Abstract
In the present study, a carbon paste electrode chemically modified with gold nanoparticles was used as a sensitive electrochemical sensor for determination of ochratoxin A. The differential pulse voltammetric method was employed to study the behavior of ochratoxin A on this modified electrode. The effect of variables such as percent of gold nanoparticles, pH of sample solution, accumulation potential and time on voltammogram peak current were optimized. The proposed electrode showed good oxidation response for ochratoxin A in 0.1 mol L−1 PBS (pH 7.2) and the peak potential was about +0.8 V (vs. Ag/AgCl). The peak current increased linearly with the ochratoxin A concentration in the range of 0.5–100 nM. The detection limit was found to be 0.2 nM and the relative standard deviation was 6.2 % (n = 7). The method has been applied to the determination of ochratoxin A in cereal derived products such as breakfast cereals, cereal-based baby foods and beer samples.
Keywords: Gold nanoparticles, Modified carbon paste electrode, Ochratoxin a determination, Real samples analysis
Introduction
Fungi play a substantial role in spoilage of food, fruits and vegetables, because of their pathogenicity to the harvested products. During the various stages of pathogenesis, however, some of these fungi may generate different mycotoxins (Baikai-Golan and Paster 1900; Magan and Olsen 2004). Mycotoxins are fungal secondary metabolites that could be present in foods and vegetables as a consequence of fungal growth and they are harmful to animals and humans (Silverio et al. 2010; Iamanaka et al. 2005; Bell et al. 2004). Five types of mycotoxins of agricultural importance occur in staple crops: aflatoxins, fumonisins, zearalenone, specific trichothecenes (deoxynivalenol and nivalenol) and ochratoxins (Miller 1995). These mycotoxins can cause various forms of poisoning in animals and in humans and some are carcinogenic. It has been reported that approximately 25 % of cereals consumed in the world are contaminated with mycotoxins (Bennett and Klich 2003; Hussein and Brasel 2001; Anukul et al. 2013, Bryden 2012). Ochratoxins (cyclic pentaketids, dihydroisocoumarin derivatives linked to an L-phenylalanine moiety) are mycotoxins produced by some Aspergillus and Penicillium species. They were originally isolated in South Africa in 1965 as metabolites from a strain of Aspergillus ochraceus (Van der Merwe et al. 1965). Although a wide range of ochratoxin derivatives can be isolated from laboratory cultures, it is usually only ochratoxin A (OTA) and occasionally ochratoxin B which occur naturally in mouldy products (Belli et al. 2002). Ochratoxin A has received particular attention because of its association with cancer promoting activity. Worldwide studies on ochratoxigenic fungi, primarily A. carbonarius, A. Niger aggregate and other Aspergillus species, as well as on ochratoxin A contamination in food derived from plants, such as coffee beans (Taniwaki et al. 2003), wine (Ratola et al. 2006; Romero-Gonzglez et al. 2010), cereals (Alarcon et al. 2006; Sugita-Konishi et al. 2006), nuts (Overy et al. 2003), spices (Goryacheva et al. 2007), in fruits and fruit juices (apple juice, grape juice, and orange juice) (Marino et al. 2009; Lasra et al. 2007), indicated that these products can be important dietary sources of ochratoxin A for people who consume large amounts them. Due to these findings many countries have established limits on OTA levels in food, typically between 1 and 10 μg kg−1, depending on the type and quality of the foodstuffs (Turner et al. 2004). There is increasing interest among researchers in determining contamination levels of OTA and among regulatory agencies to establish new or replace old OTA limits for food commodities due to its widespread occurrence, increasing health risk concerns and development of new sensitive analytical methods (Fakoor Janati et al. 2012). Many detection techniques have been used for the determination of OTA in different kind of samples. Traditional methods for OTA analysis are usually performed by thin layer chromatography (TLC), high performance liquid chromatography (HPLC), and gas chromatography (GC), coupled to UV/V, fluorescence (FL) or mass spectrometry (MS) (Romani et al. 2000; Shephard et al. 2003; Blesa et al. 2004; Aksoy et al. 2007). These laboratory techniques, unfortunately, require highly qualified personnel, tedious assay time or sophisticated instrumentation (Panini et al. 2011). A rapid, simple, and inexpensive method that does not require sophisticated laboratory equipment would be beneficial for routine determination of mycotoxins in biological samples. Electrochemical detections can be ideal for such tasks since their selectivity often requires less intensive clean-up, in turn allowing rapid and inexpensive detection (Perrotta et al. 2011, 2012; Ramirez et al. 2010, 2011). The advantages of electrochemical detection are evident in the development of chemical and biological sensors utilizing electrochemical detection devices (Taylor and Schultz 1996). Modified carbon paste electrodes (MCPEs) have several advantages such as non-toxic, low background current, wide range of used potential, rapid renewal and easy fabrication (Ashkenani and Taher 2012). In this work, we developed a method for the determination of OTA in different food samples based on its oxidation responses at a gold nanoparticles modified carbon paste electrodes (GNP/CPE).
Experimental
Apparatus
All voltammetric experiments were performed using Autolab electroanalyzer Model PG-STAT-100 from Metrohm (Switzerland). The electrochemical cell consisted of a carbon paste modified electrode as a working electrode, Ag/AgCl/KCl (saturated) as a reference electrode and a platinum wire as an auxiliary electrode. A RH B-KT/C (Germany) magnetic stirrer was employed in the deposition step. A Metrohm 827 pH meter (Switzerland) with a combined glass electrode for adjusting the pH was also used.
Chemicals
Standard solution of ochratoxin A was purchased from Fluka (Switzerland). Tetrachloroauric acid (III) trihydrate (HAuCl4·3H2O) and sodium citrate were purchased from Merck (Darmstadt, Germany) and working solutions were prepared daily by appropriate dilution. Highly pure graphite powder, acetic acid, sodium acetate and sodium dihydrogen phosphate were purchased from Merck. 0.1 M NaH2PO4/Na2HPO4 buffer solution (PBS) at pH 7.2 were used as the supporting electrolyte.
Synthesis of gold nanoparticles
Gold nanoparticles were prepared following the method of Gopu et al. (2008). 95 mL of an aqueous chloroauric acid solution containing 5 mg of Au was brought to boil and then 5 mL of 1 % sodium citrate solution was added to the boiling solution. The color of the solution first changed to bluish, then to purplish and eventually to ruby red. The solution was further boiled for 30 min and left to cool to ambient temperature. TEM image shows the spherical particles with a diameter of 10 ± 2 nm (Fig. 1a).
Fig. 1.
TEM image of a) gold nano particles and b) gold nanoparticles/carbon paste composite
Preparation of the electrode
The unmodified carbon paste was made by hand-mixing 70 mg of graphite powder and 30 μL of paraffin oil with a mortar and pestle. The CPE was constructed by packing this paste into a glass tube (3.4 mm inner diameter) and providing it with a copper contact. The CPE could be reused after each experiment by cutting a thin layer of paste and polishing of new surface. For the preparation of the modified carbon paste electrode (MCPE) 30 % GNP was added to paste and prepared as for the unmodified electrode. TEM image is shown in Fig. 1b.
Experimental procedure
A cell containing the desired concentration of OTA in PBS at pH 7.2 was subjected to the deposition voltage (+0.5 V) under stirring. Following the electrochemical deposition step and a short equilibration period (10 s), differential pulse (DP) voltammograms were recorded by potential scanning from 0.6 to 1.0 V. The resulting oxidation peak at about 0.8 V was registered and its currents used as a measure of OTA concentration. The experimental conditions for differential pulse voltammetry (DPV) were: pulse amplitude 50 mV, pulse width 70 ms, scan rate 50 mV s−1. Measurements were carried out using a modified carbon paste electrode (GNP/CPE), with a Pt wire counter electrode and an Ag/AgCl (3 M KCl) electrode as reference.
Procedure of cereal derived products preparation
A total of 9 cereal-derived samples, including 3 baby food samples, 3 breakfast cereal samples and 3 beer samples were purchased randomly from different supermarkets and small shops in Kerman, Iran. The cereal contents in the baby food samples ranged from 30 to 80 %. Breakfast cereal samples included the following major ingredients, alone or mixed: maize, bran, chocolate, cereals (wheat and/or rice), fruits and oats. All of the beer samples were non-alcohol. 25 g of each Breakfast cereals and baby foods was extracted with 100 mL of methanol: water extraction solvent (80:20, v/v). The extract was filtered through a filter paper. Then 10 mL of filtrate was diluted with 30 mL of PBS.
100 mL of beer samples were boiled for 15 min until foaming was stopped. Then the residue liquid was diluted with distilled water. Then each sample was stirred for about 10 min and the pH was measured directly and adjusted to 7.2.
Results and discussion
Electrochemical behavior of ochratoxin a at the GNP/CPE
The studies of the voltammetric behavior of the ochratoxin A were performed using differential pulse voltammetry. DPV was used in the voltammetric measurement owing to its good sensitivity. Figure 2 compares typical differential pulse voltammograms of 100 nM OTA recorded at two different working electrodes (i.e. bare CPE (curve a) and GNP/CPE (curve b)) after 180 s accumulation under close circuit condition in PBS at pH 7.2 solution. As is seen in Fig. 2b, at the GNP/CPE, compared to the bare CPE, the oxidation peak of OTA shifted to more negative values direction and also the peak current increased. The enhanced peak current response and shift in the oxidation potential are clear evidences of the catalytic effect of the GNP/CPE towards oxidation OTA.
Fig. 2.
Differential pulse anodic stripping voltammograms of 100 nM OTA after closed circuit accumulation: a CPE-GNP and, b bare CPE. Experimental conditions: stripping medium: 0.1 M PBS (pH = 7.2), deposition potential: +0.5 V, deposition time: 180 s, scan rate: 50 mV s−1, pulse amplitude: 50 mV s−1 and pulse time: 4 s
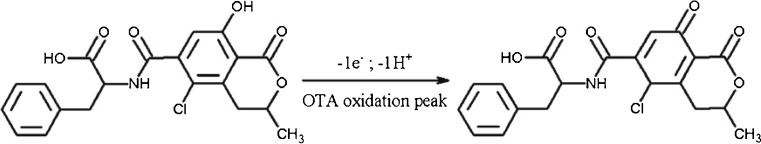
With regard to the above observations and under the experimental conditions, Fig. 3 shows the oxidation reaction of OTA.
Fig. 3.
Schematic representation of the oxidation reaction of ochratoxin
Optimization of operational parameters
Preliminary experiments showed that OTA is adsorbed onto the surface of GNP/CPE by electrostatic interaction. Therefore, pH was recognized as an important factor for the accumulation of OTA on the surface of the modified electrode. The influence of pH on the stripping peak current of OTA was studied by different buffers in the pH range of 5.0–9.0. The maximum peak current was obtained at pH = 7.2. Therefore, pH of 7.2 was chosen for subsequent experiments.
The effect of accumulation potential on the peak current of OTA was examined over the range of +0.3 to +0.8 V. It was found that the peak current of OTA increases with increasing accumulation potential in the range of +0.3 − +0.5 V and increasing from +0.5 to +0.8 V lead to decrease of peak current. Hence accumulation potential of +0.5 V was employed.
The influence of accumulation time on the anodic peak current was studied. For a 100 nM OTA solution, the peak current increases rapidly within 180 s and then remains almost unchanged, meaning that a saturated adsorption is achieved. Thus an accumulation time of 180 s was used.
Analytical characterization
Standard solutions containing different concentrations of OTA were prepared in a 0.1 M PBS and exposed to the optimized anodic stripping voltammetric procedure (Fig. 4a). The calibration curve was linear in the range of 0.5–100 nM and obeyed the equation y = 0.459 x + 0.598, where y and x are the peak current (μA) and OTA concentration (nM), respectively (Fig. 4b). The square of the linear correlation coefficient was 0.9962. The limit of detection was calculated by making seven replicate current measurements at 0.8 V for a blank solution; the detection limit based on the mean of these measurements gave a value of 0.2 nM OTA. Precisions in terms of repeatability and reproducibility (RSD%) of the proposed method were evaluated. Intra-day RSD% (N = 7) at three concentrations of 10 and 70 (nM) were 4.4 and 1.7 %, respectively and inter-day RSD% at the same concentration were 6.2 and 2.2 %.
Fig. 4.
Differential pulse anodic stripping voltammograms in a solution containing OTA; a 0.5 nM, b 1.0 nM, c 5.0 nM, d 20.0 nM, e 50.0 nM, f 75.0 nM, g 100.0 nM. Experimental condition: supporting solution: PBS, deposition potential: +0.5 V, deposition time: 180 s, scan rate: 50 mV s−1, pulse amplitude: 50 mV s−1and pulse time: 4 s. inset: Plot of I(μA) vs. OTA concentrations
Food samples analysis
The proposed method was applied to the determination of OTA in food samples. The analytical results are shown in Table 1-3. The proposed method was checked by spiking OTA in samples. The accuracy of the proposed method was evaluated by analyzing a Maize Reference Material (TET002RM) with ochratoxin A content of 4.89 ± 0.62 ng g−1. The result is given in Table 2 and is in good agreement with reference value. According to results, limits on OTA levels in food samples is below the maximum limits permitted.
Table 1.
Determination of OTA in baby food
| Sample No. | Spiked (ng g−1) | Found (ng g−1) | Recovery (%) |
|---|---|---|---|
| 1 | 0.00 | 0.080 ± 0.005 | – |
| 0.50 | 0.59 ± 0.03 | 102.0 % | |
| 1.00 | 0.90 ± 0.06 | 82.0 % | |
| 2 | 0.00 | – | – |
| 0.50 | 0.52 ± 0.02 | 104.0 % | |
| 1.00 | 0.98 ± 0.05 | 98.0 % | |
| 3 | 0.00 | 0.070 ± 0.003 | – |
| 0.50 | 0.58 ± 0.02 | 101.0 % | |
| 1.00 | 1.05 ± 0.07 | 91.1 % |
Table 3.
Determination of OTA in beer samples
| Sample No. | Spiked (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|
| 1 | 0.0 | 4.13 ± 1.82 | - |
| 1.0 | 5.04 ± 1.33 | 91.0 % | |
| 5.0 | 9.32 ± 1.15 | 103.8 % | |
| 2 | 0.0 | 5.43 ± 0.80 | - |
| 1.0 | 6.32 ± 0.71 | 89.0 % | |
| 5.0 | 10.86 ± 0.48 | 108.6 % | |
| 3 | 0.0 | 6.11 ± 0.20 | - |
| 1.0 | 7.03 ± 0.18 | 92.0 % | |
| 5.0 | 11.00 ± 0.13 | 97.8 % |
Table 2.
Determination of OTA in breakfast cereals
| Sample | Spiked (ng g−1) | Found (ng g−1) | Recovery (%) |
|---|---|---|---|
| 1 | 0.0 | 0.51 ± 0.02 | - |
| 0.5 | 0.98 ± 0.04 | 94.0 % | |
| 1.0 | 1.48 ± 0.09 | 97.0 % | |
| 2 | 0.0 | 0.52 ± 0.02 | - |
| 0.5 | 0.98 ± 0.05 | 92.0 % | |
| 1.0 | 1.55 ± 0.11 | 103.0 % | |
| 3 | 0.0 | 0.43 ± 0.02 | - |
| 0.5 | 0.93 ± 0.03 | 100.0 % | |
| 1.0 | 1.51 ± 0.10 | 108.0 % | |
| Reference material(TET002RM) | 0.0 Reference value (4.89 ± 0.62) |
4.72 ± 0.56 | 96.52 % |
Conclusions
In this work our aim was to develop a sensor method for ochratoxin A analysis that can give sensitive and accurate results in food samples. From the achieved results, we depict a sensitive procedure for the analysis of ochratoxin A in real samples such as breakfast cereals, cereal-based baby foods and beer samples. The proposed method provides a new, sensitive, selective and simple electrochemical sensor utilizing special properties of gold nanoparticles such as high specific surface area, electrocatalytic and adsorptive properties. GNP/CPE resulted in catalytic effects for the electrooxidation of ochratoxin A since it enhances the oxidation peak currents and lowers the oxidation overvoltage. This developed sensor has a wide concentration range (0.5–100.0 nM) and very good detection limit (0.2 nM).
Acknowledgments
We gratefully acknowledge the financial support provided for this project (No. 7/4248) by Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran.
Contributor Information
Daryoush Afzali, Phone: 00983426226611, Email: daryoush_afzali@yahoo.com.
Fariba Fathirad, Phone: 00983413222033, Email: Fathirad.f@gmail.com.
Sima Ghaseminezhad, Phone: 00983413222033.
References
- Aksoy U, Eltem R, Meyvaci KB, Altindisli A, Karabat S. Five-year survey of ochratoxin a in processed sultanas from turkey. Food Addit Contam. 2007;24:292. doi: 10.1080/02652030601039021. [DOI] [PubMed] [Google Scholar]
- Alarcon SH, Palleschi G, Compagnone D, Pascale M, Visconti A, Barna Veto I. Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin a in wheat. Talanta. 2006;69:1031–1037. doi: 10.1016/j.talanta.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Ashkenani H, Taher MA. Determination of cadmium (II) using carbon paste electrode modified with a Cd-ion imprinted polymer. Microchim Acta. 2012;178(1):53. doi: 10.1007/s00604-012-0803-8. [DOI] [Google Scholar]
- Anukul N, Vangnai K, Mahakarnchanakul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J Food Drug Anal. 2013;21:227–241. doi: 10.1016/j.jfda.2013.07.009. [DOI] [Google Scholar]
- Baikai-Golan R, Paster N (1900) Mycotoxins in fruts and vegetables, Elsevier, 525 B street, suite, San Diego, CA 92101-4495, USA.
- Bell N, Marin S, Duaigu A, Ramos AJ, Sanchis V. Ochratoxin a in wines, musts and grape juices from Spain. J Sci Food Agric. 2004;84:591. doi: 10.1002/jsfa.1702. [DOI] [Google Scholar]
- Belli N, Marin S, Sanchis V, Ramos AJ. Ochratoxin a (OTA) in wines, musts and grape juices: occurrence, regulations and methods of analysis. Food Sci Technol Int. 2002;8:325. [Google Scholar]
- Bennett WJ, Klich M. Mycotoxins Clin Microbiol Rev. 2003;16:497. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J, Berrada H, Soriano JM, Molto JC, Manes J. Rapid determination of ochratoxin a in cereals and cereal products by liquid chromatography. J Chromatogr A. 2004;1046:127. [PubMed] [Google Scholar]
- Bryden WL. Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim Feed Sci Technol. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. [DOI] [Google Scholar]
- Fakoor Janati S, Beheshti HR, Asadi M, Mihanparast S, Feizy J. Preliminary survey of aflatoxins and ochratoxin a in dried fruits from Iran. J. Feizy. Bull Environ Contam Toxicol. 2012;88:391–395. doi: 10.1007/s00128-011-0477-7. [DOI] [PubMed] [Google Scholar]
- Gopu CL, Aher S, Mehta H, Paradkar AR, Mahadik KR. Simultaneous determination of cinnamaldehyde, eugenol and piperine by HPTLC densitometric method. Phytochem Anal. 2008;19:116–121. doi: 10.1002/pca.1022. [DOI] [PubMed] [Google Scholar]
- Goryacheva IY, De Saeger S, Nesterenko IS, Eremin SA, Van Peteghem C. Rapid all-in-one three-step immunoassay for non-instrumental detection of ochratoxin a in high-coloured herbs and spices. Talanta. 2007;72:1230–1234. doi: 10.1016/j.talanta.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicol. 2001;167:101. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- Iamanaka BT, Taniwaki MH, Menezes HC, Vicente E, Fungaro MH. Incidence of toxigenic fungi and ochratoxin a in dried fruits sold in Brazil. Food Add. Contam. 2005;22:1258. doi: 10.1080/02652030500260447. [DOI] [PubMed] [Google Scholar]
- Lasra S, Belli N, Chebil S, Nahla Z, Ahmed M, Sanchis V. Occurrence of ochratoxigenic fungi and ochratoxin a in grapes from Tunisian vineyard. Int J Food Microbiol. 2007;114:376–379. doi: 10.1016/j.ijfoodmicro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Magan M, Olsen M (2004) Mycotoxins in foods: detection and control. CRC Press Boca Raton Boston
- Marino A., Nostro A., Fiorentino C. Ochratoxin a production by aspergillus westerdijkiae in orange fruit and juice. Int J Food Microbiol. 2009;132:185–189. doi: 10.1016/j.ijfoodmicro.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Miller JD. Fungi and mycotoxins in grain: Implications for stored product research. J. stored Prod. Res. 1995;31:1. doi: 10.1016/0022-474X(94)00039-V. [DOI] [Google Scholar]
- Overy DP, Seifert KA, Savard ME, Frisvad JC. Spoilage fungi and their mycotoxins in commercially marketed chestnuts. Int J Food Microbiol. 2003;88:69–77. doi: 10.1016/S0168-1605(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Panini NV, Salinas E, Messina GA, Raba (2011) Modified paramagnetic beads in a microfluidic system for the determination of zearalenone in feedstuffs samples. Food Chem 125(2): 791
- Perrotta PR, Arevalo FJ, Vettorazzi NR, Zon MA, Fernandez H. Development of a very sensitive electrochemical magneto immunosensor for the direct determination of ochratoxin a in red wine. Sensors & Actuatos B. 2012;162:327–333. doi: 10.1016/j.snb.2011.12.089. [DOI] [Google Scholar]
- Perrotta PR, Vettorazzi NR, Arevalo FJ, Granero AM, Chulze SN, Zon MA. Electrochemical studies of ochratoxin a mycotoxin at gold electrodes modified with cysteamine self-assembled monolayers. Electroanal. 2011;23:1585–1592. doi: 10.1002/elan.201100094. [DOI] [Google Scholar]
- Ramirez EA, Zon MA, Jara Ulloa PA, Squella JA, Nunez Vergara L, Fernandez H. Adsorption of ochratoxin a (OTA) anodic oxidation product on glassy carbon electrodes in highly acidic reaction media: its thermodynamic and kinetics characterization. Electrochim Acta. 2010;55:771–778. doi: 10.1016/j.electacta.2009.09.013. [DOI] [Google Scholar]
- Ramirez EA, Granero AM, Zon MA, Fernandez H. Development of an amperometric biosensor based on peroxidases from Brassica napus for the determination of ochratoxin a content in peanut samples. J Biosens Bioelectron. 2011;S3:1–6. [Google Scholar]
- Ratola N, Barros P, Simoes T, Cerdeira A, Venancio A, Alves A. Worldwide interlaboratory study on the determination of ochratoxin a in different wine type samples. Talanta. 2006;70:720–731. doi: 10.1016/j.talanta.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Romani S, Sacchetti G, Lopez CC, Pinnavaia GG, Rosa MD. Screening on the occurrence of ochratoxin a in coffee beans of different origins and types. J Agric Food Chem. 2000;48:3616–3619. doi: 10.1021/jf990783b. [DOI] [PubMed] [Google Scholar]
- Romero-Gonzglez R, Garrido Frenich A, Martdnez Vidal JL, Aguilera Luiz MM. Determination of ochratoxin a and T-2 toxin in alcoholic beverages by hollow fiber liquid phase microextraction and ultra high-pressure liquid chromatography coupled to tandem mass spectrometry. Talanta. 2010;82:171–176. doi: 10.1016/j.talanta.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Fabiani A, Stockenstrom S, Mshicileli N, Sewram V. Quantitation of ochratoxin a in South African wines. J Agric Food Chem. 2003;51:1102. doi: 10.1021/jf0259866. [DOI] [PubMed] [Google Scholar]
- Silverio A, Lopes MM, de Freitas G. Determination of fungal contamination, aflatoxin B1 and ochratoxin a content in peanuts available in Portuguese market. Biotechnol. 2010;150:305. [Google Scholar]
- Sugita-Konishi Y, Tanaka T, Nakajima M, Fujita K, Norizuki H, Mochizuki N, Takatori K. The comparison of two clean-up procedures, multifunctional column and immunoaffinity column, for HPLC determination of ochratoxin a in cereals, raisins and green coffee beans. Talanta. 2006;69:650–655. doi: 10.1016/j.talanta.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Taniwaki MH, Pitt JI, Teixeira AA, Iamanaka BT. The source of ochratoxin a in Brazilian coffee and its formation in relation to processing methods. Int J Food Microbiol. 2003;82:173–179. doi: 10.1016/S0168-1605(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Taylor RF, Schultz JS. Chemical and biological sensors. Philadelphia: IOP Publishing Ltd.; 1996. [Google Scholar]
- Turner NW, Piletska EV, Karim K, Whitcombe M, Malecha M, Magan N, Baggiani C, Piletsky SA. Biosens Bioelectron. 2004;20:1060. doi: 10.1016/j.bios.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Van der Merwe KJ, Steyn PS, Fourie L, Scott DB, Theron JJ. A toxic metabolite produced by aspergillus ochraceus wilh. Nature. 1965;205:1112. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]