Abstract
The effect of seven drying treatments (sun, shade, oven 60 °C, oven 80 °C, oven 100 °C, microwave and freeze-drying) were evaluated with respect total flavonoid (TFC), phenolic (TPC), antioxidant activity, vitamin C and color characteristics of green tea. In general, drying increased antioxidant activity, TPC, TFC and chlorophyll content, while it led to a decrease in vitamin C. The highest TPC (209.17 mg Gallic acid/gdw) and TFC (38.18 mg Quercitin/gdw) were obtained in oven drying at 60 and 100 °C, respectively. Among methods, oven drying at 60 °C revealed the highest radical scavenging activity (IC50 = 167.166 μg/ml), while microwave showed the lowest one (IC50 = 505.5 μg/ml). Similar trend was also observed in reducing power assay. The highest vitamin C (16.36 mg/100gDM) and Chlorophyll a (17.35 mg/l) were obtained in freeze drying. Finally, sun and freeze drying methods were considered as the least and the most desirable drying methods, respectively the final color of green tea leaves.
Keywords: Green tea, Drying, Temperature, Quality, DPPH
Introduction
Tea is one of the most popular beverages in the world (Jun 2009). It (Camellia sinensis or C.assamica) is used in different parts of the world as green, black or oolong. Among these, the most remarkable positive effects on human health have been observed by consumption of green tea (Armoskaite et al. 2011). The differences between green and black tea are in their production process. To produce green tea, freshly harvested leaves are immediately steamed or heated to destroy the enzymes responsible for breaking down the color pigments in the leaves and to allow the tea to maintain its green color during drying. These processes preserve natural polyphenols with respect to their health-promoting properties (Mazzanti et al. 2009; Komes et al. 2010; Armoskaite et al. 2011).
In recent years, much attention has been paid to the quality of foods during drying. The need for high quality dried food products has been increased all over the world. The main purpose of drying is increasing the products shelf life; minimize packaging requirements and reducing the bulk weight. Drying process increases the shelf life by slowing or stopping microorganisms’ growth and preventing certain biochemical reactions that might alter the organoleptic characteristics (Rahimmalek and Goli 2013). Furthermore, there is growing interest to find naturally occurring antioxidants for consumption in foods or medicinal materials to replace synthetic antioxidants, which are being restricted due to their carcinogenicity (Stankovic et al. 2012; Katalinic et al. 2012).
Natural drying (drying in the shade or in the sun) and hot air drying are still the most widely used methods because of their lower cost. Natural drying has many disadvantages due to the inability to control large quantities and to achieve consistent quality standards. Conventional air drying is one of the most frequently used operations for food dehydration. Total products show low porosity and high apparent density. Significant color changes occur during air drying and the final product has low sorption capacity (Hamrouni-Sallami et al. 2013). In addition, hot air drying presents some benefits such as low energy efficiency and lengthy drying time during the last stage of drying. Furthermore, in comparison to hot air drying, microwave drying techniques can greatly reduce the drying time of biological materials without quality degradation. Recently, microwave drying has gained popularity as an alternative drying method for a variety of food products such as fruits, vegetables, snack foods and dairy products (Hamrouni-Sallami et al. 2011). Freeze drying is one of the most advanced drying methods, which provides dry products with porous structure combined with small or negligible shrinkage, superior flavor and aroma retention and improved rehydration behavior compared to products of the alternative drying processes. As a result, by choosing a suitable drying method and appropriate conditions, the final product quality can be handled (Hamrouni-Sallami et al. 2013).
Green tea contains many valuable compounds such as phenolics, flavonoids (catechins), amino acids, minerals, vitamins (B, C, E), xanthic bases (caffeine, theophylline), pigments (chlorophyll, carotenoids) and volatile compounds (aldehydes, alcohols, esters, lactones, hydrocarbons) (Armoskaite et al. 2011). It is worth noting that, numerous epidemiological studies have linked these tea flavonoids with many human health benefits such as prevention of cancers, cardiovascular diseases, microbial diseases, diabetes and obesity (Vuong et al. 2011; Prathapan et al. 2011) . The tea flavonoids have also been found to prevent lipid peroxidation, a major problem in food industry, which can cause unfavorable rancidity and potentially toxic reaction in products (Vuong et al. 2011).
Most of the previous researches on green tea were carried out on limited drying methods (Hirun et al. 2014). Furthermore, drying treatments can affect nutritional and qualitative characteristics of green tea such as vitamins, color, chlorophyll, total flavonoid, total phenolic content and antioxidant activity of final product, but in most previous researches one or two qualitative aspects were studied. Moreover, to the best of our knowledge, no comprehensive reports were available on the variations in vitamin C, color characteristics, chlorophyll, total flavonoid, total phenolic content and antioxidant activity of green tea under different drying methods. Therefore, the aims of this research were (1) to study the effect of seven drying methods (Sun drying, shade drying, oven drying in 100, 80 and 60 °C, freeze drying and microwave) with respect to vitamin C, total phenolic, flavonoid and chlorophyll content, (2) to assess the color characteristics of dried samples based on L*, a*, b*parameters and (3) to compare the antioxidant activity of samples in different drying conditions.
Materials and methods
Plant material
Green tea leaves obtained from tea farms in Lahijan City in the northern region of Iran in the fall growing season. In order to preserve them from enzymatic browning, the leaves were blanched with steam at 90 °C for 1 min.
Drying methods
The leaves were dried using one of the following methods: (a) air drying at shade and room temperature (25 °C); (b) sun drying; (c) drying in a hot air oven at 60 °C; (d) drying in a hot air oven at 80 °C; (e) drying in a hot air oven at 100 °C; (f) drying in a microwave oven at 800 W; (g) freeze drying. Shade drying was carried out under natural air flow and surrounding’s temperature (mean temperature = 25 °C) for 36 h. In the case of sun drying, leaves were dried into trays under direct sunlight at temperatures between 30 and 35 °C for 7.5 h in November in Isfahan, Iran. Oven drying was conducted in a ventilated oven (Osk, Japan) at three different temperatures (60, 80 and 100 °C). Microwave drying was performed in a domestic digital microwave oven (Nikai, NMO-518 N, Japan) with technical features of 230 V, 800 W. The samples were dried for 240 s. Freeze-drying was carried out in HeltoHolten DW8 freeze dryer. The plant materials were frozen in −80 °C for 24 h and dried under vacuum and the condenser temperature at −15 °C for 24 h.
Color measurement
Five grams of each method were ground in a coffee grinder for 10 s to produce a powder of a uniform color. The samples were transferred to a 10 cm petri dish and subsequently read by a spectrophotometer Texflash (Datacolor, Swiss). The colorimeter was calibrated against a standard calibration plate of a white surface and set to CIE Standard Illuminant C. The L*, a* and b* values were determined and averaged from three readings. The color brightness, L* measures the whiteness value of a color and ranges from black at 0 to white at 100. The chromaticity coordinate a* measures red when positive and green when negative, and the chromaticity coordinate b* measures yellow when positive and blue when negative (Arslan and Ozcan 2008).
Chlorophyll content
One hundred mg of material was ground in a mortar with 4 mL of 80 % acetone. The ground material was collected in a 15 mL falcon tube. The mortar was rinsed with 4 ml of acetone. The volume of the falcon was adjusted to 10 ml with acetone. The falcons were kept in ice and dark environment. The samples were centrifuged at 3000 rpm (Sigma, Germany) for 10 min at 4 °C. Immediately after centrifugation, the falcons were transferred to ice before rapid measurement with spectrophotometer. The absorbance of the solution was read at three wavelengths including 663.2, 646.8 and 470 nm. Chlorophyll concentrations were calculated in mg/l of acetone according to the formula:
Vitamin C content
Ascorbic acid (AA) was determined by titration method using 2,6-dichlorophenol–indophenols according to AOAC method No. 967.21 (AOAC, 2000). The vitamin C content in fresh and dried samples was expressed as mg AA/100 g wet basis.
Total phenolic content
2.5 g of the finely ground sample was extracted with 50 ml methanol 80 % in falcon and was shacked in 240 rpm for 24 h. The sample was filtered twice with Whatman 0.2 μm. Total phenolic content (TPC) was determined using Folin-Ciocalteu method (Pinelo et al. 2004). 500 μl of diluted extraction (500 μl primary extraction was adjusted in the volume of 50 ml methanol 80 % for producing 1/250 dilution), 2.5 ml Folin-Ciocalteu reagent and 2 ml of 7.5 % sodium carbonate were also mixed. After heating at 45 °C for 15 min, the absorbance was measured at 765 nm against methanol 80 % as blank. TPC was expressed as Gallic acid equivalent/g dry weight of sample. The calibration equation for Gallic acid was obtained as y = 17.422x + 0.0872 (R2 = 0.998), where x is the absorbance and y is the concentration of Gallic acid in mg/l.
Total flavonoid content of the extracts
Total flavonoid content was determined spectrophotometrically using a method based on the formation of a complex flavonoid–aluminum, with some modifications. An aliquot (0.5 ml) of the extract solution was mixed with distilled water (2 ml) and subsequently, with NaNO2 solution (5 %, 0.15 ml). After 6 min, AlCl3 solution (10 %, 0.15 ml) was added and allowed to stand for further 6 min; thereafter, NaOH solution (4 %, 2 ml) was added to the mixture. Immediately, distilled water was added to bring the final volume to 5 ml. Then, the mixture was properly mixed and allowed to stand for 15 min. The intensity of pink color was measured at 510 nm.
DPPH radical-scavenging activity
The ability of the plant extract and reference substance to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals was assessed using the method described by Stankovic et al.(2012). The stock solution of the plant extract was prepared in methanol 80 % to achieve the Phenolic concentration of 5, 3, 1 and 0.5 mg/ml. Dilutions were made to obtain Phenolic concentrations of 500, 300, 100 and 50 μg/ml (ppm). 100 μl of diluted solutions was mixed with 5 ml of DPPH methanolic solution (0.1 mM) and was adjusted to the volume of 10 ml with methanol 80 %. After 30 min in darkness at room temperature (23 °C), the absorbance was recorded at 517 nm. The control samples contained all the reagents except the extract. The percentage inhibition was calculated using equation: % inhibition = 100 × (A of control − A of sample)/A of control, while concentrations of extracts required to inhibit radical by 50 % (IC50) values were estimated from the percentage of inhibition versus concentration of sigmoid curve, using a non-linear regression analysis. The data were presented as mean values ± standard deviation (N = 2) (Arslan and Ozcan 2012).
Reducing power
The extracts (2.5 ml) and Butylated hydroxytoluene (BHT) were mixed with 2.5 ml of 1 % potassium ferricyanide and 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and were incubated at 50oC for 20 min. Then, 2.5 ml of 10 % trichloroacetic acid was added, and the mixture was centrifuged at 200 g for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of deionized water and 0.5 ml of 0.1 % ferric chloride. The absorbance at 700 nm was measured against a blank. The increased absorbance of the reaction mixture correlates with greater reducing power (Ardestani and Yazdanparast 2007).
Statistical analysis
Cluster analysis and calculation of correlations among the compounds were performed using SPSS ver.11 based on Ward’s minimum variance method (Ward 1963). Cluster analysis was conducted to classify the drying methods based on their similarity of the major compounds. Statistic ver. 8 was also used to evaluate. The significance of data was calculated using SAS ver. 8.
Results and discussion
Total flavonoid and Phenolic content
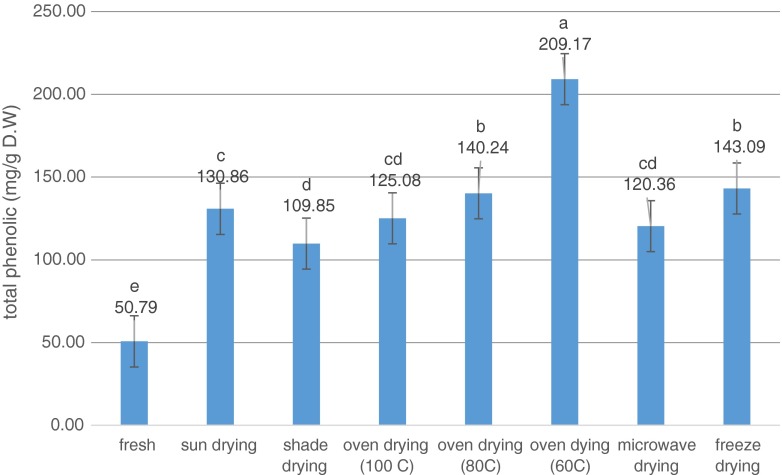
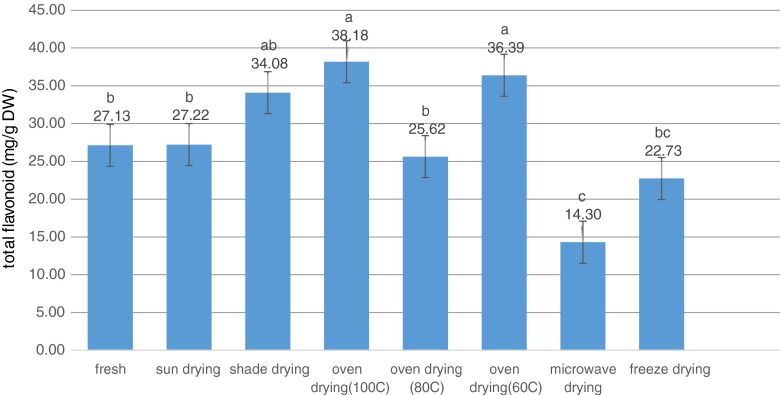
The antioxidant (phenolic compounds, vitamins, etc.) contents of fresh plant materials were higher than those of dried plant materials due to their degradation during drying, while some recent studies have shown that dried plant materials contain higher antioxidants such as polyphenolics, and antioxidant activity as compared to fresh plant materials (Pinela et al. 2012). Drying process can break down the cellular constituents leading to accelerate in releasing phenolic compounds from the food matrix (Chang et al. 2006; Arslan and Ozcan 2010). Another possibility concerning the low level of phenolic compounds in the fresh sample was the presence of an active enzyme in the sample that probably causes these compounds to be depredated. In the dried samples, due to the low water activity, destructive enzymes were inactivated and high levels of phenolic compounds remained in the extract (Hassain et al. 2010). In the present study, the highest total phenolic content (TPC) was obtained in oven 60 °C (216.16 mg Gallic acid/gdw), while the lowest one belonged to shade drying (109.85 mg Gallic acid/gdw) (Fig. 1). Similar to the present study, oven drying method possessed the highest amount of phenolic compounds among other methods as reported by Pinela et al. (2012). Rabeta and Lai were also reported that vacuum dried samples had the highest TPC, followed by freeze dried and fresh samples in Ocimum tenuiflorum (Rabeta and Lai 2013). TPC decreased in higher temperatures (80 and 100 °C). Total flavonoid content (TFC) also ranged from 14.3 mg Quercitin/gdw in microwave drying to 38.18 mg Quercitin/gdw in oven 100 °C.
Fig. 1.
Total phenolic content (TPC) variation in green tea using seven drying treatments. Bars with different letters are significantly different
Previous studies also reported the high amount of TFC in green tea compared to other types (Fig. 2). This can be due to more oxidation and polymerization by enzymes derived from tea leaves during the production of oolong and black tea from green tea by fermentation (Bae et al. 2015). Furthermore, previous reports have shown that temperature more than 50 °C leads to a decrease in essential oil yield and total phenolic content of different plant species (Braga et al. 2005; Rezaeinodehi and Khangholo 2008; Rabeta and Lai 2013). Prathapan et al. (2009) studied the effect of heat treatment (60–100 °C) on TPC, color value (yellowishness and brightness), polyphenol oxidase (PPO) activity and curcuminoid of fresh turmeric rhizome. In their study, TPC values increased gradually when samples were heated from 60 to 80 °C. Moreover, PPO was completely inactivated at 80 °C (Prathapan et al. 2009).
Fig. 2.
Total flavonoid content (TFC) variation in green tea using seven drying treatments. Bars with different letters are significantly different
Generally, plants are often dried and stored for a long time before being used in manufacturing various types of products. Dehydration is an important preservation method for plant materials, as it inhibits enzymatic degradation and minimizes microbial growth of plants (Pinela et al. 2012). Freeze-drying also maintained a high amount of total phenolics. In most previous studies, freeze-drying was considered as one of the appropriate methods to preserve the quality of plants during processing (Arslan and Ozcan 2008, 2012; Annegowda et al. 2014). Chan et al. (2009) studied the effects of five different drying methods (microwave, oven, and sun drying) on antioxidant properties of Alpinia zerumbet, Etlinger aelatior, Curcuma longa and Kaempferia galangal. Air drying of ginger leaves resulted in significant losses in total phenolic content and antioxidant activity for all four species (Chan et al. 2009). Drying process can break down the cellular constituents leading to acceleration in releasing phenolic compounds from the food matrix (Chang et al. 2006; Arslan and Ozcan 2010). Another possibility about the low level of phenolic compounds in the fresh sample is the presence of an active enzyme in the sample that probably causes these compounds to be depredated. In the dried samples, due to the low water activity, destructive enzymes are inactivated and high levels of phenolic compounds will remain in the extract (Hassain et al. 2010).
During drying, metabolically active plants would slowly lose their moisture and might sense the moisture loss as stress. Plants, in general, produce phenolic compounds as a defense mechanism in response to stress. It is reported that the synthesis of several phenylpropanoid compounds (flavonoids, isoflavonoids, psoralens, coumarins, phenolic acids, lignin and suberin) was induced in plants in response to biotic and abiotic stress, such as wounding, low or high temperature and by attacks pathogens (Dixon and Paiva 1995).
DPPH assay
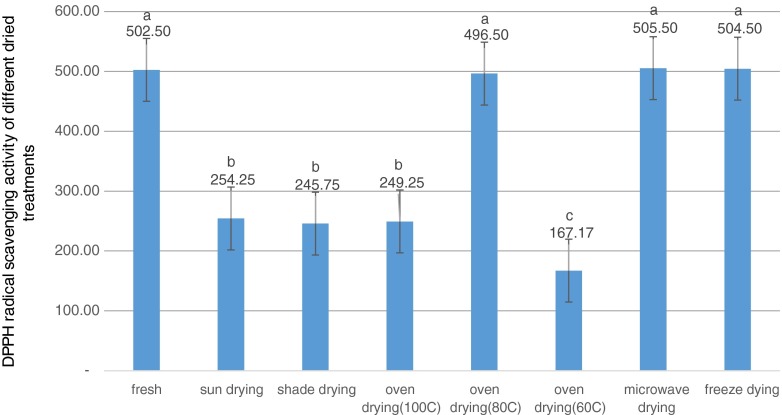
The ability of different extracts of tea drying treatments to quench DPPH free radical was measured. The extracts and BHT demonstrated a dose-dependent scavenging activity by reducing DPPH radical (Fig. 3). By plotting the graph of extract concentrations against the scavenging activity, a specific concentration of the sample that needed to provide 50 % inhibition (IC50) was calculated. Among treatments, oven drying at 60 °C revealed the highest radical scavenging activity (the lowest IC 50 = 167.166 μg/ml), while the microwave treatment showed the lowest scavenging activity (IC 50 = 505.5 μg/ml). Previous researches showed that the radical scavenging activity was elevated at higher drying temperatures using oven drying treatments (Lee Mei Ling et al. 2013; Rodriguzer et al. 2014). It is believed that the high total phenolic content might contribute to the high antioxidant activity by high temperature drying (Lou et al. 2015).
Fig. 3.
DPPH radical scavenging activity of different dried treatments in green tea. Bars with different letters are significantly different
Reducing power
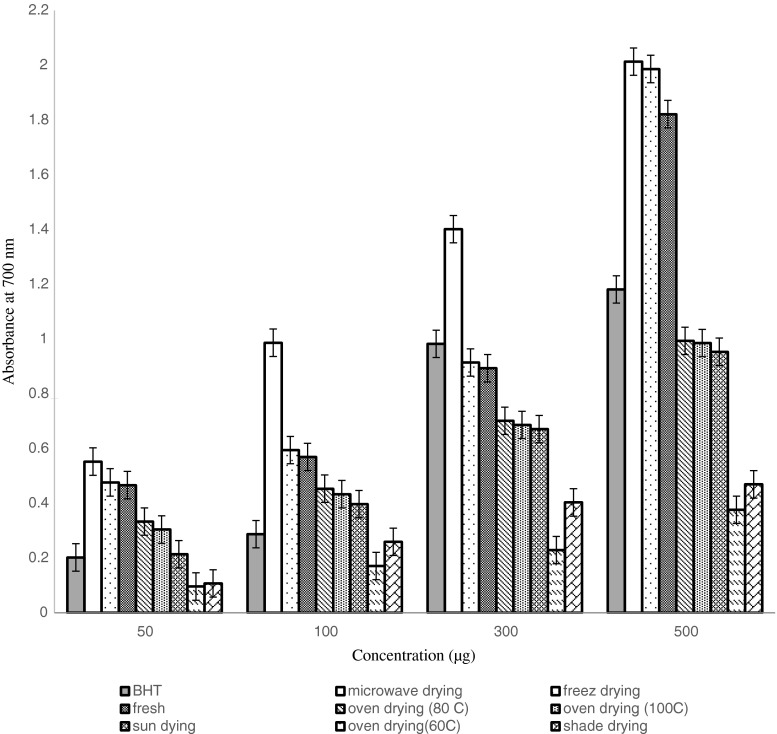
The ability of the antioxidant fraction to reduce Fe+3 to Fe+2 represents the reductive power of the antioxidant (Ardestani and Yazdanparast 2007). Color changes of the solution in the presence of the methanolic extract of green tea and control antioxidant were evaluated. Reducing capacities of methanolic extract of green tea are shown in Fig. 4. The reducing power of the extracts and the standard was elevated by increasing the concentration of the samples. Similar to DPPH assay, in FTC model system, the strongest reducing power was obtained in microwave drying method, while the weakest activity was observed in oven drying at 60 °C.
Fig. 4.
Reducing power of different dried treatments in green tea. Bars with different letters are significantly different
Reducing capacity of an extract can serve as an indicator of its potential antioxidant activity. However, the antioxidant activity of antioxidants could be attributed to different mechanisms (Yildirım et al. 2000). For example, DPPH assay is mostly attributed to such phenolic compounds that participate in scavenging free radicals, while in reducing power the inactivation of the metal ions such as ferric ions is evaluated by phenolics (Gharibi et al. 2013).
Vitamin C
Besides phenolic and flavonoid compounds, tea provides a significant dietary source of polysaccharides, vitamin B complex, vitamin C, vitamin E and fluoride, as well as trace elements such as K, Mn, Cr, Ni and Zn (Komes et al. 2010). As shown in Fig. 2, the results revealed that different drying methods led to a decrease in vitamin C in the green tea leaves. Ascorbic acid (vitamin C) is considered an important nutrient in fruits and vegetables. It is usually selected as a parameter of nutritional value because of its labile nature compared to other nutrients in foods. The degree of vitamin C loss during drying vegetables depends on the physical properties of the product and the type of process (Goula and Adamopoulos 2010). In the fresh leaves, vitamin C content was 17.72 mg/100gDM. During drying, a significant decrease was observed in the ascorbic acid content (Fig. 5). The highest vitamin C in dried leaves was obtained in freeze drying (16.36 mg/100gDM) followed by microwave drying (15.99 mg/100gDM) (P < 0.05). Two factors affect the vitamin C content in final products. Vitamin C oxidizes and disappears in high temperatures and long drying times (Vega-Galveza et al. 2009; Jin et al. 2014). In microwave method the amount of vitamin C was notably high. This might be due to the short drying time. Previous reports also illustrated that short drying time and lower temperature can maintain ascorbic acid (Negi and Roy 2000).
Fig. 5.
Vitamin C content of green tea in different drying treatments. Bars with different letters are significantly different
Chlorophyll content
The color of green vegetables is mainly determined by the chlorophyll pigments available in plant materials, which to catch the energy from the sun light. In most cases, color changes are reflected in the chemical, biochemical and microbiological reactions. Temperature and relative humidity during drying are among the reasons leading to color degradation in dehydrated products. However, the visual appearance of dehydrated product is very important for shopping (Shahabi et al. 2014). Furthermore, previous reports highlighted that in green tea leaves and non-fermented teas, chlorophyll is considered an important pigment as its amount determines thefinal color of green tea infusion (Ostadalova et al. 2014).
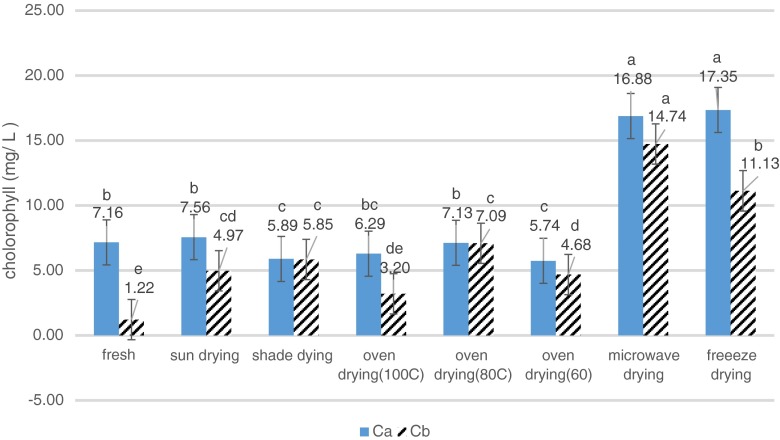
Results revealed that different methods of drying had a significant effect on the amount of chlorophyll in the leaves (Fig. 6). They showed that chlorophyll content in dried samples was higher than that in the fresh sample based on wet basis. During drying, chlorophyll content was significantly increased (Fig. 6). It is believed that chlorophyll is sensitive to heat and its retention is dependent on temperature and duration of heat treatment. For this reason, the sample dried by oven drying in 100 °C method contained the lowest chlorophyll content (6.2904 Ca mg/l and 3.2004 Cb mg/l in leaf, respectively). The results were in agreement with those obtained by Negi and Roy (Negi and Roy 2000).
Fig. 6.
Changes in chlorophyll a and b of green tea as affected by different drying treatments
The highest and the lowest chlorophyll content were obtained in freeze drying (17.35 mg/l) and oven 60 °C (5.73 mg/l), respectively. Chlorophyll is much susceptible to degradation during food processing and storage. Degradation of chlorophylls a and b to pheophytin a and b, respectively, followed first-order kinetics and chlorophyll a was more heat sensitive than chlorophyll b (Arslan and Ozcan 2012; Rahimmalek and Goli 2013). During heat treatment, the magnesium in chlorophyll is readily replaced by two hydrogen atoms in the presence of mild acids such as oxalic or acetic acid to produce pheophytin (Arslan and Ozcan 2010). Since this degradation is considered an index of loss of vegetables quality by consumers, food processors are trying to prevent or minimize the chlorophyll degradation (King et al. 2001).
Color
The color data in terms of the Hunter L*, a* and b* values of fresh and dried green tea leaves are illustrated in Table 1. The L* value of freeze drying treatment was the highest among all dried leaves (25.2), while sun drying revealed the lowest L* (20.1) value among dried treatments (P < 0.05). High temperature and long drying time is the most effective factors in color damage during drying (Arslan and Ozcan 2008; Arabhosseini et al. 2011; Rahimmalek and Goli 2013). Similar results were also obtained for b*(Table 1). Freeze drying treatment showed the highest b* values in comparison to others. So, freeze drying and sun drying possessed the highest and the lowest yellow color, respectively (b* value). For a* value (greenness) freeze drying had the highest value (more negative), while oven 80 °C showed the lowest green color. Overall, sun and freeze drying treatments were considered the least and the most desirable drying methods regarding the final color of green tea leaves.
Table 1.
Effect of different drying methods on color characteristics of green tea
| Drying methods | L* | a* | b* |
|---|---|---|---|
| Fresh | 16.97 | −1.95 | 7.58 |
| Shade drying | 21.60 | −1.19 | 8.50 |
| Sun drying | 20.10 | −1.07 | 7.09 |
| Oven drying (100 °C) | 22.94 | −1.45 | 9.01 |
| Oven drying (80 °C) | 21.63 | −1.01 | 7.84 |
| Oven drying (60 °C) | 26.63 | −1.39 | 9.97 |
| Microwave drying | 21.16 | −1.58 | 8.59 |
| Freeze dying | 25.20 | −2.81 | 10.88 |
Conclusion
In conclusion, to select the best drying method, different criteria such as plant species, energy consumption, cost, and final color of dried plant, nutritional value and the time of drying should be considered. Since the consumption of green tea has been increasing during the past decades, selecting the best treatments in respect to qualitative and nutritional characteristics of this prodct is very important. The present research introduced the best drying treatments in respect to different characteristics such as vitamin C, TPC, TFC, antioxidant activity, chlorophyll and final color of the product. The higher temperatures and longer drying time led to more color damage. Totally, freeze drying increased or maintained color characteristics and vitamin C, while oven drying was more efficient to elevate TPC, TFC and antioxidant activity of green tea.
References
- Annegowda H, Bhat R, Yeong K, LiongM KA, Monsor S. Influence of drying treatments on polyphenolic contents and antioxidant properties of raw and ripe Papaya (Carica papaya L.) Int J Food Prop. 2014;17:283–292. doi: 10.1080/10942912.2011.631248. [DOI] [Google Scholar]
- Arabhosseini A, Padhye S, Huisman W, Boxte AV, Muller J. Effect of drying on the color of Tarragon (Artemisia dracunculus L.) leaves. Food Bioprocess Technol. 2011;4:1281–1287. doi: 10.1007/s11947-009-0305-9. [DOI] [Google Scholar]
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achilleasantolina extracts. Food Chem. 2007;104:21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- Armoskaite V, Ramanaukiene K, Maruska A, Razukas A, Dagilyte A, Baranuskas A, Bredis V. The analysis of quality and antioxidant activity of green tea extracts. J Med Plants Res. 2011;5:811–816. [Google Scholar]
- Arslan D, Ozcan MM. Evaluation of drying methods with respect to drying kinetics, mineral content and color characteristics of rosemary leaves. Energy Convers Manag. 2008;49:1258–1264. doi: 10.1016/j.enconman.2007.08.005. [DOI] [Google Scholar]
- Arslan D, Ozcan MM. Study the effect of sun, oven and microwave drying in quality of onion slices. Food Sci Technol. 2010;43:1121–1127. [Google Scholar]
- Arslan D, Ozcan MM. Evaluation of drying methods with respect to drying kinetics, mineral Content, and color characteristics of Savory leaves. Food Bioprocess Technol. 2012;5:983–991. doi: 10.1007/s11947-010-0498-y. [DOI] [Google Scholar]
- Bae I, Ham H, Jeong M, Kim D, Kim H. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: Method developmentand optimization of extraction process. Food Chem. 2015;172:469–475. doi: 10.1016/j.foodchem.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Braga NP, Cremasco MA, Valle RCCR. The effects of fixed-bed drying on the yield and composition of essential oil from long pepper (Piper hispidinervium CDC) leaves. J Braz Chem Soc. 2005;22:257–262. [Google Scholar]
- Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113:16–172. doi: 10.1016/j.foodchem.2008.07.090. [DOI] [Google Scholar]
- Chang CH, Lin HY, Chang CY, Liu YC. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. Food Eng. 2006;77:478–485. doi: 10.1016/j.jfoodeng.2005.06.061. [DOI] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Am Soc Pl Biol. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibi SH, SayedTabatabaei GH, Goli SAH. Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind Crop Prod. 2013;50:154–158. doi: 10.1016/j.indcrop.2013.07.038. [DOI] [Google Scholar]
- Goula AM, Adamopoulos KG. Retention of ascorbic acid during drying of tomato halves and tomato pulp. Dry Technol. 2010;24:57–64. doi: 10.1080/07373930500538709. [DOI] [Google Scholar]
- Hamrouni-Sallami I, Rahili FZ, Rebey IB, Sriti J, Limam F, Marzouk B. Drying Sage (Salvia officinalis L.) plants and its effects on content, chemical composition, and radical scavenging activity of the essential oil. Food Bioprocess Techno1. 2011;l5:2978–2989. [Google Scholar]
- Hamrouni-Sallami I, Rahali FZ, Rebey IB, Bourgou S, Lomam F, Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying method. Food Bioprocess Technol. 2013;6:806–817. doi: 10.1007/s11947-012-0877-7. [DOI] [Google Scholar]
- Hassain MB, Barry-Byan C, MartinDiana AB, Bruntin NP. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010;123:85–91. doi: 10.1016/j.foodchem.2010.04.003. [DOI] [Google Scholar]
- Hirun S, Utama-Ang N, Vuong VQ, Scarlett C. Investigating the commercial microwave vacuum drying conditions on physicochemical properties and radical scavenging ability of Thai green tea. Dry Technol. 2014;32:47–54. doi: 10.1080/07373937.2013.811249. [DOI] [Google Scholar]
- Jin X, Oliviereo T, Sman R, Verkerk R, Dekker M. Impact of different drying trajectories on degradation of nutritional compounds in broccoli (Brassica oleracea var. italica) Food Sci Technol. 2014;59:189–195. [Google Scholar]
- Jun X. Caffeine extraction from green tea leaves assisted by high pressure processing. J Food Eng. 2009;94:105–109. doi: 10.1016/j.jfoodeng.2009.03.003. [DOI] [Google Scholar]
- Katalinic V, Mozin S, Genealic L, Skroza D, Ljubenkov I, Klancnik A. Phenolic Profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six vitisviniferaL. Varieties. Int J Food Prop. 2012;16:45–60. doi: 10.1080/10942912.2010.526274. [DOI] [Google Scholar]
- King VAE, Liu CHF, Liu YJ. Chlorophyll stability in spinach dehydrated by freeze-drying and controlled low-temperature vacuum dehydration. Food Res Int. 2001;34:167–175. doi: 10.1016/S0963-9969(00)00148-4. [DOI] [Google Scholar]
- Komes D, Horzic D, Belscak A, GanticK VI. Green tea preparation and its influence on the content of bioactive compounds. Food Res Int. 2010;43:167–176. doi: 10.1016/j.foodres.2009.09.022. [DOI] [Google Scholar]
- Lee Mei Ling A, YASIR S, Matanjun P, Bakar Abu MF. Antioxidant activity, total phenolic and flavonoid content of selected commercial Seaweeds of Sabah. Malays Int J Pharm Phytopharm Res. 2013;3:2249–2259. [Google Scholar]
- Lou S, Lai Y, Huang J, Ho C, Ferng L. Drying effect on flavonoid composition and antioxidant activity of immature kumquat. Food Chem. 2015;171:356–363. doi: 10.1016/j.foodchem.2014.08.119. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Ippolioi F, Moro P, Cassetti F, Raschetti R, SantuccioC MS. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- Negi PS, Roy SK. Effect of blanching and drying methods on β -carotene, ascorbic acid and chlorophyll retention of leafy vegetables. Food Sci Technol. 2000;33:295–298. [Google Scholar]
- Ostadalova M, Tremlova B, Pokarna J, Kral M. Chlorophyll as an indicator of green tea quality. Acta Vet Brno. 2014;83:103–109. doi: 10.2754/avb201483S10S103. [DOI] [Google Scholar]
- Pinela J, Barros L, Duenas M, Carvalho AM, Santos-Buelga C, Ferreira IC. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: effects of drying and oral preparation methods. Food Chem. 2012;135:1028–1035. doi: 10.1016/j.foodchem.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Pinelo M, Rubilar M, Sineiro J, Nunez MJ. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster) Food Chem. 2004;85:267–273. doi: 10.1016/j.foodchem.2003.06.020. [DOI] [Google Scholar]
- Prathapan A, Lukhman M, Arumughan C, Sundaresan A, Raghu KG. Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome. Int J Food Sci Technol. 2009;44:1438–1444. doi: 10.1111/j.1365-2621.2009.01976.x. [DOI] [Google Scholar]
- Prathapan A, Singh MK, Anusree SS, Kumar DRS, Sundaresan A, Raghu KG. Antiperoxidative, free radical scavenging and metal chelating activities of Boerhaavia Diffusa L. J Food Biochem. 2011;35:1548–1554. doi: 10.1111/j.1745-4514.2010.00477.x. [DOI] [Google Scholar]
- Rabeta M, Lai S. Effects of drying, fermented and unfermented tea of Ocimumtenuiflorum Linn. on the antioxidant capacity. Int Food Res J. 2013;20:1601–1608. [Google Scholar]
- Rahimmalek M, Goli SM. Evaluation of six drying treatments with respect to essential oil yield, composition and color characteristics of Thymys Daenensis subsp. daenensis. Celak leaves. Ind Crops Prod. 2013;42:613–619. doi: 10.1016/j.indcrop.2012.06.012. [DOI] [Google Scholar]
- Rezaeinodehi A, Khangholo S. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak J Biol Sci. 2008;6:946–949. doi: 10.3923/pjbs.2008.946.949. [DOI] [PubMed] [Google Scholar]
- Rodriguzer O, Santacatalina J, Simal S, Garcia-Perez J, Femenia A, Rosselleo C. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. Food Eng. 2014;129:21–29. doi: 10.1016/j.jfoodeng.2014.01.001. [DOI] [Google Scholar]
- Shahabi M, Rafiee S, Mohtasebi SS, Hosseinpour S. Image analysis and green tea color change kinetics during thin-layer drying. Food Sci Technol Int. 2014;20:465–76. doi: 10.1177/1082013213492524. [DOI] [PubMed] [Google Scholar]
- Stankovic M, Niciforovic N, Mihailovic V, Topuzovic M, Solujic S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc Bot Pol. 2012;81:117–122. doi: 10.5586/asbp.2012.010. [DOI] [Google Scholar]
- Vega-Galveza A, Di Scala K, Rodriguez K, Lemus-Mondaca R, Miranda M, Lopez J, Perez-Won M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian) Food Chem. 2009;117:647–653. doi: 10.1016/j.foodchem.2009.04.066. [DOI] [Google Scholar]
- Vuong VQ, Golding BJ, Nguyen HM, Roach DP. Production of caffeinated and decaffeinated green tea catechin powders from underutilized old tea leaves. J Food Eng. 2011;110:1–8. doi: 10.1016/j.jfoodeng.2011.12.026. [DOI] [Google Scholar]
- Ward J. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- Yildirım A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentaDesf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48:5030–5034. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]