Abstract
The pan fryings of frozen pre-fried French fries in refined rapeseed oil (RO) and professional blend (PB) were of 8 h duration. Initially, while fresh, and after every 1 h of frying, the oils were analyzed for acid and anisidine values, colour, refractive index, fatty acid composition (by GC), polar fraction content and composition (by high pressure size exclusion chromatography). Additionally the fat content and fatty acid composition of French fries were determined. Hydrolytic and oxidative changes in the frying media varied with oil composition and increased with frying time. The pace of oxidation in RO was three times higher than in PB. The loss of polyunsaturated fatty acids (sum of C18:2 and C18:3) after 8 h of frying was 7 % in PB and 20 % in RO. The polar fraction content was two times lower in PB, but did not exceed 25 % in either of the frying media. The predominant fraction in RO was oxidized triacylglycerols, while in PB – diacylglycerols. During frying the proportions of triacylglycerol polymers and dimers increased in both oils, reaching 32 % in RO and 23 % in PB.
Keywords: Pan frying, Polar fraction, Vegetable oil, Food analysis
Introduction
Frying is a very common and popular practice for the preparation and manufacture of foods. It is a fast, convenient, and energy efficient cooking procedure that increases palatability and provides crust formation together with pleasant flavours and odours (Gertz 2014).
Pan- or griddle-frying is a popular cooking method in the home and in many restaurants. Even given the emphasis on low-fat diets, people are still fond of fried foods because of their crispy texture and desirable fried food flavour.
During frying, a complex series of chemical reactions takes place in the heated fats, such as thermooxidation, hydrolysis, polymerization and fission. Oils for frying decompose to form a variety of volatile compounds as well as monomeric and polymeric products. Several factors, such as contact with air, temperature and length of heating, type of frying vessel, degree of oil unsaturation, and presence of pro-oxidants or anti-oxidants, affect the overall performance of frying oils by diminishing their original characteristics (Perkins 2007). During typical domestic frying, oil is usually used for six and in some cases for nine or ten successive sessions (Andrikopoulos et al. 2002).
Fried foods absorb the heated fats, which become part of our diet and contain total polar artefact in amounts equal to those present in the fried oils. Therefore, it is important for an average family to know the limits of use when recycling frying oils. The fat content can increase several times during frying, e.g. from 3.9 % in raw chicken to 9.9 % in fried food, in doughnuts from 5.2 to 21.9 % and in sardines from 10.0 to 35.0 %. The greatest increase is found in French fries and potato crisps, in which the amount of fat during frying increases from 0.1 % in raw potatoes up to about 10–15 % in French fries and up to 40 % in potato crisps (Matthäus 2007).
The fatty acid composition of the frying oil is a key factor influencing fried food flavour and oil stability. Ideally, frying oil should have a long frying life and good organoleptic attributes, and it should be low in saturated and trans fatty acids and relatively low in PUFA (Mehta and Swinburn 2001). Rapeseed is one of the most important oilseed crops globally. Research indicates that the fatty acid composition of rapeseed oil is especially favourable in terms of health benefits, when used as part of a nutritionally balanced diet. Rapeseed oil is rich in 18-carbon fatty acids, most notably the monounsaturated fatty acids (MUFA, 58-64 %), oleic acid (18:1n9), as well as the polyunsaturated fatty acids (PUFA, 28–31 %), linoleic (18:2n6, 20–25 %) and linolenic (18:3n3, 8–12 %) acids. Moreover, it contains the lowest concentration of saturated fatty acids (SFA, 7 %) among vegetable oils. The other nutritionally favourable property of rapeseed oil is that it has a 2:1 ratio of n6 to n3 PUFA (Brinkmann 2000; Zambiazi et al. 2007).
Specially prepared frying fats are obtained from a range of oils (e.g. rapeseed, soybean or sunflower oil) and fats (coconut, palm). These oils are subject to various modifications to improve their oxidative stability, alter the melting properties and the crystallization properties, or because of economic benefits. These modifications include hydrogenation, fractionation, interesterification and (the most simply) physical blending (Senanayake and Shahidi 2005). One of the most common oils used in the manufacture of special frying fat by physical blending is palm oil or palm-based fractions. The relatively high content of saturated fatty acids (approximately 50 %, of which palmitic acid is 45 %) and low levels of polyunsaturated fatty acids, which means that palm oil has good thermooxidative stability. These kinds of oils are widely used in the industry - for frying all kinds of snack products (including French fries) as well as in fastfood restaurants (Rossel 2003; Matthäus 2007; Rani et al. 2010). The thermooxidation stability of a blend depends strongly on those of its individual oil fractions (Isbell et al. 1999).
Due to the consistency of palm oil, requiring liquefaction before filling the fryer, frying fats are often prepared as mixtures of palm oil and high oleic refined oils. These products are called “new generation fats”. These types of frying fats have superior functional properties and preferred fatty acid composition (Matthäus et al. 2009; Kalogianni et al. 2009).
The limited availability in the market and the high price of special fats for pan- and deep-frying are the main reasons why consumers preferably choose traditional well known oils for cooking at home. However, a new generation of oils have recently appeared on the market which are dedicated to households. It is interesting to compare the thermooxidative stability of professional frying oil with rapeseed oil - the most popular oil used for frying in Poland which is available in domestic market.
The aim of the study was to compare the thermooxidative and hydrolytic stability of refined rapeseed oil with the new professional frying fat for the pan-frying of French fries.
Materials and methods
Reagents and chemicals
Iso-octane, methanol, ethanol, chloroform, diethyl ether, glacial acetic acid, methyl pentane, concentrated hydrochloric acid of analytical grade; benzene, boron trifluoride in methanol, dichloromethane of high performance liquid chromatography (HPLC) grade; p-anisidine, potassium hydroxide, benzoic acid, potassium iodide, sodium thiosulphate, sodium tetraborate, potassium dichromate, sodium bicarbonate, and starch were purchased from Merck (Darmstadt, Germany).
Materials
The materials used were refined rapeseed oil and a professional blend frying oil, as well as frozen pre-fried French fries.
The frying media were purchased from Kruszwica Company (Poland). Industrially refined rapeseed oil (liquid oil, RO) was bought in local supermarket and professional blend (liquid oil, PB) was sourced from wholesale suppliers. Both oils were purchased in packages with a volume of 10 L.
The professional oil (PB) was a blend of high-oleic sunflower oil with rapeseed and palm oils in different proportions with some additives as antioxidants and anti-foaming agent as declared the producer. All ingredients are allowed for food production. The manufacturer of PB claims that the optimal selection of components, a high content of monounsaturated fatty acids, provides high resistance to adverse changes occurring during frying, extending the lifetime of frying.
Pre-frozen French fries (made from one potato variety of identical technological parameters) were collected from the technological line of McCain Poland Manufacturers, immediately after stage I of frying. Stage II of frying was performed in the laboratory. Portions with a mass of 5 kg of product were packed in plastic bags. The material taken for the study consisted of French fries frozen at −18 °C prior to frying.
Frying process
Frozen French fries were fried in the frying oils. The oil (620 g) was placed in an uncovered stainless steel pan fryer, 24 cm diameter, 5 cm depth (Tefal, model Enjoy A04232, France) and heated to 180 °C. The thickness of the layer of oils in the pan was 1.85 cm. The French fries were fried in 100 g batches at a constant frying temperature (180 °C). The batches were fried at 4.5 min intervals at a rate of every 30 min for a total of 8 h. A sample of oil (about 20 g) was taken every hour, pured into a screw-cap vial and promptly frozen at −20 °C and kept until further analysis. A sample of French fries (about 70 g) was removed every hour and placed into screw-cap cups, promptly frozen at −20 °C, and kept until further analysis. The volume of oil was not replenished during the frying process. Frying experiments were conducted in duplicate for each frying medium.
Methods of analysis
Peroxide (PV), anisidine (AnV) and acid (AV) value contents were determined in frying media according to AOAC standards (1995).
The refractive index of oil samples was determined using a refractometer (Rudolph Research Analytical, model J157, USA) at 20 °C, for each test five repetitions were performed.
The fatty acid methyl esters of frying oils as well as fat extracted from fried French fries were prepared with BF3 in methanol as the methylating agent. The obtained methyl esters of fatty acids were separated with a PU 4410 gas chromatograph (Philips, UK), using a capillary column RTX- 2330 (Restek, USA), 105 m length, diameter 0.25 mm i.d. and film thickness 0,2 μm. Detector (FID) and injection temperature was 260 °C. Column temperature was 160 °C (30 min.) to 180 °C (17 min.) at 3 °C/min. and to 220 °C (15 min.) at 5 °C/min. The carrier gas was helium. For data handling system the Star Chromatography Workstation (version 6.6) was used, with software from Varian. The fatty acid composition was expressed as percentage of total fatty acids (AOAC 1995).
Oil degradation during frying decreased the polyunsaturated fatty acid content. Changes in the content of fatty acids in the samples, defined as a losses of C18:2 and C18:3 (L18 : x) in relation to the initial summary content of these fatty acids in fresh oils, were calculated according to:
where:
- 0
initial oil
- f
thermally treated oil
Determination of the polar fraction content was performed by chromatographic and weight methods by using columns of silica gel and diethyl ether as the eluent. The composition of the polar fraction was determined by high pressure size exclusion chromatography (HPSEC) using the liquid chromatographic system Varian (Paolo Alto, CA, USA), consisting of a ternary pump (230 Pro-Star) and an autosampler (430 Pro-Star) and Phenogel columns SEC/GPC (300 × 7.8 mm) connected in series, equipped with a precolumn (50 × 7.8 mm) (Phenomenex, USA). Detector RI was used (Knauer, Germany). Using this system, the following compounds were separated and quantified: triacylglycerol polymers and dimers (TGP and TGD), oxidised triacylglycerols (oxTAG), diacylglycerols (DAG) and free fatty acids (FFA) (Dobarganes et al. 2000a; Aniołowska and Kita 2014).
The fat content of the French fries was determined using Soxhlet’s method (AOAC). Fat was extracted using a Büchi B-811 Universal extraction system (Büchi Labortechnic AG, Flawil, Switzerland). Two grams of sample were extracted for 180 min using diethyl ether as the solvent.
Statistical analysis
All frying experiments were duplicated, and for each experiment analyses were conducted in duplicate. The results presented are the mean and standard deviation of the obtained values. Data handling and figures preparation were carried out using Microsoft Excel 2007 (Microsoft Corp., Seattle, WA).
The data were analysed statistically using Statistica 10.0 software. For comparison, the results obtained were analysed using one- and two-way analysis of variance with the application of Duncan’s test (P ≤ 0.05). One-way analysis of variance was used for determining the significance of the frying time on fatty acids profile of frying oils sampled from the pan and the oil extracted from French fries fried in those oils. Two-way analysis of variance was used for determining the significance of the kind of oil used and frying time on anisidine values, acid values, changes of refractive index and fat contents of the samples. Regression analysis (R values) was designated using Microsoft Excel 2007 (Microsoft Corp., Seattle, WA).
Results and discussion
Table 1 shows the characteristics of oils used for frying the French fries. The acid value ranged from 0.08 mg KOH/g in refined rapeseed oil (RO) to 0.20 mg KOH/g in professional blend (PB). RO had peroxide values three times higher than PB. Both oils had similar, and low, anisidine values, polar fractions and refractive indices. This means that both of the oils were characterized by good quality. The fatty acid contents of oils varied, containing a range of levels of unsaturated and saturated fatty acids (Table 2). PB exhibited higher level of oleic fatty acid than RO (respectively 68 and 62 %). The share of saturated fatty acids (SFA) in PB was on a level of 17 %, while in RO on 7.5 %. The new oil exhibited lower SFA content in comparison with quite popular palm oils, but higher in comparison with most of the oils obtained from oleaginoses seeds exhibiting around 14 % SFA.
Table 1.
Characteristics of oils used as frying media (AV - acid value, PV - peroxide value, AnV - anisidine value)
| Kind of oil | AV (mg KOH/g) | PV (meq O2/kg) | AnV | Polar fraction (g/100 g oil) | Refractive index |
|---|---|---|---|---|---|
| Rapeseed oil (RO) | 0.08 | 3.62 | 6.08 | 2.69 | 1.46424 |
| Professional blend (PB) | 0.20 | 1.16 | 6.16 | 2.88 | 1.46115 |
Table 2.
Changes of fatty acids profile of frying oils sampled from the fryer and the oil extracted from French fries fried in those oils during 8 h pan-frying
| Fatty acids | Fatty acids % in rapeseed oil | Fatty acids (%) in professional blend | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil from frying medium | Oil extracted from French fries | Oil from frying medium | Oil extracted from French fries | |||||||||||||
| 0 h | 1 h | 4 h | 8 h | 0 h | 1 h | 4 h | 8 h | 0 h | 1 h | 4 h | 8 h | 0 h | 1 h | 4 h | 8 h | |
| C14:0 | 0.07c ± 0.00 | 0.07c ± 0.00 | 0.11b ± 0.00 | 0.14a ± 0.00 | 0.07c ± 0.00 | 0.07c ± 0.00 | 0.11b ± 0.00 | 0.14a ± 0.00 | 0.22d ± 0.00 | 0.26c ± 0.00 | 0.27b ± 0.00 | 0.31a ± 0.00 | 0.21d ± 0.00 | 0.25c ± 0.00 | 0.27b ± 0.00 | 0.31a ± 0.00 |
| C16:0 | 4.82d ± 0.05 | 5.58c ± 0.01 | 6.96b ± 0.04 | 7.17a ± 0.02 | 4.96d ± 0.02 | 5.69c ± 0.02 | 6.98b ± 0.00 | 7.16a ± 0.01 | 12.84d ± 0.02 | 13.79c ± 0.01 | 14.07b ± 0.05 | 14.95a ± 0.00 | 12.83d ± 0.01 | 13.79c ± 0.02 | 14.04b ± 0.04 | 14.95a ± 0.00 |
| C18:0 | 1.94d ± 0.04 | 2.50c ± 0.01 | 2.62b ± 0.02 | 2.83a ± 0.02 | 2.07d ± 0.03 | 2.49c ± 0.01 | 2.61b ± 0.03 | 2.81a ± 0.02 | 3.08d ± 0.03 | 3.55c ± 0.05 | 3.73b ± 0.00 | 3.87a ± 0.00 | 3.07d ± 0.02 | 3.58c ± 0.04 | 3.73b ± 0.04 | 3.86a ± 0.02 |
| C20:0 | 0.40d ± 0.01 | 0.43c ± 0.01 | 0.46b ± 0.01 | 0.50a ± 0.01 | 0.41c ± 0.01 | 0.44bc ± 0.01 | 0.47ab ± 0.01 | 0.51a ± 0.02 | 0.28a ± 0.01 | 0.29a ± 0.01 | 0.29a ± 0.00 | 0.29a ± 0.00 | 0.28a ± 0.01 | 0.29a ± 0.01 | 0.29a ± 0.01 | 0.29a ± 0.00 |
| C22:0 | 0.26b ± 0.01 | 0.27b ± 0.00 | 0.33a ± 0.01 | 0.34a ± 0.00 | 0.27b ± 0.01 | 0.28b ± 0.01 | 0.34a ± 0.02 | 0.35a ± 0.01 | 0.47b ± 0.01 | 0.50a ± 0.00 | 0.51a ± 0.00 | 0.51a ± 0.00 | 0.47b ± 0.00 | 0.51a ± 0.00 | 0.51a ± 0.01 | 0.51a ± 0.00 |
| C24:0 | 0.07c ± 0.00 | 0.07c ± 0.00 | 0.09b ± 0.00 | 0.11a ± 0.01 | 0.07c ± 0.00 | 0.07c ± 0.00 | 0.10b ± 0.00 | 0.11a ± 0.00 | 0.11b ± 0.00 | 0.12a ± 0.00 | 0.12a ± 0.00 | 0.12a ± 0.00 | 0.11b ± 0.00 | 0.12a ± 0.00 | 0.12a ± 0.00 | 0.12a ± 0.00 |
| tC18:1 | 0.00c ± 0.00 | 0.00c ± 0.00 | 0.02b ± 0.00 | 0.03a ± 0.00 | 0.00b ± 0.00 | 0.00b ± 0.00 | 0.02a ± 0.00 | 0.02a ± 0.00 | 0.00c ± 0.00 | 0.01b ± 0.00 | 0.01ab ± 0.00 | 0.01a ± 0.00 | 0.00c ± 0.00 | 0.01b ± 0.00 | 0.01ab ± 0.00 | 0.01a ± 0.00 |
| tC18:2 | 0.02d ± 0.00 | 0.05c ± 0.00 | 0.06b ± 0.00 | 0.07a ± 0.00 | 0.02d ± 0.00 | 0.05c ± 0.00 | 0.06b ± 0.00 | 0.07a ± 0.00 | 0.10c ± 0.00 | 0.11bc ± 0.00 | 0.11ab ± 0.00 | 0.12a ± 0.00 | 0.11b ± 0.00 | 0.11b ± 0.00 | 0.11b ± 0.00 | 0.12a ± 0.00 |
| C16:1 | 0.25b ± 0.01 | 0.26b ± 0.00 | 0.26ab ± 0.00 | 0.27a ± 0.00 | 0.25a ± 0.00 | 0.26a ± 0.01 | 0.26a ± 0.01 | 0.27a ± 0.00 | 0.15c ± 0.00 | 0.16b ± 0.00 | 0.16b ± 0.00 | 0.17a ± 0.00 | 0.15b ± 0.00 | 0.16b ± 0.00 | 0.16b ± 0.00 | 0.17a ± 0.00 |
| C18:1 | 61.56d ± 0.01 | 62.05c ± 0.01 | 62.51b ± 0.03 | 63.62a ± 0.02 | 61.74d ± 0.00 | 62.17c ± 0.02 | 62.48b ± 0.02 | 63.60a ± 0.03 | 68.46a ± 0.01 | 67.35b ± 0.06 | 67.11c ± 0.06 | 66.37d ± 0.02 | 68.46a ± 0.01 | 67.30b ± 0.04 | 67.15b ± 0.10 | 66.38c ± 0.02 |
| C20:1 | 1.26d ± 0.01 | 1.27c ± 0.00 | 1.30b ± 0.00 | 1.37a ± 0.00 | 1.27b ± 0.00 | 1.28b ± 0.01 | 1.29b ± 0.01 | 1.37a ± 0.01 | 0.42a ± 0.00 | 0.41b ± 0.00 | 0.41b ± 0.00 | 0.41c ± 0.00 | 0.42a ± 0.00 | 0.41b ± 0.00 | 0.41b ± 0.00 | 0.41b ± 0.00 |
| C22:1 | 0.40a ± 0.01 | 0.39a ± 0.00 | 0.36b ± 0.00 | 0.35b ± 0.00 | 0.39a ± 0.00 | 0.39ab ± 0.01 | 0.37bc ± 0.00 | 0.36c ± 0.01 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 | 0.05a ± 0.00 |
| C18:2n-6 | 20.46a ± 0.09 | 19.06b ± 0.00 | 18.14c ± 0.02 | 17.39d ± 0.03 | 20.00a ± 0.02 | 18.42b ± 0.01 | 18.15c ± 0.03 | 17.41d ± 0.02 | 11.81a ± 0.02 | 11.57b ± 0.01 | 11.48c ± 0.00 | 11.37d ± 0.02 | 11.83a ± 0.01 | 11.57b ± 0.01 | 11.48c ± 0.02 | 11.39d ± 0.01 |
| C18:3n-3 | 8.49a ± 0.02 | 7.99b ± 0.01 | 6.79c ± 0.02 | 5.81d ± 0.02 | 8.48a ± 0.01 | 8.39b ± 0.02 | 6.77c ± 0.01 | 5.83d ± 0.01 | 2.01a ± 0.03 | 1.84b ± 0.02 | 1.67c ± 0.01 | 1.46d ± 0.02 | 2.01a ± 0.00 | 1.85b ± 0.00 | 1.66c ± 0.01 | 1.44d ± 0.01 |
| SFA** | 7.56 | 8.93 | 10.57 | 11.09 | 7.85 | 9.04 | 10.60 | 11.08 | 17.00 | 18.50 | 18.99 | 20.05 | 16.98 | 18.53 | 18.96 | 20.04 |
| MUFA | 63.47 | 63.97 | 64.43 | 65.61 | 63.65 | 64.10 | 64.40 | 65.59 | 69.08 | 67.97 | 67.73 | 66.99 | 69.08 | 67.93 | 67.78 | 67.00 |
| PUFA | 28.95 | 27.05 | 24.92 | 23.20 | 28.48 | 26.81 | 24.92 | 23.24 | 13.82 | 13.41 | 13.15 | 12.83 | 13.84 | 13.42 | 13.14 | 12.83 |
| Losses | ||||||||||||||||
| L18:2 | – | 6.86 | 11.35 | 15.01 | – | 7.91 | 9.24 | 12.93 | – | 2.03 | 2.83 | 3.73 | – | 2.19 | 2.92 | 3.68 |
| L18:3 | – | 5.87 | 20.09 | 31.55 | – | 1.05 | 20.16 | 31.29 | – | 8.66 | 16.99 | 27.56 | – | 7.96 | 17.36 | 28.26 |
| L18:2+18:3 | – | 6.57 | 13.92 | 19.86 | – | 5.86 | 12.49 | 18.40 | – | 2.92 | 4.81 | 7.12 | – | 3.03 | 5.02 | 7.25 |
* All values are averages of the triplicate analysis (n = 6)
**SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids
***Values followed by different letters in raws for the same type of oil are statistically different at the 95 % confidence level
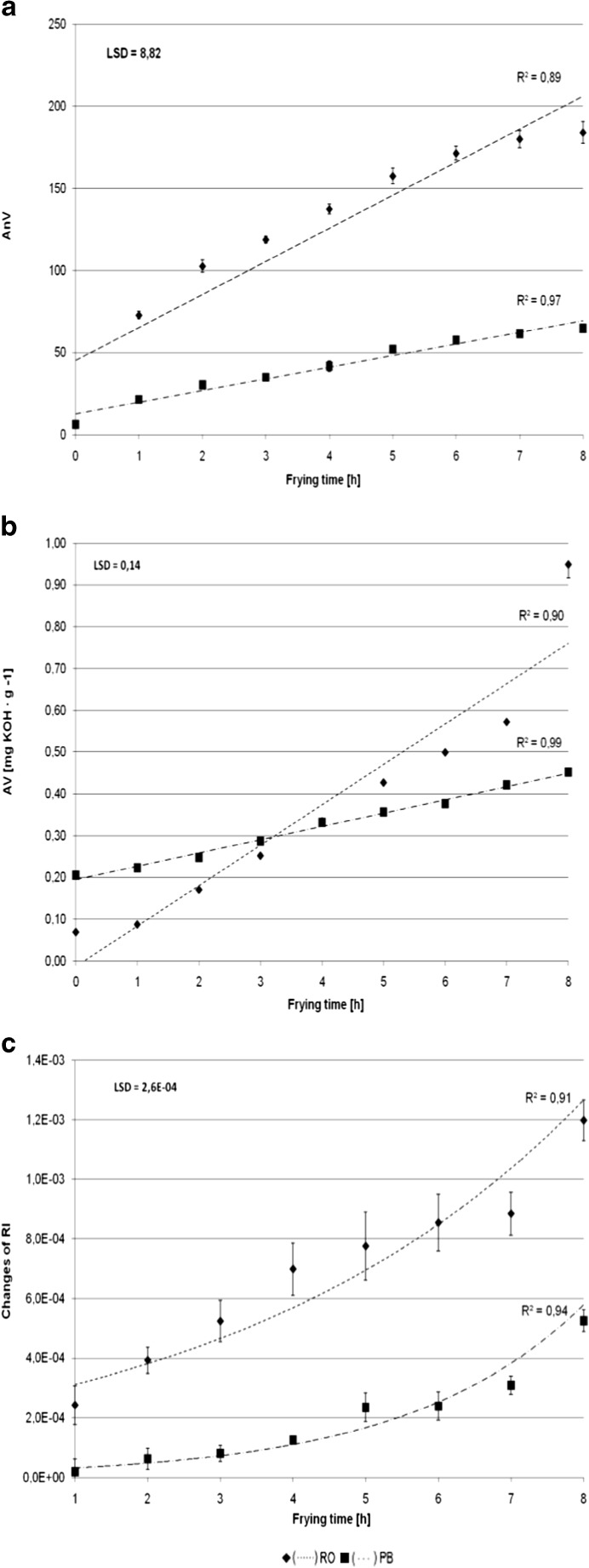
One of the most common reactions playing a key role in the degradation of a frying medium is oxidation. A change in this parameter in frying oils was observed as a change in the anisidine value (AnV) (Fig. 1a). The AnV, which mainly accounts for the amount of unsaturated aldehydes as products of degradation of peroxides, increased for every hour of frying. The highest increase in AnV was observed in RO after the first hour, while PB exhibited better oxidative stability. At the end of frying AnV of rapeseed oil was 184 while of professional blend almost 3-times lower - 65. A rapid increase in AnV was observed by other authors (Choo et al. 2007; Soheili et al. 2002a). The ratio of AnV increase depended on kind of oil as well as on frying procedure. Choo et al. (2007) observed the highest AnV increase for flaxseed cold-pressed oils used for short pan-frying, when after several minutes (3 and 6 min) AnV increased from 0.4–0.7 to 290–588 and 622–876, respectively. In another study by Soheili et al. (2002a), after 10 min of heating at 150 °C, the AnV of low-linolenic soybean oil was significantly greater (386.5 abs/g oil) than that of partially hydrogenated soybean oil after 14 min of heating (298.5 abs/g oil). On the other hand during deep-frying process oxidative changes in frying oils were not so intensive (Houhoula et al. 2002).
Fig. 1.
Changes of (a) anisidine value (AnV), (b) acid value (AV), (c) refractive index (RI) of oils during 8 h pan-frying of frozen prefried French fries
The other changes that occur in oils during frying are due to hydrolysis. A linear increase in acid value (AV) was observed (Fig. 1b). According to the Polish Directive from the Minister of Health from 25 September 2012, the acceptable level of free fatty acids in a frying medium, cannot be higher than 2.5 mg KOH/g of fat. As a result of hydrolytic reaction, the free fatty acid content of the frying medium increased. However, this did not exceed 2.5 mg KOH/g in any of the samples. The highest AV content was found in RO after 8 h of frying, while the lowest was observed in fresh RO. In this case, the professional blend (PB) is degraded to a lesser degree than RO. Our results are in agreement with other authors, who also report a linear increase in AV content with frying time in different frying media using different methods of frying (Matthäus 2006; Kiatsrichart et al. 2003; Soheili et al. 2002a; Choo et al. 2007). Kiatsrichart et al. (2003) stated that during pan-frying using mid-oleic sunflower oil (NuSunoil) and a commercial rapeseed oil more sensitive for hydrolysis was rapeseed oil, however both oils exhibited low level of free fatty acids. In another experiment Soheili et al. (2002a) found that after 10 min of heating the low linolenic soybean oil, free fatty acid content, was lower than in partially hydrogenated soybean oil after 14 min of heating (respectively 2.66 and 4.28 %).
Another of the parameters monitored in frying oils to determine the physical changes of the oil is the refractive index (RI). According to Abe et al. (2010), RI is related to the fat autoxidation reaction, and its value increases after the formation of peroxides. In oils used in the experiment, changes in RI also depended on the frying duration and the kind of oil used (Fig. 1c). During the frying time, changes in the RI calculated for the PB were always twice smaller than for the RO and increased exponentially with time.
The fatty acid composition of fresh and used in pan-frying oils as well as oils extracted from fried French fries are listed in Table 2. It was observed that the fatty acid composition of the oil extracted from the French fries was similar to that of the oil from the pan, which is in agreement with Sebedio et al. (1990). Share of each fatty acid depended on the frying duration. In all samples predominant were monounsaturated fatty acids represent mostly by oleic acid. MUFA increased constantly until the end of the frying time in every analysed sample. At the same time also increased a part of SFA, while PUFA decreased. As expected, before and after frying the professional blend as well as oils extracted from fried French fries was higher in saturated fatty acids, particularly in the contribution of palmitic acid (both from 12.8 in fresh oil and product to 15.0 % in used), showing that one of the component of this blend was palm oil.
The amount as well as composition of PUFA – especially contribution of most unstable linolenic acid influenced stability of oils during frying. After 8 h of frying, higher content of polyunsaturated fatty acids (PUFA) represented by linoleic and linolenic acids exhibited RO (respectively 17.4 % and 5.8 %) in comparison with PB (respectively 11.1 and 1.5 %). PUFA content of the both oils extracted from the French fries differed only in the 0.01 % from the result observed in analyzed oils. The changes of PUFA contribution in analysed oils during pan-frying of frozen pre-fried French fries were calculated as relative losses of C18:2 and C18:3. The loss of polyunsaturated fatty acids (sum of C18:2 and C18:3) after 8 h of frying was 7 % in PB and 20 % in RO. Such results showed that PB characterized near to 3-fold higher stability in comparison with RO. Similar results obtained other authors analysing fatty acid profile of different oils used for frying (Ali et al. 2013; Casal et al. 2010). Al-Khusaibi et al. (2012) in experiment with rapeseed oil and its blend with palm oil used for 28 h deep-frying observed in rapeseed oil a decrease in the percentage of polyunsaturated fatty acids from 27.4 to 21.7 %, while in the blend from 19.0 to 17.5 %.
In our experiment, the loss of sum of C18:2 and C18:3 obtained in oil extracted from French fries fried in PB was 0.13 % higher than in oil. The reverse situation occurred in the case of RO when the loss in oil extracted from French fries was 1.47 % lower than in pan-frying oil. It is supposed that French fries absorbed greater part of oil during frying in RO in comparison with PB. So the higher fat exchange in French fries fried in RO might caused greater difference between fatty acid composition of oil and product’s sample. Moreover, it also resulted in lower lost of PUFA by oxidation in the French fries, compared with the oil from the pan.
Isomerization is another change observed during long heating or frying. In our experiment total share of trans isomers (tC18:1 and tC18:2) increased from 0.02 to 0.10 % in RO and from 0.10 to 0.13 % in PB after 8 h of pan frying. Kalogeropoulos et al. (2007) reported the formation of cis and trans isomers during eight successive pan- and deep-frying sessions in five different types of vegetable oils (as cottonseed and sunflower oils, vegetable shortening, palm oil and virgin olive oil). These oxidized fatty acids were present at relatively low concentrations in the fresh oils and pre-fried potatoes while they increased linearly with frying time, reaching up to 1140.8 mg/g in virgin olive oil and 186.9 mg/g in potatoes pan-fried in this oil after eight pan-frying sessions. Such results confirm that quality of fried product is mostly depended on the quality of frying medium.
Table 3 shows changes in the polar content and composition of fresh oils and after 1, 4 and 8 h pan-frying of frozen pre-fried French fries. After finishing frying the polar fraction content was twice lower in PB, but didn’t exceed 25 % in either of the frying media. Such results confirm good quality of refined rapeseed oil as frying medium in comparison with professional blend dedicated for frying. Obtained results are in agreement with other study with rapeseed oil used as a frying medium (Farhoosh et al. 2008). Aladedunye and Przybylski (2009) observed that the contribution of total polar material increased consistently with frying time, achieving values of about 10 % for 7 h of deep frying. Lower contents of polar compounds in oils used for deep-frying in comparison with pan-frying indicate that the content of polar compounds depends also on type of frying.
Table 3.
Polar fraction content and composition ofoils during 8 h pan-frying of frozen pre-fried French fries
| Frying time (h) | Polar fraction content* (g/100 g oil) | Polar fraction composition (contribution in relative percentages)* | ||||
|---|---|---|---|---|---|---|
| TGP** | TGD | oxTAG | DAG | FFA | ||
| Rapeseed oil | ||||||
| 0 | 2.69g*** ± 0.05 | 0.00 ± 0.00 | 5.78 ± 0.09 | 50.21 ± 0.60 | 34.71 ± 0.31 | 9.30 ± 0.07 |
| 1 | 9.37e ± 0.17 | 0.00 ± 0.00 | 16.42 ± 0.27 | 48.75 ± 0.23 | 31.40 ± 0.25 | 3.43 ± 0.05 |
| 4 | 15.40b ± 0.20 | 2.52 ± 0.07 | 18.94 ± 0.29 | 46.90 ± 0.79 | 29.51 ± 0.64 | 2.13 ± 0.03 |
| 8 | 23.21a ± 0.18 | 5.63 ± 0.09 | 26.08 ± 0.14 | 46.43 ± 0.54 | 20.65 ± 0.21 | 1.21 ± 0.05 |
| Professional blend | ||||||
| 0 | 2.88g ± 0.08 | 0.00 ± 0.00 | 1.73 ± 0.04 | 29.31 ± 0.25 | 63.33 ± 0.44 | 5.63 ± 0.07 |
| 1 | 3.68f ± 0.10 | 0.86 ± 0.04 | 7.52 ± 0.04 | 25.02 ± 0.33 | 62.44 ± 0.32 | 4.17 ± 0.09 |
| 4 | 10.40d ± 0.16 | 1.54 ± 0.08 | 7.95 ± 0.02 | 24.87 ± 0.26 | 61.54 ± 0.51 | 4.10 ± 0.05 |
| 8 | 12.19c ± 0.12 | 3.04 ± 0.10 | 19.96 ± 0.21 | 21.33 ± 0.31 | 52.80 ± 0.54 | 2.88 ± 0.03 |
* All values are averages of the triplicate analysis (n = 6)
**TGP - triacylglycerol polymers, TGD - triacylglycerol dimers, oxTAG - oxidised triacylglycerols, DAG - diacylglycerols and FFA - free fatty acids
***Values followed by different letters are statistically different at the 95 % confidence level
The predominant fraction in RO was oxidized TAG, while in the blend, diacylglycerols (DAG). The share of those two fractions was on a level of 84 % for RO and 92 % for PB. Between polar compounds in fresh oils were also presented dimers of triacylglycerols (TGD) and free fatty acids (FFA). Most undesirable polar components formed during frying are polymers of triacylglycerols (TGP) as well dimers (TGD). TGP were firstly detected after 1 h of frying in professional blend, while after 4 h in rapeseed oil. Such results show that in short frying rapeseed oil is quite stable for polymerization. During 8 h pan-frying the share of TGP and TGD increased in both oils – up from 5.8 to 32 % in RO and from 1.8 to 23 % in PB. The share of other fractions decreased with the highest changes of FFA. In used rapeseed oil the predominant fraction was oxTAG followed by polymers and dimers, DAG and FFA, while in professional blend – DAG, followed by polymers and dimers, oxTAG and FFA.
The content and the composition of polar fraction deeply depend on kind of oil or oils used for obtaining the frying medium as well as their quality. Usually palm oils exhibit higher polar compounds content in comparison with seeds oils (Aniołowska and Kita 2014). Bansal et al. (2010) attributed the high-polar compound content of palm olein to the high concentration of DAG present in the oil. In our study fresh oils exhibited the same level of polar compounds with different share of individual fractions. Similar patterns for oils with predominating DAG or oxTAG were observed in others experiments (Przybylski et al. 2013; Marmesat et al. 2012; Houhoula et al. 2003). Aladedunye and Przybylski (2009) stated decreasing levels of oxTAG and DAG with increasing of polymerisation product concentration in canola oil used for deep-frying. In study done by Soheili et al. (2002b) fresh soybean oils had a relatively low polymer content (below 4 %), although the oil remaining in the pan after frying had a much greater polymer content (from 17.5 % up to 38.8 %).
The initial content of fat in pre-fried French fries was 4 g/100 g . Fat content in French fries fried in fresh RO was about 0.5 % higher than in PB (respectively 12.3/100 g and 11.8/100 g). The fat content in fried French fries in first 4 h was not significantly different. Changes in fat absorption were observed during next hours of frying. Subsequently, an increase in fat content of French fries fried in RO, and a decrease in PB was observed (respectively 12.6/100 g and 10.3/100 g). Some of the data in the literature suggest that fat absorption depends on the degradation of the frying media (Dobarganes et al. 2000b). The results of the present study did not show that fat absorption mainly depended on oil degradation, rather on the kind of oil.
Changes in fatty acid composition of fat extracted from fried French fries were shown in Table 2. Obtained results show that during frying followed almost total fat’s exchange between fried products and frying oils. Fats extracted from fried French fries exhibited the same fatty acid profile as used frying media. Such results confirm that taking into account the nutritional value the composition of final frying oil is the most important in fried products preparation.
Conclusions
Hydrolytic and oxidative changes in frying media varied according to oil composition and increased with frying time. The best thermooxidative stability was found with PB. The kind of oil influenced all the tested parameters. The pace of oxidation in RO was three times higher than in PB. The loss of polyunsaturated fatty acids (sum of C18:2 and C18:3) after 8 h of frying was at a level of 7 % in PB and 20 % in RO. The polar fraction content was twice lower in PB, but did not exceed 25 % in either of the frying media. The predominant fraction in RO was oxidized TAG, while in PB it was DAG. During frying the percentages of TGP and TGD increased in both oils – up to 32 % in RO and 23 % in PB. The fat content in French fries fried in fresh and used RO was about 0.5 and 2 % higher than it was observed in the case of PB, respectively. Besides lower stability of rapeseed oil, the acceptable level of free fatty acids and polar fraction contents for frying oils were not exceeded, what confirms the suitability of using this oil for pan frying. Although the professional blend was characterized by better thermooxidative stability, rapeseed oil also can be used for short-term frying when in a thin layer.
Acknowledgments
This work was supported by the National Science Center in Poland under Grant No. 2012/05/N/NZ9/01508.
References
- Abe I, Oliveira J, Simoes E, Caladas P, Frazao O. Monitoring the quality of frying oils using a nanolayer coated optical fiber refractometer. Talanta. 2010;83:291–293. doi: 10.1016/j.talanta.2010.08.040. [DOI] [PubMed] [Google Scholar]
- Aladedunye F, Przybylski R. Degradation and nutritional quality changes of oil during frying. JAOCS. 2009;86:149–156. [Google Scholar]
- Ali MA, Nouruddeen ZB, Muhamad II, Latip RA, Othman NH, Mahmood AN. Impact of palm olein addition on the thermooxidative degradation of canola oil during frying. Chiang Mai J Sci. 2013;40:643–655. [Google Scholar]
- Al-Khusaibi M, Gordon MH, Lovegrove JA, Niranjan K. Frying of potato chips in a blend of canola oil and palm olein: changes in levels of individual fatty acids and tocos. Int J Food Sci Technol. 2012;47:1701–1709. doi: 10.1111/j.1365-2621.2012.03024.x. [DOI] [Google Scholar]
- Andrikopoulos NK, Kalogeropoulos N, Falirea A, Barbagianni MN. Performance of virgin olive oil and vegetable shortening during domestic deep-frying and pan-frying of potatoes. Int J Food Sci Technol. 2002;37:177–190. doi: 10.1046/j.1365-2621.2002.00555.x. [DOI] [Google Scholar]
- Aniołowska M, Kita A. Content and composition of polar fractions in refined vegetable oils available on the Polish market. Przem Chem. 2014;93(4):492–494. [Google Scholar]
- AOAC . Official methods of analysis. Washington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Bansal G, Zhou W, Barlow PJ, H-L LO, Neo F-L. Performance of palm olein in repeated deep frying and controlled heating processed. Food Chem. 2010;121:38–347. [Google Scholar]
- Brinkmann B. Quality criteria of industrial frying oils and fats. Eur J Lipid Sci Technol. 2000;102:539–541. doi: 10.1002/1438-9312(200009)102:8/9<539::AID-EJLT539>3.0.CO;2-B. [DOI] [Google Scholar]
- Casal S, Malheiro R, Sendas A, Oliveira BPP, Pereira JA. Olive oil stability under deep-frying conditions. Food Chem Toxicol. 2010;48:2972–2979. doi: 10.1016/j.fct.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Choo WS, Birc EJ, Dufour JP. Physicochemical and stability characteristics of flaxseed oils during pan-heating. JAOCS. 2007;84:735–740. [Google Scholar]
- Dobarganes MC, Velasco J, Dieffenbacher A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats: results of collaborative studies and the standardized method (Technical report) Pure Appl Chem. 2000;72(8):1563–1575. doi: 10.1351/pac200072081563. [DOI] [Google Scholar]
- Dobarganes MC, Marquez-Ruiz G, Velasco J. Interactions between fat absorption and food during deep-fat frying. Eur J Lipid Sci Technol. 2000;102:521–528. doi: 10.1002/1438-9312(200009)102:8/9<521::AID-EJLT521>3.0.CO;2-A. [DOI] [Google Scholar]
- Farhoosh R, Esmaeilzadeh KR, Poorazrang H. Frying stability of canola oil blended with palm olein, olive, and corn oils. JAOCS. 2008;86:71–76. [Google Scholar]
- Gertz C. Fundamentals of the frying process. Eur J Lipid Sci Technol. 2014;116:669–674. doi: 10.1002/ejlt.201400015. [DOI] [Google Scholar]
- Houhoula DP, Oreopoulou V, Tzia C (2002) A kintetic study of oil deterioration during frying and a comparision with heating. JAOCS 79:133–137
- Houhoula DP, Oreopoulou V, Tzia C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. J Sci Food Agric. 2003;83:314–319. doi: 10.1002/jsfa.1314. [DOI] [Google Scholar]
- Isbell TA, Abbott TP, Carlson KD. Oxidative stability index of vegetable oils in binary mixtures with meadowfoam oil. Ind Crop Prod. 1999;9:115–123. doi: 10.1016/S0926-6690(98)00022-3. [DOI] [Google Scholar]
- Kalogeropoulos N, Salta FN, Chiou A, Andrikopoulos NK. Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur J Lipid Sci Technol. 2007;109:1111–1123. doi: 10.1002/ejlt.200700007. [DOI] [Google Scholar]
- Kalogianni EP, Karastogiannidou C, Karapantios TD. Effect of the presence and absence of potatoes under repeated frying conditions on the composition of palm oil. JAOCS. 2009;86:561–571. [Google Scholar]
- Kiatsrichart S, Brewer MS, Cadwallader KR, Artz WE. Pan-frying stability of NuSun Oil, a Mid-Oleic sunflower oil. JAOCS. 2003;80:479–483. [Google Scholar]
- Marmesat S, Morales A, Velasco J, Dobarganes CM. Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying. Food Chem. 2012;135:2333–2339. doi: 10.1016/j.foodchem.2012.06.128. [DOI] [PubMed] [Google Scholar]
- Matthäus B. Utilization of high-oleic rapeseed oil for deep-fatfrying of French fries compared to other commonly used edible oils. Eur J Lipid Sci Technol. 2006;108:200–211. doi: 10.1002/ejlt.200500249. [DOI] [Google Scholar]
- Matthäus B. Use of palm oil for frying in comparison with other high-stability oils. Eur J Lipid Sci Technol. 2007;109:400–409. doi: 10.1002/ejlt.200600294. [DOI] [Google Scholar]
- Matthäus B, Haase NU, Unbehend G. Chemical and sensory characteristic of products fried in high-oleic, low-linolenic rapeseed oil. JAOCS. 2009;86:799–808. [Google Scholar]
- Mehta U, Swinburn B. A review of factors affecting fat absorption in hot chips. Crit Rev Food Sci Nutr. 2001;41:133–154. doi: 10.1080/20014091091788. [DOI] [PubMed] [Google Scholar]
- Perkins EG. Volatile odor and flavor components formed in deep frying. In: Erickson MD, editor. Deep frying: chemistry, nutrition and practical application. 2. Urbana: AOCS Press; 2007. pp. 51–56. [Google Scholar]
- Przybylski R, Gruczynska E, Aladedunye F. Performance of regular and modified canola and soybean oils in rotational frying. JAOCS. 2013;90:1271–1280. doi: 10.1007/s11746-013-2278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani AK, Reddy SY, Chetana R. Quality changes in trans and trans free fats/oils and products during frying. Eur Food Res Technol. 2010;230:803–811. doi: 10.1007/s00217-010-1225-7. [DOI] [Google Scholar]
- Rossel JB. Developments in oils for commercial frying. Lipid Technol. 2003;1:5–8. [Google Scholar]
- Sebedio JL, Bonpunt A, Grandgirard A, Prevost J. Deep fat frying of frozen prefried french fries: influence of the amount of linolenic acid in the frying medium. J Agric Food Chem. 1990;38:1862–1867. doi: 10.1021/jf00099a017. [DOI] [Google Scholar]
- Senanayake N, Shahidi F. Modification of fats and oils via chemical and enzymatic methods. In: Shahidi F, editor. Bailey’s industrial oil and fat products. 6. Hoboken: Wiley; 2005. pp. 555–584. [Google Scholar]
- Soheili KC, Artz WE, Tippayawat P. Pan-heating of low-linolenic acid and partially hydrogenated soybean oils. JAOCS. 2002;79:287–290. [Google Scholar]
- Soheili KC, Tippayawat P, Artz WE. Comparison of a low-linolenic and a partially hydrogenated soybean oil using pan-fried hash browns. JAOCS. 2002;79:1197–1200. [Google Scholar]
- Zambiazi RC, Przybylski R, Zambiazi MW, Mendonça CB. Fatty acid composition of vegetable oils and fats. B CEPPA Curitiba. 2007;25(1):111–120. [Google Scholar]