Abstract
In the present study, active antimicrobial (AM) packaging films were prepared from chitosan (CH) incorporated with ginger (Zingiber officinale) essential oil at different concentrations (0.1, 0.2 and 0.3 % v/v) and characterized. GC-MS analysis revealed zingiberene (22.54 ± 0.13), geranial (12.34 ± 0.33), β-sesquiphellandrene (8.14 ± 0.14), camphene (7.44 ± 0.54) and neral (5.45 ± 0.23) as the major components of essential oil extracted from ginger. Addition of ginger essential oil (GEO) improved the AM activity of the CH film against food borne pathogens, without significantly (p < 0.05) affecting the mechanical properties of the film. CH film with GEO was more effective against Gram-positive bacteria than Gram-negative bacteria and maximum antibacterial property against Staphylococcus aureus and Escherichia coli was shown by 0.3 % GEO added CH film. In a further experiment, steaks of barracuda (Sphyraena jello) fish were wrapped with the CH-GEO (0.3 %) film and stored at 2 °C for 20 days. Throughout the storage period, the total volatile basic nitrogen (TVB-N) value and total mesophilic count of fish steak wrapped with the CH-GEO film were significantly (p < 0.05) lesser than both the unwrapped control fish steak and aerobically packed fish steak in synthetic multilayer film of ethylene vinyl alcohol (EVOH) (nylon, EVOH and polyethylene). Sensorily, CH-GEO film wrapped sample was acceptable till the end of storage for 20 days compared to 12 days for unwrapped control and fish steak packed in EVOH film. The results indicate that the developed CH-GEO film is efficient in extending the storage life of fish.
Keywords: Chitosan, Antimicrobial film, Essential oil, Barracuda fish, Shelf-life
Introduction
Active packaging is a concept, which can improve the functionality of the packing due to the addition of an active substance in the packaging material (Appendini and Hotchkiss 2002). In active packaging systems, product, packaging and the surrounding environment interact to extend food shelf life, improving the organoleptic properties and/or food security (Suppakul et al. 2003). Antimicrobial (AM) packaging, where an antimicrobial substance is added to the packaging material, is one of many applications of active packaging. Unlike conventional food packaging systems, they are specifically designed to control growth of microorganisms by release of antimicrobial agent incorporated into a packaging material. AM films have received great attention over the past two decades because of its ability to extend the storage life of food packed in it, by delaying the microbial spoilage of food and reducing the risk of surface contamination of food by pathogenic microorganisms (Dutta et al. 2009; Ouattara et al. 2000). The packaging material and the antimicrobial agents intended for adding to the packaging material can be natural or synthetic compounds. As a result of the recent consumer preferences and environmental problems, the current packaging research focuses on developing bio-based edible packaging material incorporated with natural compounds exhibiting antimicrobial property. Chitosan is gaining attention as an edible and environmentally friendly packaging material due to its nontoxic, biocompatible, and biodegradable properties (Caner and Cansiz 2007). Chitosan is a functional biopolymer obtained by deacetylation of chitin, which is seen chiefly in the exoskeletons of crustaceans (Rabea et al. 2003). Owing to its intrinsic antimicrobial, anti-inflammatory, antioxidant properties, and good film-forming ability (Dutta et al. 2009), chitosan has very good potential to be used as a packaging material.
Unlike synthetic films, application of biopolymers like chitosan is limited by their high affinity to water leading to textural transformations that have a strong impact on their mechanical, transport and solubility properties. Poor moisture barrier property of the hydrophilic chitosan can be improved by the addition of hydrophobic essential oils (Atarés et al. 2010; Sanchez-Gonzalez et al. 2010). Gómez-Estaca et al. (2009) have observed that antimicrobial efficiency of chitosan films can be safely enhanced by incorporation of natural antimicrobial agents. Essential oils (EOs), which are composed of volatile aromatic compounds extracted from plant parts, are well known natural antimicrobial agents (Sefidkon and Ahmadi 2000). Since essential oil is rich in volatile terpenoids and phenolic particles, it has potential to inhibit a wide spectrum of microorganisms. Essential oil, due to its hydrophobicity and antimicrobial property, will be ideal for incorporating into chitosan for producing AM active packaging film. Direct incorporation of active agents like essential oil into food results in an immediate but short-term reduction of bacterial populations, while the AM films can maintain their activity for a long period of time (Appendini and Hotchkiss 2002; Hanusova et al. 2009). Also, high concentrations of EOs are generally needed in direct food applications to achieve effective antimicrobial activity, which might have inappropriate flavors and odors in the product (Gutierrez et al. 2009). Several essential oils, especially from cinnamon, clove, fennel, garlic, ginger, or thyme are reported to possess antimicrobial and antioxidant properties. Ginger (Zingiber officinale), a member of Zingiberacea family, is a common spice used worldwide as a food and medicine (Zhang et al. 2009). In spite of that, unlike many other essential oils, there is little information available on the use of essential oil from ginger in antimicrobial edible film development. Fish, a rich source of protein and polyunsaturated fatty acids, is highly perishable because of its unique biological composition. Antimicrobial packaging is a recent approach to preserve foods like fish, which spoil mainly due to rapid microbial growth (Gómez-Estaca et al. 2009). Keeping in view of the above facts, the present study attempts to prepare AM packaging film from chitosan incorporated with GEO, without significantly modifying its strength parameters, and to evaluate its efficiency in extending the shelf life of a popular tropical fish, barracuda (Sphyraena jello) stored under chilled condition.
Materials and methods
Materials
Commercial shrimp shell Chitosan (CH) in powder form with a low molecular weight of 186 kDa and 90 % degree of deacetylation was procured from Mahatani Chitosan Pvt. Ltd. (Veraval, Gujarat, India). All the microbiological media used were purchased from HiMedia (Mumbai, India). Other reagents used were of analytical grade.
Extraction of essential oil
Fresh rhizome of ginger (Zingiber Officinale Rosc.) was collected from the local market in Veraval, India and washed thoroughly to remove the sand and dirt adhering onto them. The skin of the ginger rhizome was removed before blending in a mixer grinder (Maharaja Whiteline, India) with a ginger to water ratio of 1:3 for 2 min at low speed. Further, the ginger essential oil was extracted by hydro distillation for 3 h using a Clevenger apparatus. The extracted oil was dried over anhydrous Na2SO4 and stored in sealed glass vials at 4 °C until use.
GC-MS analysis of chemical composition of ginger essential oil (GEO)
The chemical composition of the GEO was analyzed using Gas Chromatography-Mass Spectrometer (GCMS-QP2010 Ultra, Shimadzu, Japan) equipped with a Carbowax (30 m × 0.25 mm ID; 0.25-μm film thickness) capillary column. The conditions used for GC analysis were as follows: temperature programming from 50 to 300 °C at the rate of 3 °C/min, held at 300 °C for 5 min, injection temp 250 °C, carrier gas: helium at a flow rate of 1 mL/min, split ratio of 1:50. For MS detection, conditions were: electron impact, ionizing voltage 70 eV, source temp 150 °C, electron multiplier at 2000 eV, scan speed 690 amu/s and scan speed 40–500 amu. Compounds were identified by comparing mass spectra of each compound with those of authentic samples and library. Analysis was carried out in triplicate.
Determination of antimicrobial property of GEO
Test organism
Common pathogenic foodborne bacteria, Gram positive Staphylococcus aureus and Gram negative Escherichia coli, isolated from horse mackerel fish (Megalaspis cordyla), were used for the study. S. aureus, ATCC 25923 and E. coli, ATCC 25922 served as reference strains to ensure the accuracy of testing. The cultures of bacteria were maintained in appropriate agar slants at 4 °C and used as stock cultures. Bacterial strains were sub cultured in Trypticase Soy Broth (TSB) and incubated at 37 °C for 18–24 h. The cultures were adjusted to approximately 1.5x108 CFU/ml with sterile saline solution using 0.5 McFarland standards.
Quantitative assay by disc diffusion method
The antibacterial activity of GEO was evaluated by disc diffusion method using Mueller-Hinton agar (MHA) plates. The essential oil was dissolved in 10 % aqueous dimethylsulfoxide (DMSO) with Tween 80 (0.5 % v/v for easy diffusion) and sterilized by filtration through a 0.45 μm membrane filter. Under aseptic conditions, sterilized filter paper discs (Whatman no. 5, 5 mm diameter) containing 5 μl DMSO with GEO at 0.1, 0.2, and 0.3 % concentrations were placed on the agar surface inoculated with 200 μl of microbial suspension (108 cfu/ml). Ampicillin (30 μg) was used as a positive control and 5 μl DMSO without GEO was used as a negative control. The plates were incubated at 37 °C for 18 h and the inhibition zones (mm) were measured with a scale calliper. Experiments were performed in triplicate and mean value was calculated.
Film preparation
For preparing film, 1 % CH solution was prepared by dissolving 1 g CH in 100 ml of 1 % acetic acid solution with constant stirring. The resultant CH solution was filtered using a Whatman No. 3 filter paper. GEO was added to the CH film forming solution (FFS) at three different concentrations of 0.1, 0.2 and 0.3 % and one batch of solution without adding GEO was kept as control. Tween 80 (0.05 % v/v of the film forming solution) was used as an emulsifying agent and Glycerol was added at 0.1 % v/v as plasticizer. The solution (250 ml) was then homogenized using a T18 digital ULTRA TURRAX® homogenizer (IKA®, Karnataka, India) before casting on square shaped acrylic plates (30 × 22 cm) and kept for drying at 40 °C for 24 h in a vacuum dryer. The dried films (CH-GEO) were peeled off and stored at 50 % RH and 25 °C atmosphere prior to testing.
Determination of antimicrobial property of film
Under aseptic conditions, sterilized CH film discs of 5 mm diameter with and without GEO (0, 0.1, 0.2 and 0.3 %) were placed on the agar surface inoculated with 200 μl of microbial suspension (108 cfu/ml) and the antibacterial activity of CH film incorporated with GEO was evaluated by disc diffusion method using Mueller-Hinton agar (MHA) plates. Plastic film disc of synthetic multilayer ethylene vinyl alcohol (EVOH) (nylon, EVOH and polyethylene) was used as control.
Mechanical properties of the film
Tensile strength (TS) and elongation at break (E) of films were determined according to the ASTM standard method using a texture analyzer (Lloyd Instrument, Ltd., Fareham, UK). Films were cut into 50 × 80 mm strips and held parallel with an initial grip separation of 50 mm and then pulled apart at a head speed of 25 mm/min. TS (MPa) was calculated by dividing the value of peak load (N) by cross-sectional area (mm2) of the film. Percentage elongation was obtained by dividing the elongation at break by the initial length of the specimen and multiplying by 100.
Fish packaging application of the film
The CH film containing 0.3 % GEO, which showed the best AM activity without sacrificing its strength properties, was selected for fish packaging application. Fresh barracuda (Sphyraena jello) harvested from Veraval coast (Gujarat, India) was purchased and brought to the laboratory in iced condition (Fish and ice in 1: 1 ratio) in polystyrene boxes. The average length and weight of the fish were 65 ± 5 cm and 1.7 ± 0.3 kg. The fish, after beheading and gutting, were cut into steaks of 2.5 cm length and used for the study. The fish steaks were divided into three groups. The first group of was control, which was unwrapped and the second group of fish steaks was aerobically packed in a synthetic multilayer film of ethylene–vinyl alcohol (EVOH) (nylon, EVOH and polyethylene) supplied by Sealed Air (India) Pvt. Ltd. (Bangalore, India). The 15 × 20 cm sized film had a thickness of 138–140 μm and an oxygen transmission rate of 3.86 cm3 m−2 24 h−1 at 1 atmospheric pressure. The third group of fish steaks was wrapped with the developed CH-GEO film. Further, all the three groups of fish steaks (Unwrapped Control, Wrapped in EVOH film and Wrapped in CH-GEO film) were stored at 2 °C for 20 days and the biochemical (total volatile basic nitrogen (TVB-N) value and pH), microbiological (total mesophilic count, total psychrotrophic count, Pseudomonas spp., and H2S-producing bacteria including Shewanella putrefaciens) and sensory quality of the fish steaks were evaluated at regular intervals during chilled storage.
Quality analyses
The pH was measured using a digital pH meter (Eutech Instruments, Singapore) after homogenizing the fish sample in distilled water (1:5 w/v). Conway’s (1962) micro diffusion method was used for analyzing total volatile basic nitrogen (TVB-N) and expressed as mg TVB-N/100 g fish muscle.
For microbial counts, 10 g of fish steak sample was transferred aseptically to a stomacher bag (Seward Stomacher circulator bag, Model No. 400, England) to which 90 ml of sterile normal saline (0.85 %) was added and homogenized for 60 s at 230 rpm using a lab stomacher blender (Seward Stomacher 400 Circulator, England). For each sample, appropriate serial decimal dilutions were prepared with normal saline (0.85 %) and used for the microbiological analysis. 0.5 ml samples of appropriate dilution was spread evenly on the preset sterile plates using a sterile bent glass rod. The total mesophilic and psychrotrophic counts were determined by spread plate method using plate count agar (PCA, HiMedia, HiMedia Laboratories Pvt. Ltd., Mumbai, India). For mesophilic count, plates were incubated at 37 °C for 48 h while plates were kept at 7 °C for 7 days for total psychrotrophic count. Pseudomonads were enumerated on cetrimide fusidin cephaloridine agar (CFC, Oxoid, code CM 559 supplemented with SR 103) after incubation at 20 °C for 5 days. The colonies, which retained the blue colour after reacting with cytochrome oxidase were counted as Pseudomonas spp. H2S-producing bacteria (including Shewanella putrefaciens) were enumerated on peptone iron agar (PIA) (code 289,100, BBL Difco) after incubating at 20 °C for 5 days. Black colonies formed by the production of H2S were counted as H2S-producing bacteria.
Sensory analysis of the fish steak was done by a panel of 6 regular members (laboratory trained) using a 9 point hedonic scale with 9 corresponding to the most liked sample and 1 corresponding to the least liked sample as described by Meilgaard et al. (1999).
The fish steak was cooked in a microwave oven (700 W) for 10 min and cooled for 2 min before serving to the panellists. Water was provided to the panel members for restoration of taste. Score of 1–9 was assigned for the overall acceptability of the fish sample, which was determined by assessing the appearance, colour, odour, taste, texture and flavour. The sensory score of 5 was considered as the lower limit of acceptability.
Statistical analysis
The data were analysed by SPSS (Statistical Package for the Social Sciences Inc., Chicago, USA) software version 17. One-way and two-way ANOVA was applied to compare the means. The significant difference between the treatments was determined by Duncan’s Multiple Range Test (DMRT). The level of significance was set up at p < 0.01 for microbiological quality and p < 0.05 for all other attributes.
Results and discussion
Chemical composition of GEO
Essential oils are volatile in nature and the unique flavour of ginger is attributed mainly to the volatile oil. Yield of essential oil from ginger obtained by hydrodistillation method was 0.5 ± 0.03 %. The composition of the GEO is shown in the Table 1. GC-MS analysis revealed 20 components accounting approximately for 94.64 % of the total essential oil. The composition of the GEO varies considerably, depending on the geographical origin but typically consist of compounds such as zingiberene, β-sesquiphellandrene and camphene in significant proportions. The major components of essential oil extracted from ginger in the present work were zingiberene (22.54 ± 0.13), geranial (12.34 ± 0.33), β-sesquiphellandrene (8.14 ± 0.14), camphene (7.44 ± 0.54) and neral (5.45 ± 0.23).
Table 1.
Major constituents of Ginger essential oil analysed by GC-MS
| Sl. No. | Compound | *Content (%) |
|---|---|---|
| 1 | Zingiberene | 22.54 ± 0.13 |
| 2 | Geranial | 12.34 ± 0.33 |
| 3 | β-sesquiphellandrene | 8.14 ± 0.14 |
| 4 | Camphene | 7.44 ± 0.54 |
| 5 | Neral | 5.45 ± 0.23 |
| 6 | β-bisabolene | 4.62 ± 0.12 |
| 7 | α-farnesene | 4.5 ± 0.25 |
| 8 | β-phellandrene | 4.32 ± 0.32 |
| 9 | Curcumene | 3.67 ± 0.50 |
| 10 | Cineole | 3.12 ± 0.14 |
| 11 | Citronellal | 3.05 ± 0.55 |
| 12 | Copaene | 3 ± 0.14 |
| 13 | α-pinene | 2.95 ± 0.33 |
| 14 | γ-cadinene | 1.98 ± 0.33 |
| 15 | Limonene | 1.85 ± 0.33 |
| 16 | β-pinene | 1.74 ± 0.25 |
| 17 | Linalool | 1.5 ± 0.33 |
| 18 | β-eudesmol | 1 ± 0.21 |
| 19 | p-cymene | 0.89 ± 0.66 |
| 20 | Nerolidol | 0.54 ± 0.66 |
*Values expressed are mean ± standard deviation
Antimicrobial activity of GEO
The antimicrobial activity of the GEO is presented in Table 2. In the disc diffusion assay, GEO showed antimicrobial activity against Gram-positive and Gram-negative bacteria (p < 0.05). The antimicrobial activity of GEO against both the bacteria was concentration dependent. The phenolic compounds, which are abundant in essential oils, are reported for its beneficial effects such as antioxidant, and antimicrobial properties. In the present study, the type strains of both Gram positive S. aureus and Gram negative E. coli showed more sensitivity to GEO than the bacterial strains isolated from fish. GEO of 0.1, 0.2 and 0.3 % concentration showed a halo of 21.33 ± 0.33, 24.63 ± 0.15, and 28.67 ± 0.10 mm, respectively, against S. aureus isolated from fish and 22.17 ± 0.16, 25.45 ± 0.11, and 32.33 ± 0.34 mm, respectively, against S. aureus, ATCC 25923. The highest inhibition zones of 11 ± 0.15 and 13 ± 0.46 mm were shown against E. coli isolated from fish and E. coli, ATCC 25922, respectively, by the highest concentration of 0.3 % of GEO. Prasad and Seenayya (2000) have previously reported very good inhibition of Salinococcus roceus, H. turkmenicus and Halococcus morrhuae isolated from salt cured fish by GEO. Volatile compounds like α-pinene, camphene etc., are reportedly responsible for the antimicrobial property of the GEO (Nychas and Skandamis 2003) and thus help in inhibiting the growth of bacteria. It was noted that GEO was more effective against Gram-positive bacteria than Gram-negative bacteria. Generally, the Gram positive bacteria are less resistant to essential oil than Gram negative bacteria, due to the difference in their cell wall structure (Burt 2004). The greater resistance of Gram-negative bacteria towards essential oils may be attributed to the complexity of their double-layer cell membrane, compared with the single-layer membrane of Gram-positive bacteria and the additional relatively impermeable outer external membrane surrounding the cell wall in Gram-negative bacteria restricts diffusion of hydrophobic compounds through its lipopolysaccharide covering (Sanchez-Gonzalez et al. 2011).
Table 2.
Antimicrobial activity of the GEO and CF in disc-diffusion method
| Sample | *Inhibition zone (mm) | |||
|---|---|---|---|---|
| Staphylococcus aureus, isolated from fish | Staphylococcus aureus, | Escherichia coli, isolated from fish | Escherichia coli, | |
| ATCC 25923 | ATCC 25922 | |||
| DMSO (Negative Control) | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa |
| Plastic Film | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa |
| CF | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa | 0.00 ± 0.00Aa |
| CF (A) | 6.7 ± 0.65Cb | 8.5 ± 0.29Db | 1.2 ± 0.24Ab | 2.5 ± 0.43Bb |
| CF (B) | 7.4 ± 0.05Bb | 9.9 ± 0.29Cb | 2.5 ± 0.10Ac | 2.9 ± 0.67Ab |
| CF (C) | 10.2 ± 0.31Cc | 14.5 ± 0.71Dc | 3.9 ± 0.45Ad | 4.5 ± 0.83Bc |
| GEO (A) | 21.33 ± 0.33Cd | 22.17 ± 0.16Dd | 4 ± 0.33Ad | 5 ± 0.10Bc |
| GEO (B) | 24.63 ± 0.15BCe | 25.45 ± 0.11Ce | 9 ± 0.12Ae | 9.2 ± 0.44Ad |
| GEO (C) | 28.67 ± 0.10Cf | 32.33 ± 0.34Df | 11 ± 0.15Af | 13 ± 0.46ABe |
| Ampicillin (Positive Control) | 30.45 ± 0.15Cg | 35.12 ± 0.16Dg | 20 ± 0.43Ag | 24 ± 0.12Bf |
GEO = Ginger Essential Oil, CF = Chitosan Film
A = 0.1 %, B = 0.2 %, and C = 0.3 % GEO
a-gMean ± standard deviation in the same column with different superscripts are significantly different (p < 0.05)
A-DMean ± standard deviation in the same row with different superscripts are significantly different (p < 0.05)
Antimicrobial activity of the film
When added to CH films, GEO (0.1, 0.2 and 0.3 % v/v of the FFS) inhibited the growth of both S. aureus and E. coli (Table 2). But, there was a significant (p < 0.05) difference in the diameter of the inhibition zone displayed by GEO and chitosan film added with GEO against both the organisms. GEO at 0.3 % showed an inhibition zone of 32.33 ± 0.34 mm against Staphylococcus aureus (ATCC 25923), which was reduced by 55 % when it was added to CH film. Hosseini et al. (2009) reported that clear zones are produced around the film discs as EOs incorporated in the packaging films diffuse through agar gel. The reduced antibacterial activity of GEO when added to CH film may be due to the partial loss of volatile essential oil in the film by evaporation (Abdollahi et al. 2012). CH with 0.3 % GEO showed best antibacterial property against S. aureus and E. coli. Chitosan is a versatile material with proved antimicrobial activity. While several mechanisms have been proposed for chitosan’s antimicrobial activity, the exact mechanism is still unclear. One of the reasons for the antimicrobial character of chitosan is its positively charged amino group, which interacts with negatively charged microbial cell membranes, leading to the leakage of proteinaceous and other intracellular constituents of the microorganisms. Chitosan is also reported to inhibit the growth of the microorganisms by the chelation of essential nutrients, metals and spore components. Devlieghere et al. (2004) have reported that chitosan may also enter into the nuclei of the bacteria, bind with DNA, and inhibit the synthesis of mRNA and proteins. But, in the present work, CH without GEO did not show any inhibiting effect against any of the test organisms. Similarly, other authors like Pranoto et al. (2005) have also reported that CH films showed no inhibition halos against S. aureus when not added with other additives. This is explained by Fernandez-Saiz et al. (2010); Hashem et al. (2008) and Tripathi et al. (2010) that chitosan is incapable of migrating through the agar medium and the antimicrobial property of chitosan depends on the release of protonated glucosamine fractions into the culture medium. The positive charge of amino groups of chitosan interacts with the anionic groups of the bacterial cell surface and lead to bacterial cell death (Wu et al. 2014).
Mechanical properties of the film
Strength properties are very important for a film intended for packing food materials to maintain its integrity during rigorous handling and transportation. Tensile strength, which is one of the most desired characteristics of a bio active film, is defined as the stress developed during tensile testing. The control chitosan film had a tensile strength of 32.0 ± 0.30 MPa (Table 3), which was slightly decreased by the addition of GEO. Addition of GEO in 0.1, 0.2 and 0.3 % to CH film did not cause any significant difference (p < 0.05) in the tensile strength compared to the control film, indicating that trace amounts of GEO has only a very little influence on the tensile strength of CH films. The percentage increase in the length of the material before it breaks under tension is measured as elongation-at-break. There was an increase in the elongation at break of CH film from 18.15 ± 0.2 % to 18.19 % with increase in the concentration of added GEO from 0 to 0.3 % (Table 3). Similarly, a decrease in tensile strength and an increase in elongation percentage when the EOs were introduced into the chitosan films were observed by Zivanovic et al. (2005) and Begin and Van Calsteren (1999). Atarés et al. (2010) had reported that addition of cinnamon and ginger essential oils did not significantly affect the tensile strength and elongation at break of sodium casinate-based film. The reason behind the marginal difference in the mechanical properties of CH film, while adding GEO, is probably due to the low concentrations at which it was added to CH. GEO can be used in CH films at higher concentrations for improved antimicrobial properties when a component, which improves the strength properties, is added to the film.
Table 3.
Mechanical properties of the Chitosan films incorporated with GEO
| Chitosan Film with GEO | Elongation at break (%) | Tensile strength |
|---|---|---|
| (MPa) | ||
| A | 18.16 ± 0.02a | 32.0 ± 0.30b |
| B | 18.18 ± 0.02a | 31.9 ± 0.33b |
| C | 18.19 ± 0.05a | 31.8 ± 0.52ba |
| D | 18.20 ± 0.02a | 30.9 ± 0.35ba |
*Means ± standard deviation in the same column with different superscripts are significantly different (p < 0.05)
A = 0 %, B = 0.1 %, C = 0.2 % and D = 0.3 % GEO of the film forming solution
Changes in the biochemical quality of fish steaks during chilled storage
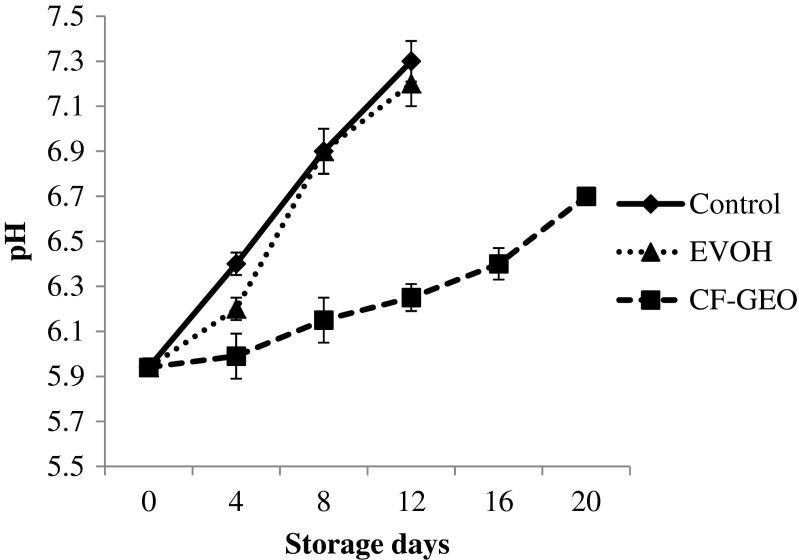
The mean pH measurements of barracuda steaks over the period of storage are shown in Fig. 1. The initial pH of the fish steak was 5.94, which increased to 7.3, 7.2 and 6.15 in control, EVOH film wrapped and CH-GEO film wrapped fish samples, respectively, on 12th day of the chilled storage study. The pH of all the three samples increased gradually, but, slowly in CH-GEO film wrapped fish samples and there was a significant (p < 0.05) difference in the pH values of the samples during storage in chilled condition. The increase in pH is due to accumulation of alkaline compounds derived mainly from microbial action in the fish muscle (Mexis et al. 2009). The pH values of EVOH wrapped fish steak samples were lower than control fish steak till 4th day of storage, but thereafter there were no significant (p < 0.05) differences in the pH values of both the samples. The lower values of pH in CH-GEO film wrapped fish samples may be due to inhibition of microbial growth by CH-GEO film resulting in decrease in the alkaline bacterial metabolite production. Value of pH of CH-GEO film wrapped fish sample on the day of sensory rejection was 6.7 (20th day).
Fig. 1.
Changes in the pH* of the fish steak during chilled storage. ∗mean ± SD, n = 3, p < 0.05. Control: Unwrapped fish steak, EVOH: Fish steak wrapped in EVOH Film, CF-GEO: Fish steak wrapped in CF-GEO Film
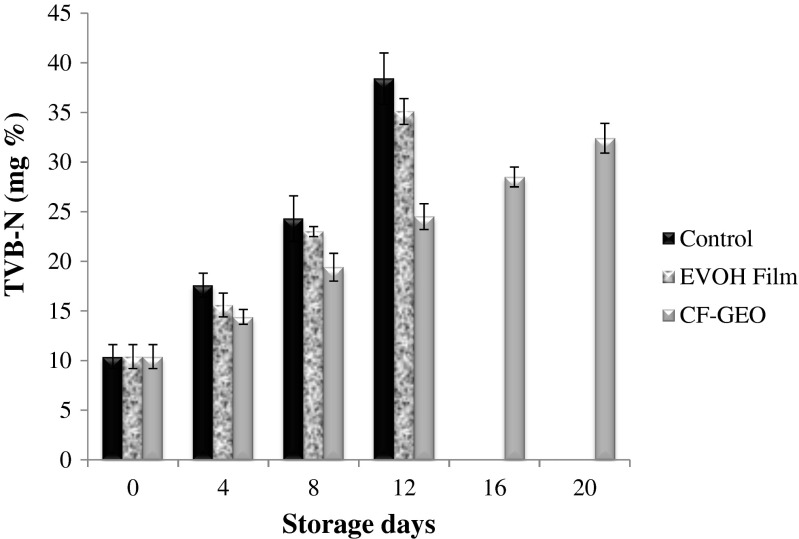
Total volatile base nitrogen (TVB-N) formed in the fish is an index of spoilage and its increase can be attributed to the activity of spoilage bacteria and endogenous enzymes. A TVB-N value of 30–35 mg N2 100 g−1 is generally regarded as the limit of acceptability. The initial TVB-N value of fish steak was 10.4 ± 1.2 mg N2 100 g−1, which upon storage increased to 38.4 ± 2.6 mg N2 100 g−1 in control sample and 35.1 ± 1.3 mg N2 100 g−1 in EVOH film wrapped sample, respectively, on 12th day of storage and 32.4 ± 1.5 mg N2 100 g−1 on 20th day of storage in CH-GEO film wrapped fish steaks (Fig. 2). The formation of volatile basic nitrogen in fish steak samples paralleled with increase in the count of total mesophilic count indicating that the volatile basic compounds are mainly generated from the metabolic activity of spoilage bacteria. The rate of volatile base formation in fish steaks wrapped around with CH-GEO film was significantly (p < 0.05) lower than the control and EVOH film wrapped sample throughout the chilled storage suggesting the inhibition of bacterial proliferation by CH-GEO film.
Fig. 2.
Changes in the TVB-N* values of the fish steak during chilled storage. ∗mean ± SD, n = 3, p < 0.05. Control: Unwrapped fish steak, EVOH: Fish steak wrapped in EVOH Film, CF-GEO: Fish steak wrapped in CF-GEO Film
Changes in the microbial quality of fish steaks during chilled storage
The fish used for the study was fresh as indicated by the initial value of total mesophilic and psychrotrophic count of barracuda steak, i.e., 3.1 and 2.5 log cfu g −1 (Fig. 3). The total mesophilic and psychrotrophic counts of the fish steak increased over the storage period. But, the psychrotrophs multiplied at a higher rate than mesophils and dominated the bacterial flora of the fish steak stored at chilled condition. The increase in total mesophilic count of the fish samples corresponded well with the formation of volatile bases and decline in sensory properties. After 12 days of storage, the total mesophilic count of control and fish steaks packed in EVOH film reached the value of 7 log cfu g −1, which is the upper acceptability limit for freshwater and marine species by ICMSF (ICMSF (International Commission on Microbiological Specifications for Foods) 1986). But, the counts of the CH-GEO film wrapped fish steaks did not exceed the value of 7 log cfu g −1 till the end of chilled storage, indicating the strong antimicrobial action of the active chitosan film. Compared to CH-GEO film sample, the counts of total mesophilic bacteria of control samples had a significantly (p < 0.01) higher increase and there was a lag phase of 4 days observed for fish packed in CH-GEO film. Han (2000) reported that antimicrobial packaging materials are capable of extending the lag phase and reduce the growth rate of microorganisms to prolong the shelf life and maintain food quality and safety. The enhanced antimicrobial activity of the CH added with GEO may be due to the ability of the essential oils and their components to partition in the lipids of the bacterial cell membrane and mitochondria while disturbing the structures and rendering it more permeable due to the hydrophobic nature of the essential oil (Sikkema et al. 1994). Based on total mesophilic count, the shelf life of control is expected to be 12 days. The CH-GEO film wrapped fish steaks were acceptable till the end of storage of 20 days, thereby extending the shelf life of barracuda steak by 8 days compared to aerobically stored control fish steaks. Previous studies on application of essential oil containing active packaging film for fish preservation are limited. Gómez-Estaca et al. (2010) has used the complex gelatin–chitosan film incorporated with clove essential oil to wrap salmon fish during chilled storage and the microbial growth was considerably reduced and corresponded with the delay in total volatile base nitrogen (TVB-N) production, as observed in the present study. Similarly, Wu et al. (2014) have also reported that the total plate count of grass carp muscle packed with gelatin-chitosan-oregano essential oil film was always lower than parafilm packaging and control samples during storage at 4 ± 0.5 °C for 12 days.
Fig. 3.
Changes in the total mesophilic and psychrotrophic bacteria counts* of the fish steak during chilled storage. ∗mean ± SD, n = 3, p < 0.01. TPC: Total Plate Count/Total Mesophilic Bacteria Count, Psychrotrophs: Total Psychrotrophic Bacteria Count. Control: Unwrapped fish steak, EVOH: Fish steak wrapped in EVOH Film, CF-GEO: Fish steak wrapped in CF-GEO Film
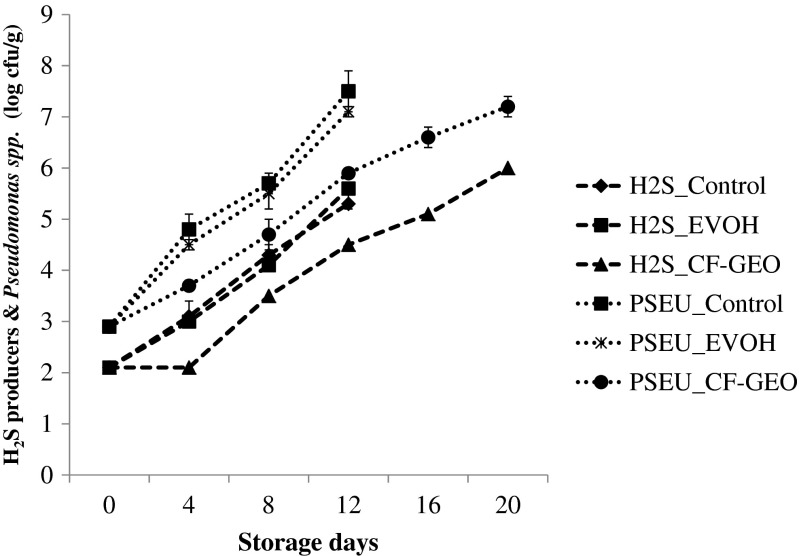
The initial counts of Pseudomonas spp. and H2S producing bacteria mainly S. putrefaciens were 2.9 and 2.1 log cfu g −1. During storage, the primary wrap of CH-GEO film significantly decreased the count of Pseudomonas spp. compared to unwrapped sample and fish steaks wrapped in EVOH film (Fig. 4). The count of H2S producing bacteria mainly S. putrefaciens was lower than Pseudomonas spp. during storage and a lag phase of 4 days observed for H2S producing bacteria in CH-GEO film wrapped samples. Pantazi et al. (2008) have reported that the initial lag phase of H2S-producing bacteria may be the result of inhibition by Pseudomonas spp. since both are strongly competitive psychrotrophic microorganisms. Gram and Melchiorsen (1996) have reported that Pseudomonas spp. can inhibit the growth of H2S producing bacteria since it produce siderophores. The counts of Pseudomonas spp. and H2S producing bacteria of CH-GEO film wrapped fish steaks were 7.2 and 6 log cfu g −1 on 20th day of storage. The microbial counts of Pseudomonas spp. of all the three samples crossed the value of 7 log cfu g −1 on the day of sensory rejection.
Fig. 4.
Changes in the H2S-producing bacteria & Pseudomonas spp. counts* of the fish steak during chilled storage. ∗mean ± SD, n = 3, p < 0.01. H2S: H2S-producing bacteria count, PSEU: Pseudomonas spp. count. Control: Unwrapped fish steak, EVOH: Fish steak wrapped in EVOH Film, CF-GEO: Fish steak wrapped in CF-GEO Film
Changes in the sensory quality of fish steaks during chilled storage
The result of the sensory evaluation of fish steak during chilled storage is presented in Fig. 5. The initial overall acceptability score of fish steak was 9, which decreased significantly (p < 0.05) during storage in all the three samples. But, the decrease was more rapid in control sample. The control fish pack and EVOH film sample were rejected sensorily on 12th day of chilled storage, when the overall acceptability scores of both samples were below 5. The fish steaks wrapped with the CH-GEO antimicrobial film was acceptable till 20th day of storage, thus prolonging the shelf life by 12 days compared to the control sample. Addition of GEO did not negatively affect the sensory properties of the fish steak, but, the spicy taste and freshness provided by the slight ginger aroma to the sample was well received by the panellists.
Fig. 5.
Changes in the overall sensory score* of the fish steak during chilled storage. ∗mean ± SD, n = 12, p < 0.05. Control: Unwrapped fish steak, EVOH: Fish steak wrapped in EVOH Film, CF-GEO: Fish steak wrapped in CF-GEO Film
Conclusion
Development of antimicrobial active packaging films, which can enhance the storage life of perishable foods by controlling the surface microbial growth, is a promising concept. Chitosan is a biopolymer extracted mainly from the crustacean shell, which is mostly thrown out as waste from the fish processing industries and is a suitable candidate for active film development because of the numerous favourable properties like biodegradability, antimicrobial property and excellent film forming ability. The present study showed that incorporation of ginger essential oil enhanced the antimicrobial and moisture barrier properties of the chitosan film without negatively modifying its physical and mechanical properties and could improve the keeping quality of the fish stored under chilled condition. This kind of active films has great potential to be used for food packaging applications since it extends the shelf life of the food it carries without risking the consumer’s health.
References
- Abdollahi M, Rezaei M, Farzi G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J Food Eng. 2012;111(2):343–350. doi: 10.1016/j.jfoodeng.2012.02.012. [DOI] [Google Scholar]
- Appendini P, Hotchkiss JH. Review of antimicrobial food packaging. Innov Food Sci Emerg Technol. 2002;3(2):113–126. doi: 10.1016/S1466-8564(02)00012-7. [DOI] [Google Scholar]
- Atarés L, Bonilla J, Chiralt A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J Food Eng. 2010;100:678–687. doi: 10.1016/j.jfoodeng.2010.05.018. [DOI] [Google Scholar]
- Begin A, Van Calsteren MR. Antimicrobial films produced from chitosan. Int J Biol Macromol. 1999;26:63–67. doi: 10.1016/S0141-8130(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Caner C, Cansiz O. Effectiveness of chitosan-based coating in improving shelf-life of eggs. J Sci Food Agric. 2007;87:227–232. doi: 10.1002/jsfa.2698. [DOI] [Google Scholar]
- Conway EJ. Micro diffusion analysis and volumetric error. 5th. London: Crosby Lockwood and Son Ltd; 1962. [Google Scholar]
- Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–714. doi: 10.1016/j.fm.2004.02.008. [DOI] [Google Scholar]
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- Fernandez-Saiz P, Ocio MJ, Lagaron JM. Antibacterial chitosan-based blends with ethylene–vinyl alcohol copolymer. Carbohydr Polym. 2010;80:874–884. doi: 10.1016/j.carbpol.2009.12.046. [DOI] [Google Scholar]
- Gómez-Estaca J, López De Lacey A, Gómez-Guillén MC, López-Caballero ME, Montero P. Antimicrobial activity of composite edible films based on fish gelatin and chitosan incorporated with clove essential oil. J Aquat Food Prod Technol. 2009;18:46–52. doi: 10.1080/10498850802581252. [DOI] [Google Scholar]
- Gómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Gram L, Melchiorsen J. Interaction between fish spoilage bacteria Pseudomonas spp. and S. putrefaciens in fish extracts and on fish tissue. J Appl Bacteriol. 1996;80:589–595. doi: 10.1111/j.1365-2672.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Barry-Ryan C, Bourke P. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26:142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Han JH. Antimicrobial food packaging. Food Technol. 2000;54:56–65. [Google Scholar]
- Hanusova K, Dobias J, Klaudisova K. Effect of packaging films releasing antimicrobial agents on stability of food products. Czech J Food Sci. 2009;27:347–349. [Google Scholar]
- Hashem HM, Razavi SH, Mousavi AMS, Shaidi YS, Ghorbani HA. Improving antibacterial activity of edible films based on chitosan by incorporation of thyme and clove essential oils and EDTA. J Appl Sci. 2008;8:2895–2900. doi: 10.3923/jas.2008.2895.2900. [DOI] [Google Scholar]
- Hosseini M, Razavi S, Mousavi M. Antimicrobial, physical and mechanical properties of chitosan based films incorporated with thyme, clove and cinnamon essential oils. J Food Process Pres. 2009;33:727–743. doi: 10.1111/j.1745-4549.2008.00307.x. [DOI] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods) (1986) Sampling plans for fish and shellfish. In: ICMSF, Microorganisms in Foods. Sampling for Microbiological Analysis: Principles and Scientific Applications, 2nd edn. vol. 2. University of Toronto Press, Canada, pp181-196
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. 3rd. Boca Raton, Fla: CRC Press; 1999. p. 387. [Google Scholar]
- Mexis SF, Chouliara E, Kontominas MG. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009;26:598–605. doi: 10.1016/j.fm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Nychas GJE, Skandamis PN. Antimicrobials from herbs and spices. In: Roller S, editor. Natural antimicrobials for the minimal processing of foods. Cambridge, UK: Woodhead Publishing; 2003. pp. 176–200. [Google Scholar]
- Ouattara B, Simard RE, Piette G, Bégin A, Holley RA. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol. 2000;62(1):139–148. doi: 10.1016/S0168-1605(00)00407-4. [DOI] [PubMed] [Google Scholar]
- Pantazi D, Papavergou A, Pournis N, Kontominas M G, Savvaidis IN. Shelf-life of chilled fresh Mediterranean swordfish (Xiphias gladius) stored under various packaging conditions: microbiological, biochemical and sensory attributes. Food Microbiol. 2008;25:136–143. doi: 10.1016/j.fm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Pranoto Y, Rakshit SK, Salokhe VM. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Sci Technol. 2005;38:859–865. doi: 10.1016/j.lwt.2004.09.014. [DOI] [Google Scholar]
- Prasad MM, Seenayya G. Effect of spices on the growth of red halophilic cocci isolated from salt cured fish and solar salt. Food Res Int. 2000;33:793–798. doi: 10.1016/S0963-9969(00)00100-9. [DOI] [Google Scholar]
- Rabea EI, Badawy ET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromulecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez L, Cháfer M, Chiralt A, González-Martínez C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr Polym. 2010;82:277–283. doi: 10.1016/j.carbpol.2010.04.047. [DOI] [Google Scholar]
- Sanchez-Gonzalez L, Chafer M, Hernandez M, Chiralt A, Gonzalez-Martinez C. Antimicrobial activity of polysaccharide films containing essential oils. Food Control. 2011;22:1302–1310. doi: 10.1016/j.foodcont.2011.02.004. [DOI] [Google Scholar]
- Sefidkon F, Ahmadi S. Essential oil of Tanacetum pathenium L. J Essent Oil Res. 2000;12:427–428. doi: 10.1080/10412905.2000.9699556. [DOI] [Google Scholar]
- Sikkema J, De Bont JAM, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269(11):8022–8028. [PubMed] [Google Scholar]
- Suppakul P, Miltz JM, Sonneveld K, Bigger SW. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J Food Sci. 2003;68:408–420. doi: 10.1111/j.1365-2621.2003.tb05687.x. [DOI] [Google Scholar]
- Tripathi S, Mehrotra GK, Dutta PK. Preparation and physico-chemical evaluation of chitosan ⁄ poly (vinyl alcohol) ⁄ pectin ternary film for food-packaging applications. Carbohydr Polym. 2010;79:711–716. doi: 10.1016/j.carbpol.2009.09.029. [DOI] [Google Scholar]
- Wu J, Shangying G, Hui L, Shuang W, Shanfei C, Jianhua W, Jianhua L, Qiqing Z. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packaging and Shelf Life. 2014;2:7–16. doi: 10.1016/j.fpsl.2014.04.004. [DOI] [Google Scholar]
- Zhang GF, Yang ZB, Wang Y, Yang WR, Jiang SZ, Gai GS. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult Sci. 2009;88:2159–2166. doi: 10.3382/ps.2009-00165. [DOI] [PubMed] [Google Scholar]
- Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of chitosan films enriched with essential oils. J Food Sci. 2005;70:M45–M51. doi: 10.1111/j.1365-2621.2005.tb09045.x. [DOI] [Google Scholar]