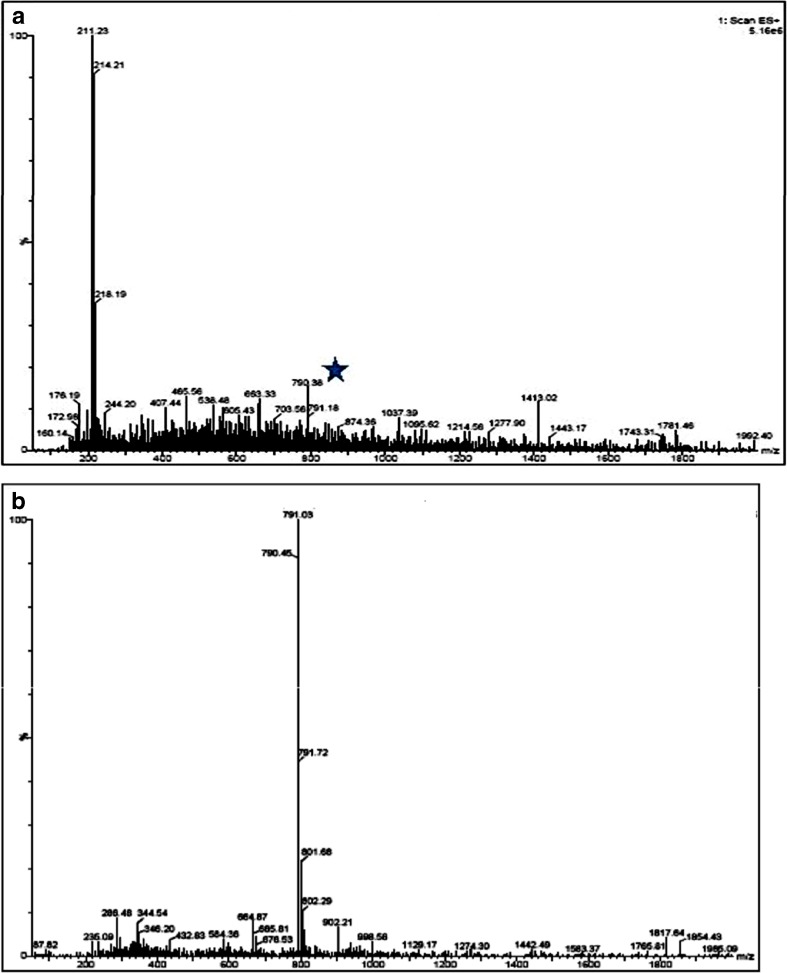
The LC-MS analysis of the standard and the purified sample was carried out. The MS TIC (total ion chromatogram) observed for Adl-Cbl standard showed the ionization in ESI positive mode. The UV data and the corresponding full scan (MS) data of standard were compared with the purified samples to confirm the presence of Adl-Cbl in the ESI positive mode. Though the mass of Adl-Cbl is 1579.58 m/z, the spectra showed that it is doubly charged and thus mass 790 m/Z was observed instead of 1581 m/Z. (Fig. 2a). The MS/MS of the standard Adl-Cbl showed the doubly charged ion (Fig. 2b). The purified sample of Dunaliella also showed the doubly charged ion of Adl-Cbl (Fig. 3a). Since the intensity of the sample was low, MS/MS, selected ion recording (SIR) and multiple reaction monitoring (MRM) were performed for the mass to confirm the presence of Adl-Cbl (Fig. 3b). Adenosylcobalamin is one of the two active forms of vitamin B12. It plays an essential role in the production of blood and maintenance of normal cerebral and nervous functioning in the human body. Also known as cobamamide or dibencozide, adenosylcobalamin occurs naturally in animal derived food types such as fish, meat, eggs and milk.
Fig. 2.
a Mass spectrum of standard adenosylcobalamin. b MS/MS of standard adenosylcobalamin
Fig. 3.
a Mass spectrum of Dunaliella extract. b MS/MS of Dunaliella extract
Not many algae have been studied extensively for the vitamin B12 content, its forms and bioavailability. Porphyra yezoensis, an ebible puple laver is one among few algae to be characterized and reported to contain five types of biologically active B12 compound. Spirulina, a cyanobacteria is also reported to contain methylcobalamin, a form of vitamin B12 (Kumudha et al. 2010). Marine algae are known to be extremely rich in vitamin B12 (Croft et al.2005), but many of them have not been characterized for vitamin B12. In the present study, the marine alga, Dunaliella contains a total of 49 ± 2 μg of vitamin B12 and 34 ± 4 μg of Adl-cbl per 100 g dry biomass of Dunaliella. Adl-cbl being one of the biologically active forms of vitamin B12 it is of great relevance to human health.
Conclusion
The recognition of algae as a source of vitamin B12 will necessitate a change in our understanding of algal communities and is likely to have profound implication for the exploitation of algae, both as a food source and for biotechnological application. The present findings highlight the presence of adenosylcobalamin in Dunaliella salina which was quantified and characterized using microbiological, chemiluminescence assay along with MS/MS and gold nanoparticle based aptamer studies. Vitamin B12 is known to be deficient in the vegetarian diet, and algae could be an alternative source of vitamin B12. The present results suggest that Dunaliella can be used as a source of vitamin B12 apart from its other nutrients.
Electronic supplementary material
(PDF 232 kb)
Acknowledgments
The authors thank the Director CSIR-CFTRI for his encouragement. KA acknowledges CSIR for providing Senior Research Fellowship. The authors also express gratitude to Gopal Vaidyanathan and Dilshad of Waters, Bangalore, India for analyzing the sample through LC-MS and MS/MS.
References
- Baz FKE, Abul-Enein AM, El-Baroty GS, Youssef AM, -Baky Abdel. Accumulation of antioxidant vitamins in Dunaliella salina. Online J Biol Sci. 2002;2:220–223. doi: 10.3923/jbs.2002.220.223. [DOI] [Google Scholar]
- Challem JJ. Beta-carotene and other carotenoids: promises failures, and a new vision. J Orthomol Med. 1997;12:11–19. [Google Scholar]
- Cho M, Han S, Ban C. Detection of mismatched DNAz via the binding affinity of muts using gold nanoparticle-based competitive colorimetric method. Chem Commun. 2008;38:4573–4575. doi: 10.1039/b811346g. [DOI] [PubMed] [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature Letters. 2005;438:90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- Fenton WA, Rosenberg LE. Improved techniques for the extraction and chromatography of cobalamins. Anal Biochem. 1978;90:119–125. doi: 10.1016/0003-2697(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Herbert V (1996) Vitamin B12. In Ziegler E.E, Filer L. J, eds. Present Knowledge in Nutrition, International Life Science Institute Washington DC, 191–205.
- Heudi O, Kilinc T, Fontannaz P, Marley E (2006) Determination of vitamin B12 in food products and in premixes by reversed-phase high performance liquid chromatography and immunoaffinity extraction. J Chromatogr A 1101:63–68 [DOI] [PubMed]
- Kralova B, Rauch P, Cerna J. Use of vitamin B12 radioassay in the analysis of biological materials, mainly of foods. Nahrung. 1982;26:803–810. doi: 10.1002/food.19820260920. [DOI] [Google Scholar]
- Kumar SS, Chouhan RS, Thakur MS. Enhancement of chemiluminescence for vitamin B12 analysis. Anal Biochem. 2009;388:312–316. doi: 10.1016/j.ab.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Kumudha A, Kumar SS, Thakur M S, Ravishankar GA, Sarada R. Purification, identification and characterization of methylcobalamin from Spirulina platensis. J Agric Food Chem. 2010;58:9925–9930. doi: 10.1021/jf102159j. [DOI] [PubMed] [Google Scholar]
- Kumudha A, Kumar SS, Dilshad P, Vaidyanathan G, Thakur MS, Sarada R. Methylcobalamin- a form of vitamin B12 identified and characterized in Chlorella vulgaris. Food Chem. 2015;170:316–320. doi: 10.1016/j.foodchem.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Lorsch JR, Szostak JW. In vitro selection of RNA aptamers specific for cyanocobalamin. Biochem. 1994;33:973–982. doi: 10.1021/bi00170a016. [DOI] [PubMed] [Google Scholar]
- Marley EC, Mackay E, Young G. Characterisation of vitamin B12 immunoaffinity columns and method development for determination of vitamin B12 in a range of foods, juices and pharmaceutical products using immunoaffinity clean-up and high performance liquid chromatography with UV detection. Food Addit Contam. 2009;26:282–288. doi: 10.1080/02652030802429104. [DOI] [PubMed] [Google Scholar]
- Murthy KNC, Vanitha A, Rajesha J, Swamy MM, Sowmya PR, Ravishankar GA. In vivo antioxidant activity of carotenoids from Dunaliella salina-a green microalga. Life Sci. 2005;76:1381–1390. doi: 10.1016/j.lfs.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Poppel GV, Goldbohm RA. Epidemiologic evidence for beta-carotene and cancer prevention. Am J Clin Nutr. 1995;62:1393S–1402S. doi: 10.1093/ajcn/62.6.1393S. [DOI] [PubMed] [Google Scholar]
- Raja R, Hemaiswarya S, Balasubramanyam D, Rengasamy R. Protective effect of Dunaliella salina against experimentally induced fibrosarcoma on wistar rats. Microbiol Res. 2007;162:177–184. doi: 10.1016/j.micres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Stabler SP. B12 and nutrition. In: Banerjee R, editor. Chemistry and biochemistry of B12. New York: John wiley and sons; 1999. pp. 343–365. [Google Scholar]
- Takenaka S, Sugiyama S, Ebara S, Miyamoto E, Abe K, Tamura Y, Watanabe F, Tsuyama S, Nakano Y. Feeding dried purple laver (nori) to vitamin B12 deficient rats significantly improves vitamin B12 status. Brit J Nutr. 2001;85:699–703. doi: 10.1079/BJN2001352. [DOI] [PubMed] [Google Scholar]
- Vonshak JA. Laboratory techniques for cultivation of microalgae. In: Richmond A., editor. Handbook of microalgal mass culture CRC press. Florida: Baca Raton; 1986. pp. 117–145. [Google Scholar]
- Wald NJ, Thompson SG, Densem JW, Boreham J, Beiley A. Serum beta-carotene and subsequent risk of cancer: results from the BUPA study. Brit J Nutr. 1988;57:428–433. doi: 10.1038/bjc.1988.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe F, Nakano Y, Tamura Y, Yamanaka H. Vitamin B12 metabolism in a photosynthesizing green alga, Chlamydomonas reihardtii. Biochim Biophys Acta. 1991;1075:36–41. doi: 10.1016/0304-4165(91)90071-N. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Abe K, Takenaka S, Tamura Y, Maruyama I, Nakano Y. Occurrence of cobalamin coenzymes in the photosynthetic green alga, Chlorella vulgaris. Biosci Biotechnol Biochem. 1997;61:896–897. doi: 10.1271/bbb.61.896. [DOI] [PubMed] [Google Scholar]
- Watanabe F, Takenaka S, Abe K, Tamara Y, Nakano Y. Comparison of a microbiological assay and a fully automated chemiluminescent system for the determination of vitamin B12 in food. J Agric Food Chem. 1998;46:1433–1436. doi: 10.1021/jf970807j. [DOI] [Google Scholar]
- Watanabe F, Takenaka S, Katsura H, Miyamoto E, Abe K, Tamura Y, Nakatsuka T, Nakano Y. Characterization of a vitamin B12 compound in edible purple laver, Porphyra yezoensis. Biosci Biotechnol Biochem. 2000;64:2712–2715. doi: 10.1271/bbb.64.2712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 232 kb)