Abstract
Edible films and coatings have been proposed as viable alternatives for the preservation of fresh food such as fruit, meat, fish and cheese. They can be designed to contain natural antioxidants, vitamins and antimicrobials in order to extend shelf life of the product keeping the natural sensorial properties. Essential oils have been targeted as potential active principles for edible films and coatings given their well-recognized antioxidant, antimicrobial and sensory properties. In the present work, lemongrass oil (LMO) microcapsules were prepared by the emulsification-separation method using sodium caseinate as wall material. Microcapsules had an average size of 22 μm and contained over 51 % oil in their nucleus. The release kinetics of the LMO components was studied for both, microcapsules and microcapsule containing films. Experimental data for the controlled release of LMO components showed good correlation with Peppas and Weibull models. The effect of the alginate matrix on the release parameters of the mathematical models could be detected by the modification of the b constant of the Weibull equation which changed from 0.167 for the microcapsules to 0.351 for the films. Films containing LMO at concentrations of 1250, 2500 and 5000 ppm were able to inhibit growth of Escherichia coli ATCC 25922 and Listeria monocytogenes ISP 65–08 in liquid cultures. A possible future application of these films for shelf life extension of fresh food is discussed.
Keywords: Lemongrass oil microcapsule; Edible alginate film; Controlled release; Escherichia coli, Listeria monocytogenes; Growth inhibition
Introduction
Research on edible films and coatings started around 50 years ago inspired by the evident preserving effect of the old practice of applying wax on apples and other fruits to extend their shelf life (Pavlath and Orts 2009). Edible films and coatings is probably one of the most active research fields in food technology today. The main advantage of edible films over traditional synthetic food packaging polymers is that they can be consumed with the food, reducing the problem of waste generation. However, research on edible films and coating as gone far beyond what anybody could have imagined 3 o 4 decades ago (Quirós-Sauceda et al. 2014). Today, it is possible to suppress respiration, improve textural quality and retain volatile flavour and aroma compounds (Mauer et al. 2000; Yang and Paulson 2000; Han et al. 2004). Films and coating can also be loaded with functional and active ingredients, such as antioxidants, flavours, colours, antimicrobial agents and nutraceuticals (Guilbert et al. 1997; Cagri et al. 2001) to make the food more nutritive, safe and tasty.
Many essential oils have been used for years because of their antioxidant and antimicrobial properties. Among them thyme oil, oregano oil and, lately, lemongrass oil have been evaluated for their antioxidant and antimicrobial properties (Tongnuanchan and Benjakul 2014). There are several reports where different bacteria have been tested for their sensitivity to LMO components (Taweechaisupapong et al. 2012; Starliper et al. 2015; Olorunnisola et al. 2014). In one of the most extensive studies, Singh et al. (2011) tested the antimicrobial activity of LMO against 1114 strains belonging to 29 genera and 105 species of microbes (molds, yeasts and bacteria) isolated from clinical cases, environment (water, air, soil, droppings of lizards and birds), food and healthy animals. The authors reported that 38.2 % of all the microorganisms were sensitive to LMO by the agar diffusion and inhibition zone assay. All molds, yeasts, Lactobacillus acidophilus, Morganella morganii, were inhibited. Most of the Bacillus spp. strains (84.3 %), aeromonads (78 %), Edwardsiella spp. (73.9 %) were also sensitive. Besides, 53.6 % pseudomonads, 53.1 % streptococci and 50 % of Budvicia aquatica and Leminorella ghirmontii strains were also sensitive to LMO. In general, the antimicrobial properties of LMO and its practical application potential are comparable to oregano oil and only surpassed by thyme oil (Starliper et al. 2015).
The effectiveness of the antimicrobial properties of LMO has also started to be evaluated in edible films. In the study by Rojas-Graü et al. (2006), apple puree edible films containing LMO at concentrations of 1000 and 5000 ppm were able to inhibit growth of E. coli O157:H7 utilizing the method of agar diffusion and zone of inhibition. Maizura et al. (2007) studied the antibacterial activity and mechanical properties of partially hydrolysed sago starch–alginate edible film containing LMO oil against E. coli O157:H7. The bacteria were inhibited at concentrations between 1000 and 4000 ppm. In another article, the same group (Maizura et al. 2008) also tested these films on E. coli O157:H7, Salmonella enteritidis and Staphylococcus aureus. Both E. coli and S. enteritidis were sensitive to LMO whereas only Staphylococcus aureus did not show any response even at the highest concentrations (4000 ppm).
Most of the antimicrobial essential oils studied for food applications have poor water solubility whereas most film forming polymers (alginate, carrageenan, chitosan, starch, modified cellulose, etc.) are water soluble. Due to this phase incompatibility, the manufacture of active films and coatings containing essential oils requires the utilization of emulsifying agents to ensure even distribution of the oil micro-droplets throughout the film (Hambleton et al. 2009). In these systems the oil droplets can be unstable and grow by coalescence until a fraction of the lipids separate off and rise to the upper surface of the film rendering it less effective for shelf life extension (Jiménez et al. 2010). It is obvious that an edible film that has that type of instability problems will be of little practical value for the food industry. One way to overcome such limitations would be to introduce the oil within a polymer microcapsule and then formulate the active edible film. Since the microcapsule wall material can be chosen to suit the physicochemical requirements of whatever is necessary in the system, a hydrophilic wall would easily disperse in a hydrophilic polymeric film leading to a stable and homogenous controlled release of LMO onto the food surface.
The main goal of this work was to produce films of microencapsulated lemongrass oil capable of delivering the active principles over long periods and expressing their antimicrobial properties on some food borne pathogenic bacteria.
Materials and methods
Chemicals
Lemongrass oil (Indian origin) was purchased from SAFC Flavors & Fragrances (Milwaukee, WI, USA). Alginic acid sodium salt (medium viscosity), sodium caseinate, calcium carbonate and Tween 20 were all from Sigma-Aldrich (St. Louis, MO, USA). Ethanol, sorbitol and acetic acid were from Blumos (Santiago, Chile). Mueller Hinton broth was from Biokar Diagnostics (Beauvais Cedex, France) and 100 % Soy oil was purchased from Nutrisa (Santiago, Chile). All the other chemicals utilized were of the best quality available in the laboratory.
Microencapsulation of LMO in sodium caseinate
Lemongrass oil microcapsules were prepared by the emulsification-separation method as described by Bustos et al. (2003) with some modifications. 30 g of emulsion with oil to water ratio of 3:7 by weight was utilized for the microencapsulation process. The oil phase was made of LMO dissolved in soy oil at concentrations between 2000 and 10000 ppm to make up a total amount of 9 g. The aqueous phase (21 g) was made of 2 % (w/w) sodium caseinate and 2 % (w/w) Tween 20.
The emulsification process was carried out utilizing an ultraturrax model T45S7 (Janke & Kunkel, IKA Works GmbH & Co., Staufen, Germany), operating at a rate of 8000 rpm for 5 min. The emulsion was immediately transferred to cold distilled water and the microcapsules were recovered by filtration trough 47 mm GA100 glass fibre filter (Advantec MFS Inc., Dublin, CA, USA) using a Büchner funnel.
Determination of microencapsulation yield
The total mass of the mixture of ingredients and the mass of every batch of microcapsules recovered after filtration were determined in a balance (Chyo Model JK-180, YMC Co, Tokyo, Japan).
Total yield was calculated from the formula:
Determination of the microencapsulation efficiency and oil content of microcapsules
Samples of accurately weighed microcapsule suspensions (between 2 and 3 g) within test tubes were subjected to ultrasound in an ultrasonic bath (Model KQ-50B, Kunshan Ultrasonic Instruments Co., Ltd., Kunshan, China) at 50 ° C and at the maximum frequency (50 Hz) for 60 min, in order to destroy the microparticles and release their oil content. The microcapsule debris was extracted with hexane to dissolve all the lipids present and the organic extracts obtained were evaporated overnight at room temperature to constant weight within a fume cabinet. Simultaneously, a similar amount of microcapsule suspension was dried to constant weight at 50 °C, under vacuum to determine dry weight (dw) of the microcapsules. The encapsulation efficiency was calculated from the equation:
The total oil content was determined from the equation:
Determination of size and size distribution of microcapsules
For determination of size distribution 10 samples were taken from each of the replicates of the LMO/caseinate microcapsules and analysed under the light microscope (Motic Model BA 310, Causeway Bay, Hong Kong) using a 100X augmentation. Images were captured with a digital camera (Canon EOS Rebel T3, 12.2 MPixeles, Canon Inc., Tokyo, Japan) and microcapsule size was determined from those images using the software Motic Images Plus 2.0 (Motic, Causeway Bay, Hong Kong). At least 200 microcapsules were measured from each replicate.
Determination of LMO concentration
The concentration of lemongrass oil in the different samples and experiments performed was determined by UV spectrophotometry in a Shimadzu UV-mini 1240 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at a wave length of 247 nm according to what has been described by Miron et al. (2012). An extinction coefficient of 0.0129 mg−1 l cm −1 was obtained from an 8 points calibration curve covering a range from 0 to 100 mg l−1 of LMO. This value was utilized to transform UV readings into LMO concentrations.
Controlled release of LMO from microcapsules
2 ml of microcapsules suspension within a dialysis bag (cut off of 12000 daltons, Thomas Scientific, Swedesboro, NJ, USA) were placed in 100 ml of a 50:50 ethanol:water within glass bottles. This solvent is a standard simulant (simulant D2) for migration studies according to CREU (2011). Samples were incubated in an orbital shaking incubator (Model NB-205, N-Biotek, Gyeonggi-do, Korea) set up at 25 °C and an agitation rate of 20 rpm. Aliquots of the solution were taken at different time intervals and the absorbance at 247 nm was measured in a Shimadzu UV-Mini 2140 spectrophotometer. After measuring the absorbance each sample was put back into the system in order to keep constant conditions throughout the experiment.
Film casting procedure
Films were prepared according to the method of Hambleton et al. (2009) with some modifications. The film-forming solution was prepared by dispersing 1 g of sodium alginate in distilled water at 50 °C for 30–45 min under magnetic stirring (Arex Hot plate stirrer, VELP Scientifica, Usmate, Italy) connected to a temperature controller (VTF digital termorregulator, VELP Scientifica, Usmate, Italy). 1 g of sorbitol was then added and left stirring until a homogeneous solution was obtained. After cooling down the mixture to 25 °C, the microencapsulated LMO was added and the mixture was homogenized at 5000 rpm for 5 min in an ultraturrax (Janke & Kunkel model T45S7, IKA Works GmbH & Co., Staufen, Germany). Finally, 0.02 g of calcium carbonate were also added and the mixture was made up to 100 ml. Aliquots of 40 ml were applied into 10 cm petri dishes and dried in an oven at 40 °C to constant weight (15–17 h).
Controlled release of LMO from films
The same procedure explained for microcapsules was utilized for measuring LMO release from films. The only difference was that, in this case, pieces of film of 2 × 2 cm were introduced within the dialysis bags to perform the release studies.
Kinetic analysis of LMO release from microcapsules and films
Release data from microcapsules and films were fitted to three different models that have been utilized to interpret experimental data: Higuchi (Siepmann and Peppas 2011), Peppas (Ritger and Peppas 1987a, b) and Weibull (Papadopoulou et al. 2006) according to the following equations.
| 1 |
| 2 |
| 3 |
Q = Mt/ M∞ where, Mt is defined as the quantity of LMO released at any time t, and M∞ is the maximum concentration that would be reached if all the LMO was released from the microcapsules. Applying those equations to the experimental data the different constants of each model were obtained and can be seen in Tables 2 and 3 respectively.
Table 2.
Kinetics parameters obtained from release curves of 2500 ppm microencapsulated lemongrass oil in ethanol:water 50:50
| Release Model | Regresion coefficient (r2) | Model constants | ||||
|---|---|---|---|---|---|---|
| Higuchi | Peppas | Weibull | ||||
| k | kP | n | a | b | ||
| Higuchi | 0.920 ± 0.014a | 0.028 ± 0.003c | ||||
| Peppas | 0.992 ± 0.005b | 0.128 ± 0.005 | 0.205 ± 0.005d | |||
| Weibull | 0.983 ± 0.008b | 0.160 ± 0.006 | 0.167 ± 0.004e | |||
Table 3.
Kinetics parameters obtained from release curves in ethanol:water 50:50 of films containing microencapsulated lemongrass oil at 1250, 2500 and 5000 ppm
| Release Model | Regression coefficient (r 2) | Model constants | ||||
|---|---|---|---|---|---|---|
| Higuchi | Peppas | Weibull | ||||
| k | k P | n | A | b | ||
| Higuchi | 0.906 ± 0.021a | 0.024 ± 0.003c | ||||
| Peppas | 0.901 ± 0.030a | 0.099 ± 0.011 | 0.311 ± 0.027f | |||
| Weibull | 0.916 ± 0.053a | 0.107 ± 0.013 | 0.351 ± 0.020g | |||
Antimicrobial activity against Escherichia coli
ATCC 25922 and Listeria monocytogenes ISP 65–08 The antimicrobial activity of the films containing microencapsulated LMO was tested against E. coli ATCC 25922 and L. monocytogenes ISP 65–08 in a 96 well plates utilizing a Multiscan Go device (Thermo Scientific, Waltham, MA USA). Alginate film pieces of the appropriate size (approximately 30 μl) were placed within each well along with 290 μl of concentrated (1.24X) Mueller Hinton broth. 40 μl of inoculum containing 1.5 × 10 5 UFC ml−1 of each bacterial strain were also added to each well before starting the incubation. Identical alginate films but containing microcapsules with no LMO inside were used to prepare the blank and positive control. The blank samples contained all the components except the bacterial inoculum which was replaced by 40 μl of 1X Mueller Hinton broth. Positive control were the same as the blanks but inoculated with 40 μl 1.5 × 105 UFC ml−1 of each bacterial strain instead of 40 μl of broth. Incubation was performed at 37 ° C for 24 h and bacterial growth was recorded as the optical density at 625 nm. All the experiments were run by quadruplicate.
Statistical analysis
To determine the statistical differences among the r2 values for the different models and the statistical differences between the model constants for microcapsules and films, an analysis of variance (ANOVA) was performed for a 95 % confidence interval. When statistical differences from ANOVA were significant (p < 0.05), differences between the respective means were determined using least significant difference (LSD) test and considered significant when p < 0.05. All statistical analyses were performed using Statgraphics Plus software version 5.1 (Statpoint Technologies Inc., Warrenton, VA, USA).
Results and discussion
Microencapsulation of LMO within sodium caseinate shell
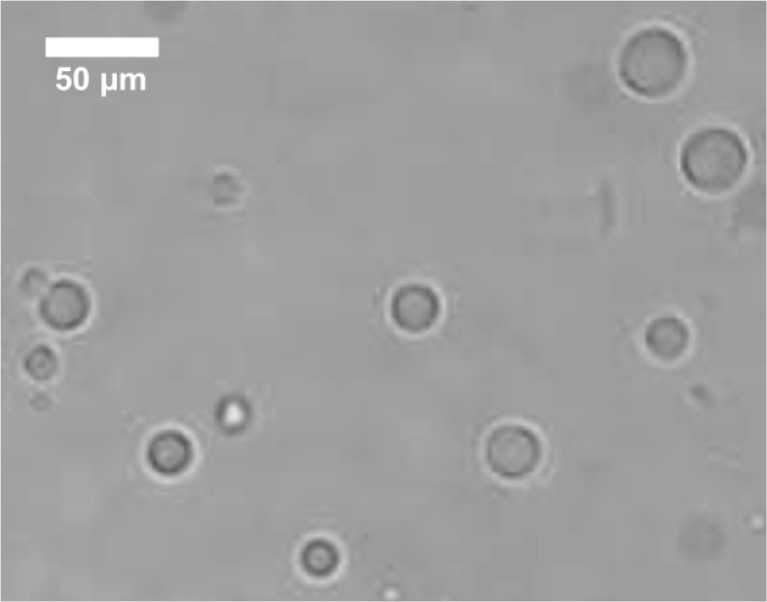
Lemongrass oil was successfully microencapsulated within a sodium caseinate shell. The size and morphology of the microcapsules can be observed in Fig. 1. The main properties of the microcapsules obtained are summarized in Table 1.
Fig. 1.
Photomicrography of lemongrass oil microcapsules under the light microscope (100X)
Table 1.
Main characteristics of lemongrass oil/caseinate microcapsules containing 2500 ppm
| Microcapsules properties | Value |
|---|---|
| Microcapsules diameter (μm) | 22.2 ± 5.4 |
| Lipid phase content (g 100 g−1) | 51.6 ± 3.2 |
| Total yield | 93.8 ± 4.3 |
| Microencapsulation efficiency | 97.3 ± 2.7 |
Mean ± standard deviation of 12 replicates
Average size and size distribution were not affected by LMO concentration (1250, 2500 and 5000 ppm) in the microcapsules (p > 0.05) and therefore data from Table 1 is applicable to all the microcapsules utilized in the experiments described in this paper.
There are very few publications about the microencapsulation of lemongrass oil. Weisheimer et al. (2010) studied the microencapsulation of LMO in β-cyclodextrin (β-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) by two different methods: spray drying and precipitation. The microparticles obtained had irregular shape and were between 5.5 and 66 μm in diameter, depending on the type of cyclodextrin and process applied. The authors utilized 100 % LMO in their preparation and the inclusion percentages of the resulting microparticles were between 23 a 60 %. Anitha et al. (2011) prepared LMO microcapsules in order to use them as mosquito repellent in textiles. Since the main goal of that study was the effectiveness of the repellent, no characterization of the physical properties of the microcapsules obtained was reported. However, from the light microscopy photograph (100X) in the article, a microcapsule size between 50 and 100 μm can be estimated. In the study by Leimann et al. (2009) utilizing poly(vinyl alcohol) as encapsulating agent, the size of the LMO microcapsules covered the range of 10 to 210 μm in average diameter, depending on the agitation rate of the reaction media and the LMO content of the microcapsules. The inclusion percentages reported by these authors were between 3.7 and 7.4 % by volume. The size of the lemongrass oil microcapsules obtained in this study was 22.2 μm with a range between 14 and 34 μm and the lipid content was above 51 %, which can be considered high when compared to literature.
Since the successful microencapsulation of a lipid active principle by the emulsification-separation process applied in this work is highly dependent on the physicochemical properties of the oil phase and the wall material, the present results can also be compared with microencapsulation studies where sodium caseinate was utilized to microencapsulate other lipids. In the study by Hogan et al. (2001a) on the microencapsulating properties of caseinate utilizing soy oil as core material, the size of the microcapsules obtained by the emulsification method was around 10 μm, but when pressure homogenization was additionally applied to the microcapsule suspension, sizes as low as 0.5 μm were obtained. After spray dying, most microcapsule systems increased their sizes up to a range of 14–32 μm in diameter. The oil contents were between 20 and 75 % depending on oil/protein ratio and homogenization speed. When carbohydrates were also included in combination with caseinate as wall materials for soy oil microencapsulation, micro-particle diameters were from 0.41 to 0.78 μm for the emulsions and increased to 13–32 μm in the dry powder. The oil content was also between 20 and 75 %, depending on the ratio of caseinate to carbohydrate utilized (Hogan et al. 2001b). Finally, it might also be interesting to analyse the microencapsulation of other bioactive essential oils such as thyme oil. In the work by Martins et al. (2009) thyme oil was microencapsulated within a polylactide shell by a coaservation methodology to produce microcapsules with diameters between 29 and 42 μm in diameter, with an oil content between 30 and 65 % depending on the type and concentration of surfactant utilized. According to what has been described in the literature for microencapsulation of essential oils, our system produces microcapsules with diameters within the range obtained in other publications and a total lipid content (51 %) better that what has been reported by Leimann et al. (2009), but there might still be room for improvements in both smaller microcapsule size and larger oil contents (Hogan et al. 2001b).
Lemongrass oil release kinetic from microcapsules
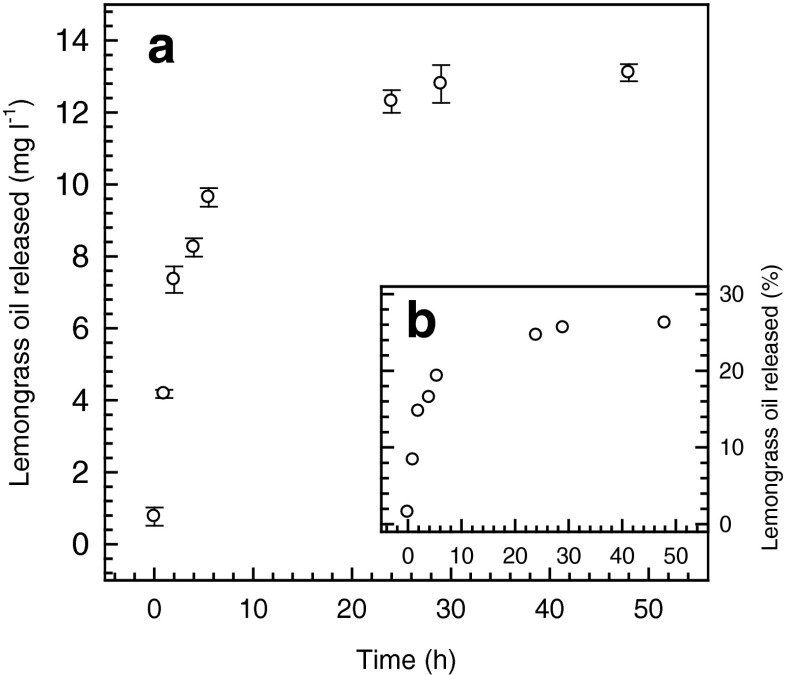
The release kinetics of lemongrass oil (Fig. 2) followed a typical exponential pattern in which a maximum concentration of around 13.1 mg l−1 was reached after approximately 40 h (Fig. 2a). At that time 26.2 % of the total LMO present was released into the medium.
Fig. 2.
Lemongrass oil (2500 ppm) release profile from caseinate microcapsules in ethanol:water 50:50, expressed as: a lemongrass oil concentration and b percentage of the total lemongrass oil content
Data from column 2 in Table 2 shows that the best regression coefficients were not statistically different (p < 0.05) between Peppas and Weibull models and, therefore, the release kinetics of LMO from caseinate microcapsules will be analysed on the basis of these two models. The value for the n exponent of the Peppas model was 0.205 (<0.43), which is within what is expected for the controlled release from spherical particles (Ritger and Peppas 1987a) that follow predominantly a fickian diffusion mechanism. In the Weibull model, the b constant is related to the release mechanism of the active principle. For the case of the LMO microencapsules, the b constant had a value of 0.167 (<0.35) which indicates that caseinate shell through which the migration of LMO components took place would behave as a highly disordered space very different to a percolation cluster model (Papadopoulou et al. 2006). Such structural concept is in good agreement with what was proposed by Fang and Dalgleish (1993) in relation the organization of casein at interfaces. These authors, applying a technique of enzymatic breakdown together with light scattering, concluded that it is possible for flexible proteins like caseins to adopt a number of different conformational states (disorder) when they are adsorbed to an oil–water interface.
Lemongrass oil release kinetic from films
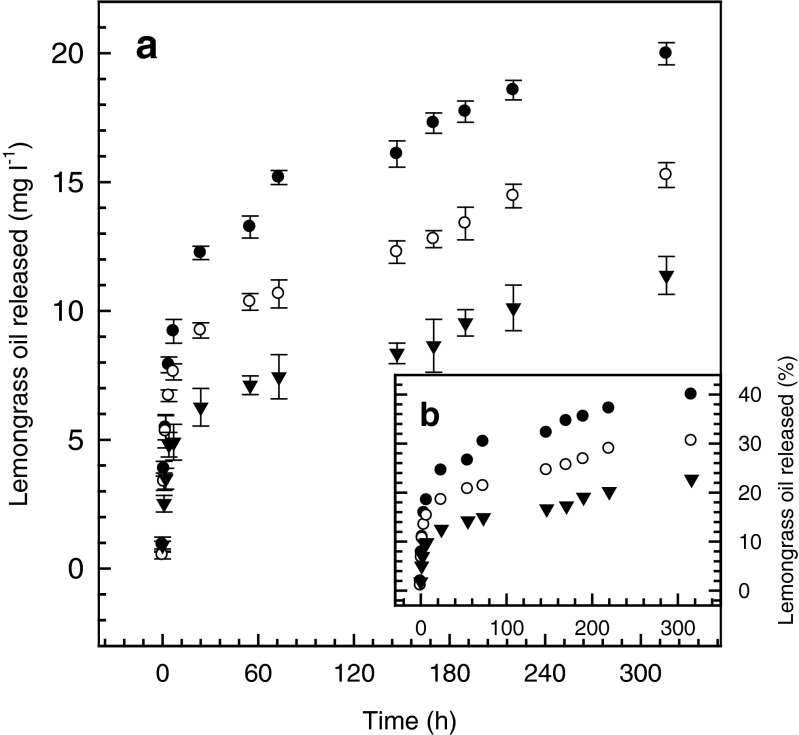
The release kinetics of microencapsulated LMO from films (Fig. 3) also followed an exponential pattern similar to the one observed for the microcapsules alone (Fig. 2). The maximum concentrations reached at the end of the release study were 11.4, 15.3 and 19.9 mg l−1 for the films containing 1250, 2500 and 5000 ppm of LMO, respectively. The fractions of LMO released were 22.7 %, 30.5 % and 39.9 % of the total amount initially present.
Fig. 3.
Release kinetics from films containing microencapsulated lemongrass oil, in ethanol:water 50:50. ▼ 1250 ppm, ○ 2500 ppm and ● 5000 ppm
When r2 values for the Higuchi model from Tables 2 and 3 are compared, there was no statistically significant difference (p > 0.05) between the degree of data correlation for microcapsules and films. No statistically significant difference in the k constant of the Higuchi model was found either and, therefore, this model would not be able to give account of any differences in the release behaviour of LMO from microcapsules and films. The values of r2 for release data correlation from films (Table 3) were smaller than r2 values of microcapsules alone (Table 2) for the Peppas and Weibull models and therefore, there was a better correlation between experimental data and mathematical models for microcapsules than for films. This result might not be unexpected since on the one hand, the film casting procedure introduces more variability to the system and, on the other hand, the alginate matrix of the films act as an additional barrier to the caseinate wall of the microcapsules and, therefore, the migration behaviour of the LMO components from films should also have a greater variability.
In relation to the model constants both the n exponent of the Peppas equation and the b exponent of the Weibull equation were statistically different (p < 0.05) between microcapsules and films (Tables 2 and 3), which suggest that the alginate matrix does have an effect on the release behaviour of the LMO components. For to the Peppas model, the value of the n exponent in both cases was in the zone indicative of a fickian release mechanism: n = 0.205 < 0.43 for microcapsule spheres, (Table 2); and n = 0.311 < 0.5 for thin films, (Table 3) (Siepmann and Peppas 2001). However, the most interesting findings come from the analysis of the b constant of the Weibull model since in the films, a b value greater than 0.35 was obtained and this means that the combined system of caseinate shell and alginate matrix would behaves as a fractal substrate morphologically similar to a percolation cluster. This system is more ordered (b = 0,351 > 0.35, Table 3) than the highly disordered matrix of the caseinate shell model mentioned before (b = 0.167 < 0.35, Table 2). Therefore, based on the meaning of the b constant of the Weibull model, the migration of the LMO components through the alginate matrix of the film suffers a slight modification with respect to what was observed in the caseinate shell of the microcapsules alone (Papadopoulou et al. 2006).
Antimicrobial activity of LMO films against Escherichia coli ATCC 25922 and Listeria monocytogenes ISP 65–08
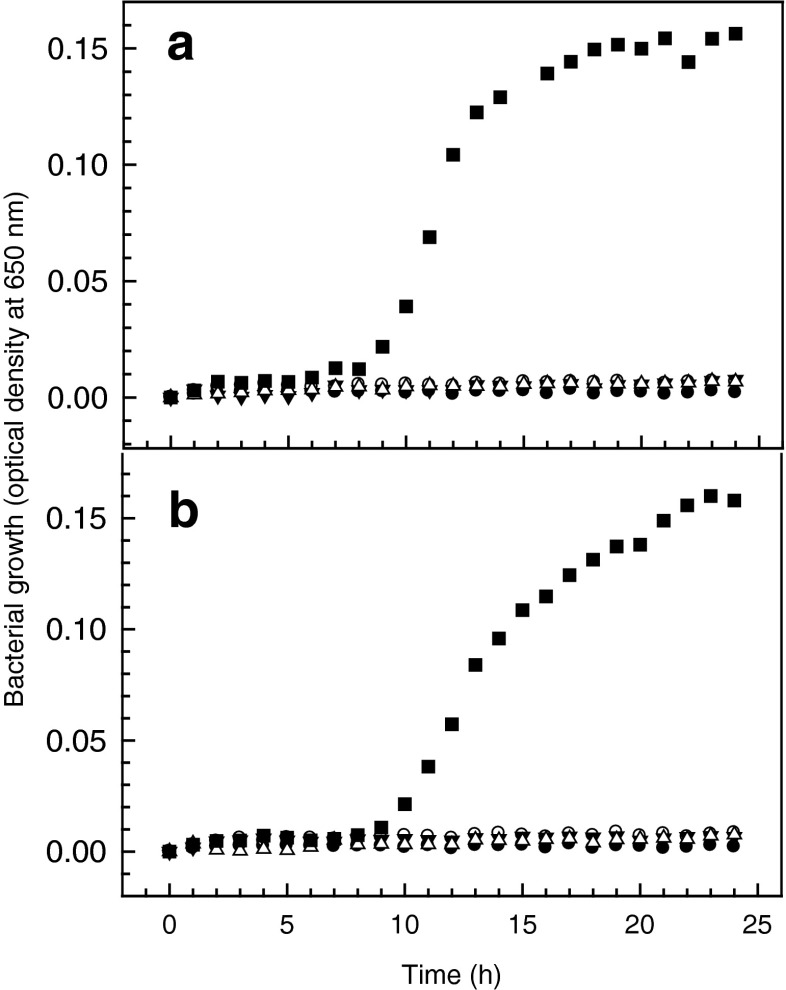
Lemongrass oil has been reported to have inhibitory capacity against several food borne pathogens when evaluated as the pure liquid by the traditional agar diffusion assay and inhibition zone quantitation. Vázquez-Sánchez et al. (2014) demonstrated the effectiveness of LMO against pathogenic Staphylococcus aureus at a minima inhibitory concentration (MIC) below 10 ppm. In that study, the antimicrobial capacity of LMO was only matched by thyme oil. In the study by Saddiq and Khayyat (2010), concentrations of 50 ppm were able to inhibit growth of a methicillin resistant Staphylococcus aureus (MRSA) strain. For Helicobacter pylori, the study of Ohno et al. (2003) with 13 essential oils demonstrated that lemongrass oil was the only able to inhibit growth even after 10 simultaneous passages, with a MIC of 4–18 ppm. However, in the study by Leimann et al. (2009), MIC values of about 2,000 and 20,000 ppm were informed for Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922, respectively. The reported information on LMO antimicrobial activity shows very clearly that its effectiveness is largely dependent on the specie of microorganism and, within the same specie, on the specific strain to be considered. Such a conclusion is in agreement with what has also been established for most of the natural components (polyphenols, polysaccharides, bacteriocins, etc.) assessed for antimicrobial activity (Kraśniewska and Gniewosz 2012). Even though essential oils in either, their natural state or as a liquid extract have been applied for many centuries as preserving agents in many cultures of the world, it is desirable to find ways of utilizing lemongrass oil and other essential oils on a larger scale. Edible films are one of the technologies through which many natural substances could be utilized on an industrial scale in a safe and reliable manner to extend shelf life on many fresh food products. The results of this work are a small step in that direction. Figure 4 shows that when incorporated into films microencapsulated LMO is able to inhibit the growth of L. monocytogenes and E. coli even at the lowest (1250 ppm) concentration in the film. From Fig. 2, it can be observed that after around 10 h of incubation a concentration of around 5 ppm is reached in the medium at any of the three LMO concentrations in the films assessed. This LMO concentration is compatible with the MIC of several of the pathogenic bacteria mentioned above that have been described as sensitive to this essential oil; therefore, these films have the potential to be applied to pathogenic microorganisms other that the ones considered in this work; for example: E. coli O157:H7 (Rojas-Graü et al. 2006; Maizura et al. 2007) and Salmonella enteritidis (Maizura et al. 2008).
Fig. 4.
Growth inhibition of a L. monocytogenes and b E. coli by films containing different concentrations of microencapsulated lemongrass oil. ● Blank, ■ bacteria, ▼ bacteria + 1250 ppm film, ○ bacteria + 2500 ppm film and, ∆ bacteria + 5000 ppm film; n = 4)
On the light of this results, the next steps to follow should be producing larger quantities of films in a reliable and reproducible manner and test its antimicrobial properties against the greatest number and wider variety of microorganism. We should also optimize the physical properties of the films in order to be applied to real food products such as fresh fish, chesses and fresh meat to extend shelf life.
Conclusions
Lemongrass oil dissolved in soy oil at concentrations between 1250 and 5000 ppm can be microencapsulated within a caseinate wall to produce homogeneous and stable microparticles with a diameter of 22.2 μm and an average oil content of 51 %. Lemongrass oil components can be released to an ethanol:water 50:50 medium following a kinetic that fit to the Peppas and Weibull models with r2 values greater than 0,98.
Microcapsules can be introduced into alginate edible films retaining their controlled release properties. Experimental data for the release of LMO from films correlated well with both Peppas and Weibull models; however, since the b constant of the Weibull model give more information about the microstructure of the migration media, only this model was able to reflect the effect that alginate gel had on the release behaviour of microencapsulated LMO components.
Lemongrass oil films were able to inhibit growth of Escherichia coli ATCC 25922 and Listeria monocytogenes ISP 65–08 in liquid medium at any of the concentrations evaluated and, therefore there could be potential for practical application of these films for shelf life extension of some fresh fatty food such as fish, meat or cheese.
Acknowledgments
The authors thank the financial support of the Chilean National Commission of Science and Technology (CONICYT), through the Research Grant FONDECYT N° 1131017.
Footnotes
Research highlights
1. Lemongrass oil was microencapsulated within a sodium caseinate wall to generate microparticles of 22 μm in diameter containing 51 % oil by weight.
2. Microencapsulated lemongrass oil was introduced into alginate films and their controlled released kinetics data were fitted to Higuchi, Peppas and Weibull models.
3. The Weibull model was the best suited for the study of these systems since it allows that the microstructural differences between the caseinate shell of the microcapsules and the alginate matrix of the film can be expressed in the value of the b exponent of the model equation.
4. Films containing microencapsulated lemongrass oil were able to inhibit growth of Escherichia coli ATCC 25922 and Listeria monocytogenes ISP 65–08 in liquid cultures.
References
- Anitha R, Ramachandran T, Rajendran R, Mahalakshmi M. Microencapsulation of lemon grass oil for mosquito repellent finishes in polyester textiles. Elixir Bio Phys. 2011;40:5196–5200. [Google Scholar]
- Bustos R, Romo L, Yanez K, Daz G, Romo C. Oxidative stability of carotenoid pigments and polyunsaturated fatty acids in microparticulate diets containing krill oil for nutrition of marine fish larvae. J Food Eng. 2003;56:289–293. doi: 10.1016/S0260-8774(02)00272-8. [DOI] [Google Scholar]
- Cagri A, Ustunoi Z, Ryser ET. Antimicrobial, mechanical, and moisture barrier properties of low pH whey protein-based edible films containing aminobenzoic or sorbic acids. J Food Sci. 2001;66:865–870. doi: 10.1111/j.1365-2621.2001.tb15188.x. [DOI] [Google Scholar]
- CREU (2011) Commission regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance. Off J Eur Union L 12/89
- Fang Y, Dalgleish DG. Dimensions of the adsorbed layers in oil-in-water emulsions stabilized by caseins. J Colloid Interface Sci. 1993;156:329–334. doi: 10.1006/jcis.1993.1120. [DOI] [Google Scholar]
- Guilbert S, Cuq B, Gontard N. Recent innovations in edible and/or bidegradable packing materials. Food Addit Contam. 1997;6:741–751. doi: 10.1080/02652039709374585. [DOI] [PubMed] [Google Scholar]
- Hambleton A, Debeaufort F, Bonnotte A, Voilley A. Influence of alginate emulsion-based films structure on its barrier properties and on the protection of microencapsulated aroma compound. Food Hydrocoll. 2009;23:2116–2124. doi: 10.1016/j.foodhyd.2009.04.001. [DOI] [Google Scholar]
- Han C, Zhao Y, Leonard SW, Traber MG. Edible coating improve storability and enhance nutritional value of fresh strawberries (Frogaria ananassa) and raspberries (Rubus idenus) Postharvest Biol Technol. 2004;33:67–78. doi: 10.1016/j.postharvbio.2004.01.008. [DOI] [Google Scholar]
- Hogan SA, McNamee BF, O’Riordan ED, O’Sullivan M. Microencapsulating properties of sodium caseinate. J Agric Food Chem. 2001;49:1934–1938. doi: 10.1021/jf000276q. [DOI] [PubMed] [Google Scholar]
- Hogan SA, McNamee BF, O’Riordan ED, O’Sullivan M. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blends. Int Dairy J. 2001;11:137–144. doi: 10.1016/S0958-6946(01)00091-7. [DOI] [Google Scholar]
- Jiménez M, Fabra J, Talens P, Chiralt A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr Polym. 2010;82:585–593. doi: 10.1016/j.carbpol.2010.05.014. [DOI] [Google Scholar]
- Kraśniewska K, Gniewosz M. Substances with antibacterial activity in edible films – a review. Pol J Food Nutr Sci. 2012;62:199–206. [Google Scholar]
- Leimann FV, Gonçalves OH, Machado RAF, Bolzan A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater Sci Eng C. 2009;29:430–436. doi: 10.1016/j.msec.2008.08.025. [DOI] [Google Scholar]
- Maizura M, Fazilah A, Norziah MH, Karim AA. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch-alginate edible film containing lemongrass oil. J Food Sci. 2007;72:C324–C330. doi: 10.1111/j.1750-3841.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- Maizura M, Fazilah A, Norziah MH, Karim AA. Antibacterial activity of modified sago starch-alginate based edible film incorporated with lemongrass (Cymbopogon citratus) oil. Int Food Resh J. 2008;15:233–236. [Google Scholar]
- Martins IM, Rodrigues SN, Barreiro F, Rodrigues AE. Microencapsulation of thyme oil by coacervation. J Microencapsul. 2009;26:667–675. doi: 10.3109/02652040802646599. [DOI] [PubMed] [Google Scholar]
- Mauer LJ, Smith DE, Labuza TP. Water vapor permeability, mechanical, and structural properties of edible P-Casein films. Int Dairy J. 2000;10:353–358. doi: 10.1016/S0958-6946(00)00061-3. [DOI] [Google Scholar]
- Miron D, Battisti F, Ten C, Mayorga P, Scherman E. Spectrophotometric simultaneous determination of citral isomers in cyclodextrin complexes with partial least squares supported approach. Curr Pharm Anal. 2012;8:401–408. doi: 10.2174/157341212803341735. [DOI] [Google Scholar]
- Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, Kodama T, Kashima K, Imanishi J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- Olorunnisola SK, Asiyanbi HT, Hammed AM, Simsek S. Biological properties of lemongrass: an overview. Int Food Res J. 2014;21:455–462. [Google Scholar]
- Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309:44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Pavlath AE, Orts W. Edible films and coatings: why, what, and how? In: Embuscado ME, Huber KC, editors. Edible films and coatings for food applications. New York: Springer; 2009. pp. 1–23. [Google Scholar]
- Quirós-Sauceda AE, Ayala-Zavala JF, Olivas GI, González-Aguilar GA. Edible coatings as encapsulating matrices for bioactive compounds: a review. J Food Sci Technol. 2014;51:1674–1685. doi: 10.1007/s13197-013-1246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- Rojas-Graü MA, Avena-Bustillos RJ, Friedman M, Henika PR, Martín-Belloso O, Mchugh TH. Mechanical, barrier, and antimicrobial properties of apple puree edible films containing plant essential oils. J Agric Food Chem. 2006;54:9262–9267. doi: 10.1021/jf061717u. [DOI] [PubMed] [Google Scholar]
- Saddiq AA, Khayyat SA. Chemical and antimicrobial studies of monoterpene: citral. Pestic Biochem Physiol. 2010;98:89–93. doi: 10.1016/j.pestbp.2010.05.004. [DOI] [Google Scholar]
- Siepmann J, Peppas NA. Modelling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–157. doi: 10.1016/S0169-409X(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA. Higuchi equation: derivation, applications, use and misuse. Int J Pharm. 2011;418:6–12. doi: 10.1016/j.ijpharm.2011.03.051. [DOI] [PubMed] [Google Scholar]
- Singh BR, Singh V, Singh RK, Ebibeni N. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int Res J Pharm Pharmacol. 2011;1:228–236. [Google Scholar]
- Starliper CE, Ketola HG, Noyes AD, Schill WB, Henson FG, Chalupnicki MA, Dittman DE. An investigation of the bactericidal activity of selected essential oils to Aeromonas spp. J Adv Res. 2015;6:89–97. doi: 10.1016/j.jare.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taweechaisupapong S, Ngaonee P, Patsuk P, Pitiphat W, Khunkitti W. Antibiofilm activity and post antifungal effect of lemongrass oil on clinical Candida dubliniensis isolate. S Afr J Bot. 2012;78:37–43. doi: 10.1016/j.sajb.2011.04.003. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci. 2014;79:R1231–R1249. doi: 10.1111/1750-3841.12492. [DOI] [PubMed] [Google Scholar]
- Vázquez-Sánchez D, Cabo ML, Rodríguez-Herrera JJ. Antimicrobial activity of essential oils against Staphylococcus aureus biofilms. Food Sci Technol Int. 2014 doi: 10.1177/1082013214553996. [DOI] [PubMed] [Google Scholar]
- Weisheimer VD, Miron CB, Silva SS, Guterres EE, Schapoval S. Microparticles containing lemongrass volatile oil: preparation, characterization and thermal stability. Pharmazie. 2010;65:885–890. [PubMed] [Google Scholar]
- Yang L, Paulson AT. Mechanical and water vapor properties of edible gellan films. Food Res Int. 2000;33:63–570. doi: 10.1016/S0963-9969(00)00024-7. [DOI] [Google Scholar]