Abstract
A solid-phase microextraction followed by gas chromatography-mass spectrometry method was developed to determine the volatile compounds in Shanxi aged vinegar. The optimal extraction conditions were: 50 °C for 20 min with a PDMS/DVB fiber. This analytical method was validated and showed satisfactory repeatability (0.5 %<RSD<12 %), reproducibility (2.5 %<RSD<15.6 %), accuracy and linearity in analysis of volatile compounds. According to the calculation of odor activity value, 19 volatile compounds were identified as aroma-active compounds. Among them, propanoic acid, acetic acid, trimethyl-oxazole, butanoic acid, acetoin, 3-methylbutanoic acid and furfural were the most powerful odorants. The aroma wheel of Shanxi aged vinegar showed that the classes of sensory descriptors are first fatty and roasty, next woody and nutty and minor fruity and floral. Principal component analysis enabled us to investigate dissimilarity/similarity of Shanxi aged vinegar sample of different raw material and ageing time.
Keywords: Shanxi aged vinegar, volatile compounds, SPME-GC-MS; Odor activity value; Aroma wheel
Introduction
Shanxi aged vinegar (SAV) is one of the most important traditional Chinese vinegars originated in Shanxi province, China, which has special flavor. The flavor is one of the most important characteristics of food influencing its quality and consumer preference (Lattey et al. 2010). The flavor or sensory qualities of vinegar varies due to raw material, processing procedure during fermentation and ageing time (Charles et al. 2000). Therefore, aroma characterization can provide an important index to improve the quality of SAV. Vinegar aroma characterization is generally dealt with chemical-analytical or sensory descriptive methodologies (Capone et al. 2013). The first is normally based on gas chromatographic methods (GC-MS), which provides a complete chemical profile of vinegar volatile fraction. The second is based on expert panel, who provides a sensory profile of vinegar by using specific descriptive sensory tests (Murray et al. 2001). However, the sensory analysis is not always available or affordable because training of the expert panel is time-consuming and expensive (Murray et al. 2001). As an alternative method to sensory analysis, aroma wheel is a simple and easy approach to portray the aroma profile of vinegar (Capone et al. 2013).
The solid-phase microextraction followed by gas chromatography-mass spectrometry method (SPME-GC-MS) was widely used to identify and quantify the volatile compounds in liquid food, such as wine (Heaven and Nash 2012). The accuracy of the SPME-GC-MS method is affected by matrix effect, which is mainly due to compounds in the sample matrix, such as sugars, organic acids, amino acids, phenolic compounds, proteins, and inorganic ions (Mitropoulou et al. 2011). Some researchers have found an effective solution, namely matrix-matched calibration curve, to minimize matrix effect in wine volatile determination (Antalick et al. 2010; Burin et al. 2013). The matrix-marched calibration curve is built in real sample or model system rather than pure solution in order to compensate matrix effect of SPME. In terms of vinegar, acetic acid is the major reason of matrix effect (Guerrero et al. 2007). Consequently, the determination of some volatile compounds in vinegar, such as acetoin, was not accuracy enough in previous studies, which did not use matrix-matched calibration curve to minimize matrix effect (Natera Marín et al. 2002). Thus, matrix-matched calibration curve was employed in our study in order to improve accuracy of SPME-GC-MS method.
The odor activity values (OAV) and aroma wheel allow to link quantitative information get by chemical analysis to sensory perception, and provide a valid tool to compare aroma profiles of different vinegar (Capone et al. 2013). The OAV is defined as the ratio of the concentration of the flavor compound to its odor threshold. Compounds with a ratio value ≥1 are considered to be responsible for aroma, and the greater their OAV is, the more they contribute to the aroma profile (Rothe and Thomas 1963). Aroma wheel is plotted based on OAV. The role of each volatile compound as odorant in vinegar aroma components can be described as one or several sensory descriptors, and similar descriptors belong to the same odorant (or aromatic) series (Gómez-Míguez et al. 2007). Thus, the generalized OAV for each aromatic series can be calculated by adding the OAV of each aromatic series component. In such a way an aroma profile of vinegar can be established by plotting in a radar graph the generalized OAV of all the aromatic series. The aroma wheel has been used to investigate red and sherry wines (Capone et al. 2013; Muñoz et al. 2007) However, available information about the sensory properties of SAV is scant. In the few reported studies on SAV (Wang et al. 2012a; Wang et al. 2012b; Xiao et al. 2011), the volatile compounds of SAV were semi-quantified and aroma-active compounds were identified by GC-Olfactometry. Therefore, in this study, the OAV and aroma wheel will reveal the aroma profile of SAV by analyzing quantitative information of SPME-GC-MS determination.
Principal component analysis (PCA) has been widely used to discover dissimilarity/similarity of foods according to their sensory and chemical data (Lan-Phi et al. 2009; Mahattanatawee and Rouseff 2014). In our study, the data get from SPME-GC-MS determination and OAV calculation will be applied to PCA in order to investigate dissimilarity/similarity of SAV samples. As organic acids also have big influence on sensory quality of vinegar (Callejon et al. 2008), the organic acids are determined by ion chromatography and the results are applied to PCA.
In this work, a SPME-GC-MS method was developed to identified and quantified volatile compounds in SAV, and a combination OAV calculation and aroma wheel method was used to portray the SAV aroma profile of different raw material or ageing time. A PCA was also conducted to investigate dissimilarity/similarity among SAV samples.
Materials and methods
Standard regents
All volatile standards (listed in Table 2) and organic acid standards (list in Table 5) were obtained from Sigma-Aldrich Co. Ltd. (Shanghai, China) and J&K Co. Ltd. (Shanghai, China). The stock solution consisted of methanol from J&K. The internal standard (Octanol) was purchased from Sigma-Aldrich.
Table 2.
Volatile compounds identified in Shanxi aged vinegar
| No. | Compounds | tR a (min) | Selected Ionsb | Odour descriptionc | Nature of the Identificationd |
|---|---|---|---|---|---|
| 1 | Ethyl acetate | 3.025 | 70/88/73 | Solvent, sweet | MS, GC, odour |
| 2 | 2,3-Butanedione | 4.323 | 57/43/86 | Yoghurt | MS, GC, odour |
| 3 | 3-Methylbutyl acetate | 8.422 | 70/43/55 | Grass, glue | MS, GC, odour |
| 4 | Trimethyloxazole | 10.417 | 111/68/82 | Sweet, green | MS, GC, odour |
| 5 | 3-Methyl-1-butanol | 10.733 | 55/70/42 | MS, GC | |
| 6 | Acetoin | 12.65 | 45/88/43 | mushroom | MS, GC, odour |
| 7 | Ethyl lactate | 14.275 | 45/75/43 | MS, GC | |
| 8 | 2,3-Dimethylpyrazine | 14.342 | 67/108/40 | Roasted | MS, GC, odour |
| 9 | 2,3,5-Trimethylpyrazine | 15.908 | 122/81/54 | Baked potato | MS, GC, odour |
| 10 | Furfural | 17.308 | 96/39/95 | Roasted, caramel | MS, GC, odour |
| 11 | Tetramethylpyrazine | 17.75 | 54/136/42 | Burnt coffee | MS, GC, odour |
| 12 | 2-Ethylhexanol | 18.158 | 57/41/70 | MS, GC | |
| 13 | 2-Furyl methyl ketone | 18.367 | 95/110/67 | MS, GC | |
| 14 | Benzaldehyde | 18.783 | 77/106/105 | Almond | MS, GC, odour |
| 15 | 5-Methyl furfural | 19.975 | 110/53/109 | Roasted, coffee | MS, GC, odour |
| 16 | Furfuryl alcohol | 21.692 | 98/81/97 | MS, GC | |
| 17 | Ethyl benzoate | 21.775 | 105/77/150 | MS, GC | |
| 18 | Ethyl benzeneacetate | 23.617 | 91/164/65 | MS, GC | |
| 19 | Phenethyl acetate | 24.033 | 104/43/91 | Flower | MS, GC, odour |
| 20 | Guaiacol | 24.592 | 109/81/124 | Rad date, sweet | MS, GC, odour |
| 21 | Phenylethyl Alcohol | 25.258 | 91/122/92 | Rose-like | MS, GC, odour |
| 22 | Creosol | 25.758 | 123/95/67 | Sweet, spicy | MS, GC, odour |
| 23 | Phenol | 26.283 | 94/66/65 | Fruity, wine | MS, GC, odour |
aRetention time was acquired on a DB-WAX column
bQuantitative ions are marked in bold and qualitative ions are marked in normal character
cOdour description at the olfactory detection port
dEach compound was identified based on the following criteria: MS, mass spectrometry; GC, injection of the authentic compound on the same column to compare mass spectrometry and retention time; Odour, compare the odour descriptions with literature
Table 5.
Content of organic acids in Shanxi aged vinegar samples
| Compound (mgL−1 ) | TB-3-Z | M-3-Z | W-3-Z | S-3-S | S-3-S’ | S-3-D | S-5-Q | S-5-S |
|---|---|---|---|---|---|---|---|---|
| Lactic acid | 24,265.3 | 26,991.6 | 30,336.6 | 12,179.5 | 4612.9 | 14,310.3 | 8218.9 | 2575.7 |
| Acetic acid | 60,610.6 | 48,804.7 | 45,945.9 | 70,002.4 | 87,723.5 | 64,814.1 | 52,965.9 | 54,836.5 |
| Propanoic acid | 110.3 | 71.1 | 60.5 | 302.3 | nd | 60.5 | nd | nd |
| Butanoic acid | 229.0 | 92.2 | 83.7 | 86.2 | 16.9 | 33.4 | 14.3 | 3.5 |
| 3-Methylbutanoic acid | 13.4 | 20.0 | 24.4 | 26.0 | 23.3 | 48.0 | 22.8 | 20.6 |
| Pentanoic acid | 307.0 | 193.1 | 184.4 | 242.6 | 121.5 | 283.9 | 51.5 | 62.8 |
| Hexanoic acid | 5.7 | 0.1 | nd | 0.2 | 0.1 | 2.4 | 0.1 | 0.1 |
| Succinic acid | 933.8 | 577.8 | 505.9 | 212.8 | 249.8 | 469.1 | 125.2 | 166.2 |
| Tartaric acid | 115.2 | 136.9 | 144.0 | 139.6 | 112.5 | 123.2 | 97.0 | 117.2 |
| Citric acid | 530.8 | 229.0 | 354.7 | 45.8 | 3796.6 | 1078.7 | 4441.1 | 4474.2 |
Spiking and sample preparation
The information of all SAV samples was listed in Table 1. Eight vinegar samples were analyzed in this study. Five commercial sorghum SAV samples, with ageing time (3 or 5 year), were produced by Shuita, Donghu and Qiannianjing Co. Ltd. The other three SAV samples were made from tartary buckwheat, millet and wheat. S-3-S and S-3-S’ were from different batches of the same product.
Table 1.
The basic information of each Shanxi aged vinegar sample
| No. | Code | Raw material | Ageing year | Brand name |
|---|---|---|---|---|
| 1 | TB-3-Z | tartary buckwheat | 3 | Ziyuan |
| 2 | M-3-Z | millet | 3 | Ziyuan |
| 3 | W-3-Z | wheat | 3 | Ziyuan |
| 4 | S-3-S | sorghum | 3 | Shuita |
| 5 | S-3-S’ | sorghum | 3 | Shuita |
| 6 | S-3-D | sorghum | 3 | Donghu |
| 7 | S-5-Q | sorghum | 5 | Qiannianjing |
| 8 | S-5-S | sorghum | 5 | Shuita |
The optimization and validation of the SPME-GC-MS method were carried out on diluted S-3-D (20 times). For validation, repeatability and reproducibility assays were carried out with diluted S-3-D (20 times) spiked at 10 μgL−1 with all the compounds studied. The accuracy was evaluated by calculating the recovery from standard. SAV was spiked at two levels, namely low and high levels. The low level was as follows: 0.5 μgL−1 with compounds (No. 17); 5 μgL−1 (No. 12, 18); 50 μgL−1 (No. 3, 5, 13, 14, 16, 19, 20, 22, 23); 500 μgL−1 (No. 2, 4, 7, 8, 9, 15, 21); 5000 μgL−1 (No. 1, 6, 10, 11). The high level was two-folds of the low level. The number of compounds is listed in Table 2. Before analysis, vinegar was diluted 20 and 5000 times for SPME-GC-MS or ion-chromatography analysis, respectively.
Optimization of solid phase-microextraction
Four fibers tested (Supelco, Bellefonte, PA, USA) were coated with various stationary phases and film thicknesses: divinylbenzene-carboxen-polydimethylsiloxane 50/30 μm (DVB/CAR/PDMS), carboxen-polydimethylsiloxane 75 μm (CAR/PDMS), polydimethylsiloxane-divinylbenzene 60 μm (PDMS/DVB), polydimethylsiloxane 30 μm (PDMS). The extraction efficiency was tested as a function of extraction temperature (30–70 °C) and extraction time (10–60 min).The fibers were conditioned prior to use according to instructions by inserting them into the GC injector. Six milliliter prepared sample was mixed with 1.5 g NaCl by a vortex shaker and then loaded onto an auto-sampling device. The SPME program consisted of shaking the vial at 500 rpm at 50 °C for 20 min, then inserting the fiber into the headspace at 50 °C for 20 min as the solution was swirled again, and then transferring the fiber to the injector for desorption at 200 °C for 3 min.
GC-MS analysis
Gas chromatographic analyses were carried out with a Shimadzu GC system coupled 2010 mass spectrometer and equipped with an AOC-5000 autosampler. Injections were in the splitless mode for 3 min. A DB-wax packed with polyethylene glycol capillary column (30 m × 0.25 mm, and 0.25 μm film thickness) was used and the carrier gas was helium with a column-head pressure of 8 psi. The oven temperature started at 40 °C and held for 3 min, increased to 120 °C at a rate of 5 °Cmin−1, increased to 200 °C at a rate of 10 °Cmin−1, and then held for 5 min. A full scan mode (m/z 35–300) was applied for the identification of all the target compounds. The mass spectrometer was operated in electron ionization mode at 70 eV with selected-ion-monitoring (SIM) mode for quantitative determination. Monitored ions are listed in Table 2.
The volatile compounds were identified by comparing their mass spectra to those of commercial spectra databases (NIST 11) in the first place. Then the identifications were confirmed by the injection of the authentic compounds into the GC–MS based on comparing mass spectra and GC retention time. Some identification were also confirmed by GC-O analysis, which procedure was reported in a previous study (Wang et al. 2012a), based on comparing ordour perception with those in literatures. For the quantitative study, 20 μL of a stock solution (octanol at 1000 mgL−1 in methanol solution) was added to 6 mL of the prepared samples.
Organic acid analysis by ion-chromatography
The Ion-chromatography analysis was carried out with ICS-3000 (DIONEX, USA) to detect organic acids in SAV. One milliliter sample was injected to 25 μL loop, and then separated on the IonPac AS11-HC 4 × 250 mm column. The packing composition of column is supermacroporous polyvinylbenzyl ammonium polymer cross-linked with divinylbenzene. An isocratic mobile phase of water was used for the ASRS 300 4 mm self-regenerating suppressor at a flow rate of 1.0 mLmin−1. The gradient elution of the mobile phase program was as follows: 0–12 min, 0.8 mmolL−1 KOH; 12–40 min, 0.8–34.0 mmolL−1 KOH; 40–50 min, 34.0 mmolL−1 KOH.
Statistical analysis
Principal component analysis was performed using SAS software for Windows.
Results and discussion
Extraction mode
The comparison among four different fibers (DVB/CAR/PDMS 50/30 μm, CAR/PDMS 75 μm, PDMS/DVB 60 μm and PDMS 30 μm) was based on relative peak areas (the peak area of target compound versus total peak area) of volatile compounds. What’s more, the linearity of four main volatile compounds (ethyl acetate, furfural, tetramethylpyrazine and acetoin) in SAV was applied as an additional factor for fiber selection.
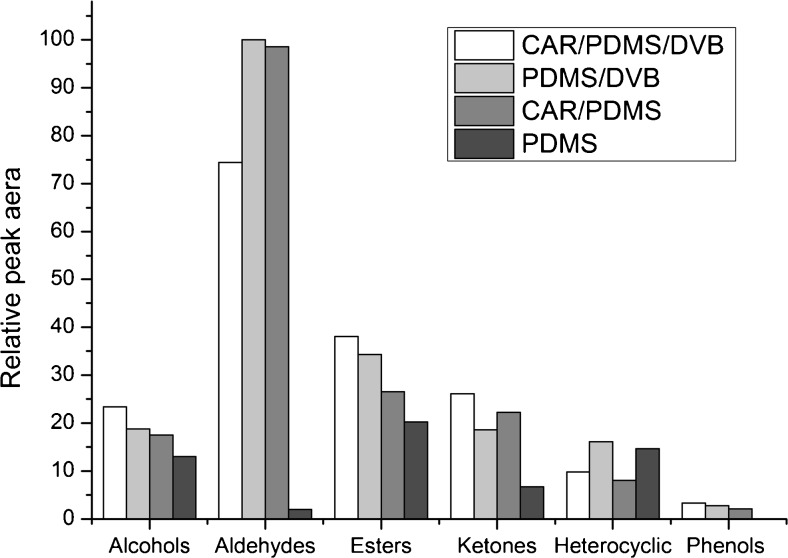
Figure 1 shows the relative peak areas of six groups of volatile compounds extracted by the four different fibers. CAR/DVB/PDMS and PDMS/DVB were efficient among four fibers. CAR/DVB/PDMS fiber was more effective in extraction of alcohols, esters, ketones and phenols, while PDMS/DVB was more effective in extraction of aldehydes and heterocyclic. The linearity of PDMS/DVB and CAR/DVB/PDMS were compared at a concentration range listed in Table 3. The coefficients of determination (R2) of ethyl acetate, furfural, tetramethylpyrazine and acetoin were 0.78, 0.69, 0.79 and 0.55, respectively. They did not reach the standard in our experiment which should be at least above 0.90, so the CAR/DVB/PDMS fiber was not suitable for our experiment. Hence, the PDMS/DVB was selected for extraction.
Fig. 1.
Relative peak area (the peak area of target compound versus total peak area) of different extraction mode for Shanxi aged vinegar
Table 3.
Linearity, detection and quantification limits, repeatability, reproducibility and accuracy of the method
| Compounds | Range (μgL−1) | Slope | R2 | LOD (μgL−1) | LOQ (μgL−1) | Repeatabilitya (RSD %) | Reproducibilityb (RSD %) | Recovery (%) c | |
|---|---|---|---|---|---|---|---|---|---|
| Low | High | ||||||||
| Ethyl Acetate | 500–20,000 | 8.2e-5 | 0.977 | 12.365 | 41.218 | 11.1 | 15.6 | 98 | 92 |
| 2,3-Butanedione | 50–1000 | 0.0008 | 0.995 | 0.646 | 2.152 | 10.6 | 14.1 | 78 | 91 |
| 3-Methylbutyl acetate | 2–100 | 0.0305 | 0.976 | 0.214 | 0.714 | 7.7 | 12.4 | 107 | 91 |
| TrimethylOxazole | 50–1500 | 0.0014 | 0.989 | 0.087 | 0.291 | 11.9 | 12.0 | 107 | 115 |
| 3-Methyl-1-butanol | 2–100 | 0.0147 | 0.990 | 0.158 | 0.526 | 10.0 | 6.7 | 101 | 104 |
| Acetoin | 5000–500,000 | 6.0e-6 | 0.927 | 257.858 | 859.528 | 7.6 | 10.2 | 101 | 96 |
| Ethyl lactate | 200–5000 | 0.0001 | 0.985 | 1.856 | 6.185 | 5.8 | 14.6 | 98 | 100 |
| 2,3-Dimethylpyrazine | 20–1000 | 0.0005 | 0.987 | 0.442 | 1.473 | 4.5 | 13.9 | 98 | 96 |
| 2,3,5-Trimethylpyrazine | 100–2000 | 0.0010 | 0.995 | 0.037 | 0.125 | 5.2 | 14.0 | 94 | 99 |
| Furfural | 500–20,000 | 0.0003 | 0.974 | 0.352 | 1.174 | 6.9 | 16.6 | 103 | 100 |
| Tetramethylpyrazine | 500–10,000 | 0.0011 | 0.991 | 0.052 | 0.174 | 5.3 | 14.5 | 89 | 100 |
| 2-Ethylhexanol | 0.50–15 | 0.0399 | 0.997 | 0.001 | 0.005 | 4.5 | 3.4 | 98 | 99 |
| 2-Furyl methyl ketone | 10.00–250 | 0.0011 | 0.991 | 0.060 | 0.200 | 6.9 | 12.5 | 99 | 103 |
| Benzaldehyde | 10.00–500 | 0.0052 | 0.989 | 0.486 | 1.621 | 7.7 | 9.1 | 105 | 103 |
| 5-Methyl furfural | 50–1500 | 0.0010 | 0.996 | 0.150 | 0.500 | 5.3 | 7.2 | 101 | 101 |
| Furfuryl alcohol | 20–100 | 4.4e-5 | 0.994 | 1.452 | 4.838 | 2.3 | 8.3 | 90 | 97 |
| Ethyl benzoate | 0.20–5.00 | 0.3114 | 0.999 | 0.001 | 0.002 | 2.3 | 2.5 | 98 | 98 |
| Ethyl benzeneacetate | 0.50–15.00 | 0.0449 | 0.997 | 0.005 | 0.016 | 0.5 | 2.6 | 96 | 99 |
| Phenethyl acetate | 5–150 | 0.0162 | 0.997 | 0.009 | 0.031 | 1.6 | 3.7 | 95 | 99 |
| Guaiacol | 10–200 | 0.0013 | 0.999 | 0.083 | 0.277 | 6.5 | 7.4 | 101 | 100 |
| Phenylethyl Alcohol | 200–10,000 | 0.0004 | 0.998 | 0.118 | 0.395 | 1.9 | 12.4 | 98 | 100 |
| Creosol | 10–500 | 0.0016 | 0.999 | 0.172 | 0.573 | 4.6 | 7.4 | 99 | 99 |
| Phenol | 10–500 | 0.0008 | 0.998 | 0.014 | 0.047 | 1.7 | 6.9 | 100 | 104 |
aThe repeatability expressed as RSD of the area ratios for all the identified volatile compounds in five identical samples (spiked at 10μgL−1)
bThe reproducibility expressed as RSD of the area ratios for all the identified volatile compounds in one sample (spiked at 10μgL−1) over 2-week period
cRecovery rate is calculated based on two standard addition levels, namely low and high
Adsorption temperature and time
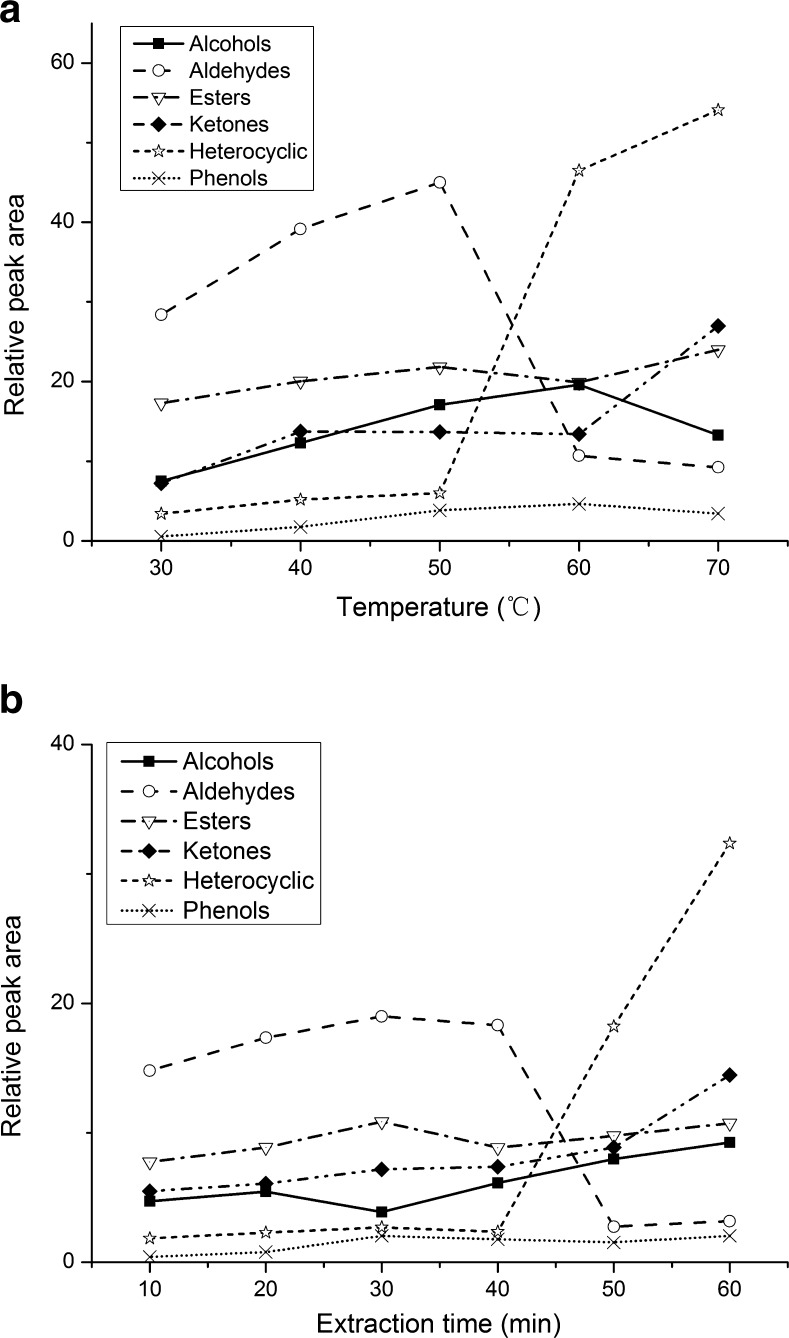
The effect of temperature was examined at different temperature using the DVB/PDMS fiber with a constant extraction time of 20 min. Figure 2(a) indicates that the maximum absorption of most families of compounds was at 50 °C. However, the absorption of heterocyclic compounds is stable until 50 °C, and then increases after that. In theory, higher temperature would increase the partial vapor pressure of analytes in the headspace but at the same time decrease the sorption onto the fiber, particularly for compounds with high volatility. Therefore, an adsorption temperature of 50 °C was selected.
Fig. 2.
Relative peak area (the peak area of target compound versus total peak area) in function of extraction parameters. a extraction temperature and b extraction time
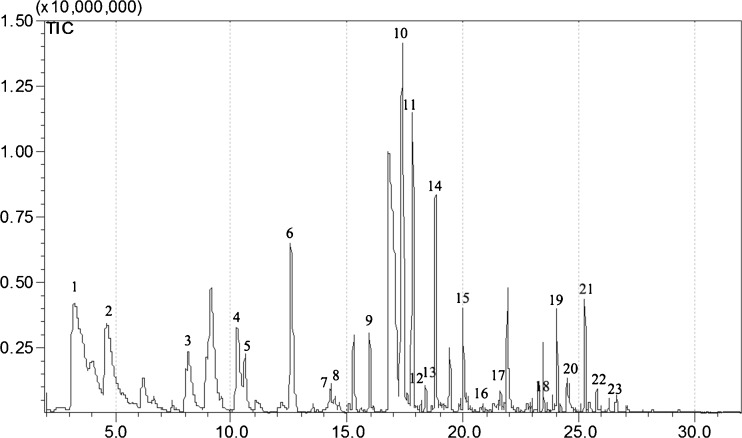
Figure 2(b) shows the extraction time profiles (10–60 min) for the volatile compounds. For the ketones, esters, alcohols and phenols, steady-state equilibrium between the fiber and the vapor phase was reached between 20 and 60 min. The steady-state equilibrium of aldehydes was between 20 and 40 min and then decreased. The absorption of heterocyclic compounds increased gradually until 60 min, especially increased markedly from 40 to 60 min. In order to a robust and effective adsorption for most of the volatile compounds, the absorption time was set as 20 min for the targeted volatile compounds in SAV. Based on the optimized extraction condition, the total ion current (TIC) chromatogram of the volatile compounds of SAV was presented in Fig. 3.
Fig. 3.
Total ion current (TIC) chromatogram of the volatile compounds of Shanxi aged vinegar. The main peaks were assigned as in Table 2
Linearity
The linearity was determined by matrix-marched calibration curves. In order to minimize the matrix effect in vinegar analysis, matrix-marched calibration curves was built as follows: diluted S-3-D (20 times) was spiked with target compounds as listed in Table 3, using five levels of concentration (depending on linearity range of each compound) prepared in duplicate. A correction was applied by subtracting the peak ratios of the non-spiked vinegar samples from the spiked vinegar sample. Then the calibration curves were plotted as relative peak areas (analyte versus octanol as the internal standard) as a function of compound concentration ratio (analyte concentration versus internal standard concentration). The linearity of the method was evaluated in a representative range of volatile compounds (Table 3). Linearity was effective in the range of studied concentrations, as R2 was in general above 0.98 except ethyl acetate, 3-methyl butyl acetate, acetoin and furfural.
Limits of detection and quantification
The limits of detection (LOD) and quantification (LOQ) were estimated as the volatile concentration which gave a signal equal to 3 or 10 times of the noise, respectively. The values were manually calculated from the ratio of the peak areas to the average noise before and after each peak (Table 3). In general, the LODs of most volatile compounds were less than 1 μgL−1. Acetoin gave poor result and showed considerably high LOD (257.8 μgL−1) compared to other volatile compounds. This was in agreement with previous studies that showed no existence of acetoin from red wine by SPME and high LOD of acetoin (2200.33 μgL−1) from sherry vinegar by stir bar extraction (Andujar-Ortiz et al. 2009; Guerrero et al. 2007). This was mainly because of low efficiency of fiber extraction and low response of GC-MS of acetoin.
Repeatability and reproducibility
To evaluate the repeatability of the method, five identical samples of spiked vinegar (10 μgL−1) were analyzed. The relative standard deviations (RSD) of the area ratios for all the identified volatile compounds were calculated (Table 3). The RSDs were less than 10 % for all compounds, except for ethyl acetate, 2, 3-butandione and trimethyloxazole (11.1, 10.6 and 11.9 %, respectively).
Spiked vinegar (10 μgL−1) was measured five times during 2 week period to test the reproducibility. The samples were individually frozen on the day of the first measurement. As shown in Table 3, the RSD of the area ratios were less than 15 % for most volatiles, except ethyl acetate and furfural (15.6 and 16.6 %, respectively).These results confirmed the good precision of the method.
Accuracy and recovery
The accuracy of the analytical method was evaluated by calculating the recoveries for the spiked samples. As shown in Table 3, the recoveries were about 100 % for most of the volatile compounds at both addition levels, especially for compounds with longer retention time, indicating good accuracy of the method.
These results for the validation of the method were in good agreement with data from other researchers who also developed and validated SPME-GC-MS methods for the determination of volatile compounds in fluid matrices. A SPME-GC-MS method was developed and validated for the determination of heterocyclic compounds (including pyrazine and furfural derivates) in red wines (Burin et al. 2013). In addition, a SPME-GC-MS method was validated to analyze volatile compounds (including phenethyl acetate and phenylethyl alcohol) in wines (Paula Barros et al. 2012). The results for both of these validation methods, which all used matrix-matched calibration curve to minimize matrix effect of SPME, are in good agreement with those observed in this study. However, inconsistence results were also found in studies of Sherry vinegar and wine determined by SPME-GC-MS method, and the value of recovery for acetoin was 57 % and 215 %, respectively (Andujar-Ortiz et al. 2009; Natera Marín et al. 2002). As the SPME-GC-MS methods used in these two studies did not use the matrix-marched calibration curves, the poor performance may be due to the matrix effect of SPME. This point of view could be further testified by a previous study. In case of SPME, recovery values actually reflected matrix effect due to minor components of sample (e.g. proteins, amino acids, sugars and polyphenols) which were not contained in normal calibration curves (Metafa and Economou 2013). In conclusion, the matrix-marched calibration curves minimized the matrix effect in SPME extraction and improved the accuracy of the SPME-GC-MS method.
Volatile profile of vinegar samples
The validated SPME-GC-MS method was applied to eight SAV to analyze the volatile profile of vinegar of different raw material or ageing time. The quantification of volatile compounds was calculated from the matrix-marched calibration curves. Each sample was analyzed in triplicate, and results of each volatile compound were shown in Table 4. The major volatile compounds quantified in SAV were acetoin, ethyl acetate, ethyl lactate, phenylethyl alcohol. Acetoin is an important volatile compound in vinegar, formed from butyleneglycol during the acetic fermentation, serves as a quality index of fermented products. The concentration of acetoin in SAV was 686.84–4036.59 mgL−1. Similarly, it has been found ranged from 265.29 mgL−1 to 1326.9 mgL−1 in sherry vinegar and balsamic vinegar (Guerrero et al. 2007). The content of phenylethyl alcohol in SAV ranged from 14.5 to 39.0 mgL−1 which was similar to sherry vinegar (Natera Marín et al. 2002).
Table 4.
Content of volatile compounds in Shanxi aged vinegar samples (n = 3)
| Compound | TB-3-Z | M-3-Z | W-3-Z | S-3-S | S-3-S’ | S-3-D | S-5-Q | S-5-S |
|---|---|---|---|---|---|---|---|---|
| Esters (μgL−1 ) | ||||||||
| Ethyl acetate | 3703.9 ± 251.3 | 80,854.7 ± 7919.6 | 90,785.0 ± 12,014.8 | 45,465.0 ± 1472.1 | 37,387.7 ± 3886.8 | 158,030.9 ± 29,217.8 | 93,177.8 ± 16,845.5 | 13,821.8 ± 2731.4 |
| 3-Methylbutyl acetate | nd | nd | 32.8 ± 2.9 | 23.1 ± 1.8 | 21.2 ± 1.9 | 141.0 ± 1.7 | 1.4 ± 0.1 | 2.0 ± 0.1 |
| Ethyl lactate | 7553.2 ± 164.1 | 40,952.2 ± 376.8 | 57,241.5 ± 453.1 | 51,394.6 ± 360.7 | 17,231.5 ± 190.1 | 50,019.6 ± 595.0 | 15,123.3 ± 124.0 | 13,020.3 ± 188.3 |
| Ethyl benzoate | 2.2 ± 0.02 | 5.1 ± 0.1 | 5.9 ± 0.1 | 2.4 ± 0.1 | 13.0 ± 2.0 | 2.5 ± 0.1 | 3.5 ± 0.1 | 2.4 ± 0.1 |
| Ethyl benzeneacetate | 10.1 ± 0.02 | 18.4 ± 0.3 | 22.7 ± 0.3 | 12.9 ± 0.1 | 14.9 ± 0.9 | 22.4 ± 0.3 | 13.3 ± 0.3 | 11.2 ± 0.1 |
| Phenethyl acetate | 466.7 ± 17.1 | 254.0 ± 4.5 | 236.6 ± 3.0 | 361.3 ± 5.6 | 326.3 ± 11.0 | 624.4 ± 0.4 | 230.7 ± 9.7 | 204.8 ± 2.3 |
| Heterocyclic (mgL−1 ) | ||||||||
| Trimethyl-Oxazole | 25.45 ± 0.12 | 10.26 ± 0.65 | 6.42 ± 0.72 | 1.44 ± 0.24 | 1.02 ± 0.36 | 6.33 ± 0.3 | 0.40 ± 0.03 | 0.30 ± 0.08 |
| 2,3-Dimethylpyrazine | 1.96 ± 0.05 | 1.96 ± 0.13 | 1.81 ± 0.14 | 2.70 ± 0.20 | 1.72 ± 0.05 | 3.50 ± 0.28 | 1.06 ± 0.06 | 1.10 ± 0.07 |
| 2,3,5-Trimethylpyrazine | 12.05 ± 0.25 | 5.72 ± 0.34 | 5.46 ± 0.27 | 4.79 ± 0.13 | 4.49 ± 0.04 | 9.34 ± 0.49 | 2.72 ± 0.11 | 2.69 ± 0.16 |
| Tetramethylpyrazine | 127.13 ± 2.95 | 20.34 ± 1.41 | 23.44 ± 0.77 | 33.05 ± 1.35 | 19.65 ± 1.00 | 69.07 ± 5.97 | 9.38 ± 0.22 | 9.71 ± 0.81 |
| Alcohols (μgL−1 ) | ||||||||
| 3-Methyl-1-butanol | 1472.5 ± 234.0 | 627.7 ± 57.9 | 370.2 ± 28.3 | 50.5 ± 3.9 | 56.8 ± 3.2 | 390.1 ± 10.0 | 7.7 ± 0.6 | 3.9 ± 0.3 |
| 2-Ethylhexanol | 25.3 ± 2.0 | 28.1 ± 2.8 | 16.8 ± 2.9 | 18.0 ± 7.5 | 27.1 ± 14.5 | 17.1 ± 7.6 | 15.5 ± 2.6 | 13.8 ± 1.2 |
| Furfuryl alcohol | 1621.7 ± 10.7 | nd | nd | 7325.8 ± 54.2 | 3895.2 ± 24.2 | 2332.5 ± 50.3 | 1730.1 ± 17.2 | 1049.4 ± 15.2 |
| Phenylethyl alcohol | 24,814.5 ± 24.5 | 14,839.7 ± 508.8 | 14,468.4 ± 513.5 | 28,098.0 ± 293.2 | 24,694.1 ± 714.5 | 39,014.4 ± 2997.9 | 15,515.0 ± 313.1 | 15,075.7 ± 197.7 |
| Aldehydes (mgL−1 ) | ||||||||
| Furfural | 88.39 ± 2.29 | 27.57 ± 5.21 | 20.14 ± 1.9 | 110.78 ± 7.14 | 41.28 ± 5.25 | 236.34 ± 25.89 | 38.21 ± 3.7 | nd |
| Benzaldehyde | 1.07 ± 0.04 | 3.01 ± 0.15 | 3.17 ± 0.34 | 0.73 ± 0.03 | 0.57 ± 0.02 | 3.22 ± 0.41 | 0.73 ± 0.02 | 0.75 ± 0.02 |
| 5-Methyl furfural | 4.08 ± 0.15 | nd | 0.67 ± 0.05 | 0.78 ± 0.14 | nd | 2.92 ± 0.12 | nd | nd |
| Ketones (mgL−1 ) | ||||||||
| 2,3-Butanedione | 12.38 ± 2.54 | 8.94 ± 1.00 | 6.06 ± 0.82 | 3.07 ± 0.02 | 3.78 ± 0.60 | 6.80 ± 0.71 | 3.26 ± 0.96 | 2.75 ± 0.06 |
| Acetoin | 1880.61 ± 27.61 | 745.63 ± 30.63 | 686.84 ± 59.06 | 3302.52 ± 93.12 | 2093.34 ± 103.51 | 4036.59 ± 664.34 | 1694.62 ± 182.20 | 1550.90 ± 168.93 |
| 2-Furyl methyl ketone | 1.88 ± 0.01 | 0.93 ± 0.05 | 1.03 ± 0.05 | 1.09 ± 0.01 | 0.67 ± 0.02 | 1.77 ± 0.23 | 0.65 ± 0.04 | 0.53 ± 0.02 |
| Phenols (μgL−1 ) | ||||||||
| Guaiacol | 516.7 ± 5.9 | 318.8 ± 12.6 | 308.7 ± 7.2 | 619.9 ± 8.3 | 291.0 ± 6.3 | 775.2 ± 46.7 | 222.1 ± 5.9 | 211.8 ± 2.0 |
| Creosol | 242.8 ± 1.2 | 304.2 ± 3.7 | 275.3 ± 5.5 | 1524.9 ± 36.5 | 507.1 ± 8.4 | 837.6 ± 57.8 | 919.8 ± 16.1 | 323.7 ± 4.5 |
| Phenol | 257.5 ± 2.1 | 573.7 ± 55.2 | 448.0 ± 40.9 | 167.8 ± 9.7 | 35.4 ± 14.1 | 656.6 ± 66.6 | 208.6 ± 30.7 | 235.2 ± 25.7 |
Heterocyclic compounds, such as furfural, tetramethylpyrazine, trimethylpyrazine and furfural alcohol, and phenols were first quantified in SAV in our studies, which were only identified and semi-quantified in the literature (Lu et al. 2011; Xiao et al. 2011). Among heterocyclic compounds, the concentration of tetramethylpyrazine was the highest, which was 127.13 mgL−1. Tetramethylpyrazine accounted for 6.77 % of volatile compounds of Zhenjiang aromatic vinegar (Lu et al. 2011). The concentration of furfural ranged from 20.14 to 88.39 mgL−1. It was the most abundant aldehyde in SAV. This result is similar to a previous study, which proposed semi-quantitative result of furfural ranged from 21 to 27 mgL−1 (Xiao et al. 2011). In addition, our study identified and quantified 2-Furyl methyl ketone, which was first found in vinegar.
Organic acid analysis
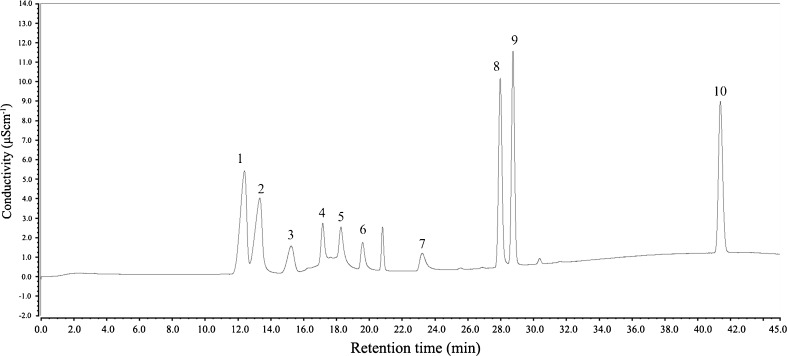
In order to know more about the difference among the SAV samples, the organic acid was analyzed by ion-chromatography. Ten acids of SAV were identified and separated in ion chromatography and their concentrations were listed in Table 5. Figure 4 shows the ion chromatography chromatogram of the ten acids. Six of the acids were volatile acid, which were also aroma-active compounds in SAV, namely acetic acid, propanoic acid, butanoic acid, 3-methylbutanoic acid, pentanoic acid and hexanoic acid. Acetic acid was the most abundant which ranged from 45,945.9 to 87,723.5 mgL−1. The concentration of propionic acid of SAV ranged from 60.5 to 302.3 mgL−1. The contents of butanoic acid (3.5–229 mgL−1), 3-methylbutanoic acid (13.4–48.0 mgL−1) and hexanoic acid (0.1–5.7 mgL−1) were close to those in sherry vinegar determined by GC-MS (Guerrero et al. 2007). The other four organic acids were non-volatile, which were lactic acid, succinic acid, tartaric acid and citric acid. Lactic acid was also high in vinegar samples (2575.7–30,336.6 mgL−1), which was similar to 3470 mgL−1 of sherry vinegar (Palacios et al. 2002).
Fig. 4.
Ion-chromatography chromatogram of organic acids of Shanxi aged vinegar. Peaks: 1, Lactic acid; 2, Acetic acid; 3, Propanoic acid; 4, Butanoic acid; 5, 3-Methylbutanoic acid; 6, Pentanoic acid; 7, Hexanoic acid; 8, Succinic acid; 9, Tartaric acid; 10, Citric acid
Although enough analytical data were got from the SPME-GC-MS determination and ion chromatography analysis, it is still hard to investigate sensory quality of SAV samples and compare dissimilarities/similarities among the SAV samples. This reason is that the content of volatile compounds did not always consist with their contribution to the whole aroma profile (Pang et al. 2012), and the variables are too much and need reduction before classification (Lan-Phi et al. 2009). Therefore, the odor activity values of each volatile compound were calculated, and the “aroma wheel” was plotted in order to investigate the sensory quality of SAV samples. What’s more, the principal component analysis was conducted on all the date acquired for samples classification.
OAV calculation and aroma wheel
OAV was obtained by dividing the concentration of the compound in a matrix by its odor threshold in that matrix (Table 6). So it is generally assumed that the odorants with higher OAVs contribute in a stronger manner to the overall aroma (Callejon et al. 2008). The odor threshold has been previously reported in related literatures (Burdock 2004; Callejon et al. 2008; Marchand et al. 2000; Peinado et al. 2004; Piccino et al. 2013; Taylor et al. 2013; Vilanova and Martínez 2007). Compounds in the Table 6 are ranked according to the maximum OAV reached in the eight SAV samples. Altogether, 19 odorants reached concentrations above their odor thresholds. The highest odor activity value of 2799 was calculated for propanoic acid, followed by acetic acid and trimethyl-Oxazole. Other compounds such as butanoic acid, acetoin, and 3-methylbutanoic acid also reached high OAVs in the eight samples. The highest OAVs of each aroma compounds were mostly calculated in S-3-D and TB-3-Z, indicating these two SAV samples had stronger aroma effect than other samples. Furthermore, the number of aroma-active compounds (OAV>1) was largest in S-3-D, which means the aroma profile of S-3-D was more complicated. Our odorants identification were in agreement with the a previous GC-O analysis of SAV (Wang et al. 2012a), while the opinions were divided on the contribution of some specific odorants to the aroma profile. Compared to the GC-O analysis, which considered furfural and tetramethylpyrazine as the most aroma-active compounds in SAV, our study believed trimethyl-oxazole was more aromatic. The differences may be due to the different application principles of GC-O analysis and OAV calculation (Pang et al. 2012), or the variation between the SAV samples used in these two studies.
Table 6.
Odor Activity Value (OAV) of the would-be impact odorants of Shanxi aged vinegar
| Odor descriiption | Odor thresholda mg/L | Ref. | OAVb | sensory descriptorsc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB-3-Z | M-3-Z | W-3-Z | S-3-S | S-3-S’ | S-3-D | S-5-Q | S-5-S | |||||
| Propanoic acid | Fatty | 0.108 | f | 1021 | 658 | 560 | 2799* | 560 | 4 | |||
| Acetic acid | Vinegar, fatty | 34 | h | 1782 | 1435 | 1351 | 2058 | 2580* | 1906 | 1557 | 1612 | 4 |
| Trimethyl-Oxazole | Boiled beef | 0.017 | g | 1497* | 603 | 377 | 84 | 60 | 372 | 23 | 17 | 2 |
| Butanoic acid | Cheese, rancid | 0.4 | d | 572* | 230 | 209 | 215 | 42 | 83 | 35 | 8 | 4 |
| Acetoin | Mushroom | 8.8 | e | 213 | 84 | 78 | 375 | 237 | 458* | 192 | 176 | 4,2 |
| 3-Methylbutanoic acid | Rancid | 0.4 | h | 33 | 50 | 61 | 65 | 58 | 120* | 57 | 51 | 4 |
| Guaiacol | Phenolic, sweet | 0.007 | h | 73 | 45 | 44 | 88 | 41 | 110* | 31 | 30 | 6 |
| Creosol | Sweet, spicy | 0.02 | h | 12 | 15 | 13 | 76* | 25 | 41 | 45 | 16 | 6 |
| Furfural | Roasted, caramel | 6.2 | e | 14 | 4 | 3 | 17 | 6 | 38* | 6 | 2 | |
| Benzaldehyde | Almond | 0.158 | e | 6 | 19 | 20 | 4 | 3 | 20* | 4 | 4 | 3 |
| 2,3,5-Trimethylpyrazine | Baked potato | 0.6 | h | 20* | 9 | 9 | 7 | 7 | 15 | 4 | 4 | 2 |
| 2,3-Butanedione | Yoghurt | 1.07 | f | 11* | 8 | 5 | 2 | 3 | 6 | 3 | 2 | 4,2 |
| Furfuryl alcohol | Burnt | 1.415 | e | 1 | 5* | 2 | 1 | 1 | 0.74 | 2 | ||
| Ethyl lactate | Fruity, buttery | 14 | d | 0.54 | 2 | 4* | 3 | 1 | 3 | 1 | 0.93 | 5,4 |
| Hexanoic acid | Fatty, cheese | 2.6 | e | 2.19* | 0.04 | 0.08 | 0.04 | 0.92 | 0.04 | 0.04 | 4 | |
| Ethyl acetate | Fruity, sweet | 91 | e | 0.04 | 0.89 | 1 | 0.50 | 0.41 | 1* | 1 | 0.15 | 5 |
| 5-Methyl furfural | Roasted, coffee | 2.4 | h | 1* | 0.28 | 0.33 | 1 | 2 | ||||
| Phenol | Fruity, wine | 0.4249 | d | 0.61 | 1 | 1 | 0.39 | 0.08 | 1* | 0.49 | 0.55 | 5 |
| Tetramethylpyrazine | Burnt coffee | 124 | f | 1* | 0.16 | 0.19 | 0.27 | 0.16 | 0.56 | 0.08 | 0.08 | 2 |
| 3-Methylbutyl acetate | Grass, glue | 0.17 | e | 0.22 | 0.15 | 0.14 | 0.94* | 0.01 | 0.01 | 5 | ||
| 2,3-Dimethylpyrazine | Roasted | 7.7 | h | 0.25 | 0.25 | 0.24 | 0.35 | 0.22 | 0.45* | 0.14 | 0.14 | 2 |
| Phenylethyl alcohol | Sweet, rose-like | 125 | i | 0.20 | 0.12 | 0.12 | 0.22 | 0.20 | 0.31* | 0.12 | 0.12 | 1 |
| 2-Ethylhexanol | Rose, sweet | 0.1 | h | 0.25 | 0.28* | 0.17 | 0.18 | 0.27 | 0.17 | 0.16 | 0.14 | 1 |
| 3-Methyl-1-butanol | Fusel | 40 | i | 0.04* | 0.02 | 0.01 | 0.01 | 5 | ||||
aOdor threshold as reported in the literature reference (Ref)
bOdor activity value defined as ratio between odor concentration and odor threshold
cEach compound was attributed to 1 or more classes of the following 6 classes of sensory descriptors: (1), floral, (2), roasty, (3), nutty, (4), fatty, (5), fruity, (6), woody
dBurdock (2004)
eCallejon et al. (2008)
fTaylor et al. (2013)
gMarchand et al. (2000)
hPiccino et al. (2013)
iVilanova and Martínez (2007)
*The highest odor activity values of the aroma compounds among the eight Shanxi aged vinegar samples
In order to link volatile composition to aroma descriptors, we grouped aroma compounds with similar sensory descriptors into class (aromatic series). In Table 6 for each compound we affiliated one or more membership of aromatic series. As aromatic series we considered the standard classes of sensory descriptors used in the well known aroma wheel, developed at the University of California at Davis by Ann C. Noble (http://winearomawheel.com/). The wheel breaks down vinegar aromas into 6 basic categories (floral, roasty, nutty, fatty, fruity, woody) and then further subdivided into different aromas that can fall into those main categories. Afterward, in reference to the six defined standardized classes of sensory descriptors, an OAV for each class of sensory descriptors (i.e. generalised OAV) was calculated by adding up the OAVs of all the compounds that form the class. In this computation, also the compounds with OAV < 1 were also considered, because of the acknowledged theory that sub-thresholds compounds may also contribute to aroma profile through additive effects of compounds with similar structure or odor (Francis and Newton 2005). Finally, we graphed them in a radar plot called aroma wheel, which is based on hybrid chemical/sensory parameter as generalised OAV is.
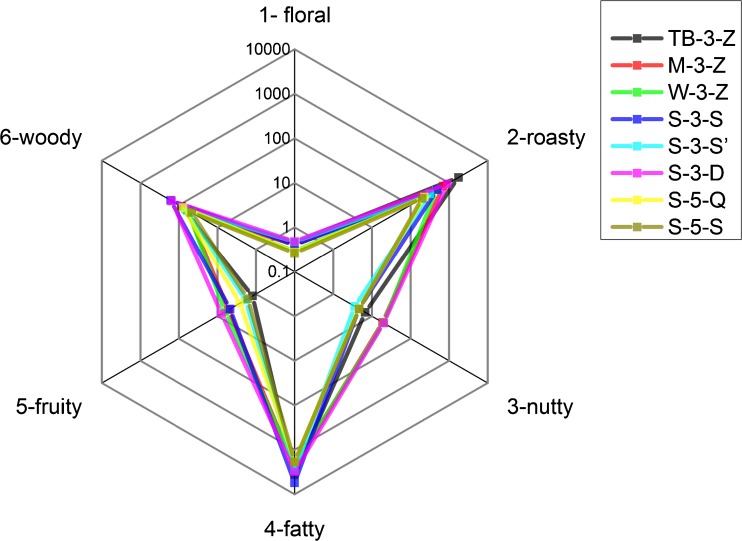
Figure 5 shows a global aroma wheel overlapping the radar plots of all the SAV samples. In particular, we chose a logarithmic scale in order to enhance minor variables flattened by predominant variables. As it can be observed, all the aroma wheels of SAV show high 2-roasty and 4-fatty characteristics, as well as typical 3-nutty and 6-woody sensory classes with minor harmonious 1-floral and 5-fruity features. Comparing aroma wheel of SAV to sensory analysis of Sherry vinegar (Callejon et al. 2008), we found roasty was a unique flavor for SAV. This preliminary comparison between aroma wheel and sensory analysis demonstrated a previous viewpoint: aroma wheel could be a replacement when a sensory analysis is not available or affordable (Capone et al. 2013). Among these SAV samples, TB-3-Z and S-3-D presented relatively high scores on most sensory descriptors. S-5-Q and S-5-S exhibited comparatively low scores on the six descriptors and were similar to each other. M-3-Z and W-3-Z also had similar performance on the aroma wheel. The aroma wheel could be used by chemical analysts to draw the aroma fingerprint of any vinegar by linking the chemical information to sensory parameters. This fast and simplified comparison between vinegar is preliminary to pure sensory analysis.
Fig. 5.
Aroma wheel of Shanxi aged vinegar based on the odor activity values of each class of sensory descriptors
Principal component analysis
PCA was the multivariate data analysis technique used for dimensionality reduction and display relationships/correlations between the chemical analytical data and the considered SAV samples. PCA was applied to four set of the data matrix: (1) volatile profile in Table 4; (2) OAVs in Table 6; (3) the generalized OAVs of each aromatic series; (4) volatile profile and organic acid content in Tables 4 and 6.
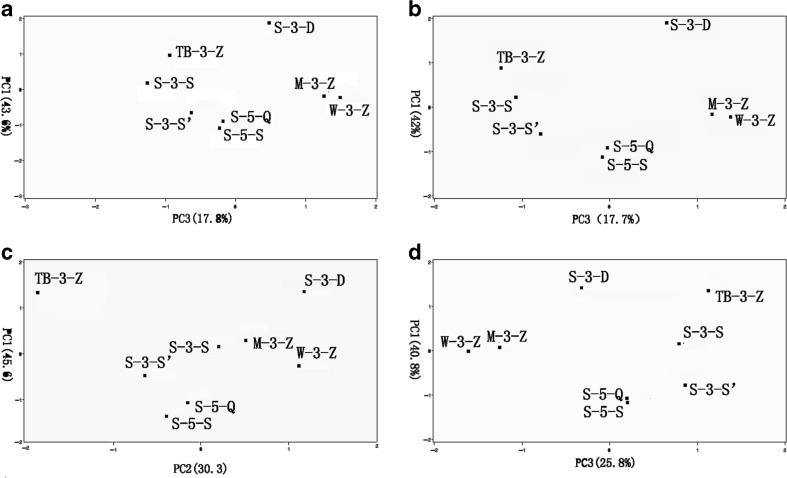
Figure 6 shows the PCA score plot of two principal components (PCs) from the first three PCs. The first three PC1, PC2 and PC3 accounted for 85.9–93.4 % of total variance and they were considered sufficient for further discussion. A relatively clear and consistency clustering of SAV samples according to raw material and ageing time was observed in the four PCA score plots. The four plots illustrated clear similarities between S-5-Q and S-5-S, and similarities between M-3-Z and W-3-Z, and the four plots also demonstrated a clear separation of TB-3-Z and S-3-D from other six samples. Furthermore, Fig. 6(c) is consistent with result from aroma wheel, which also indicted dissimilarities/similarities of the eight samples. This consistency results of the four PCA score plots indicated that the dissimilarity/similarity of volatile profile, OAV profile, the generalized OAVs and organic acid according to raw material and ageing time were consistency. PCA was used to classify different samples in previous studies (Ubeda et al. 2011; Xiao et al. 2011). However, most of these authors took volatile profile as variables in PCA. Variables in our research included not only volatile profile, but OAV profile, the generalized OAVs and organic acid content. Thus, classification results in our PCA would be more comprehensive and reliable.
Fig. 6.
Principal component analysis results based on: a volatile profile, b odor activity values, c generalized odor activity values, d volatile profile and organic acid profile
Conclusions
The SPME-GC-MS method was developed for identification and quantification of volatile compounds in SAV and was validated in terms of the linearity, repeatability, reproducibility and accuracy. Twenty-three volatile compounds were identified and quantified, and the matrix effect of SPME was minimized in this SPME-GC-MS method with matrix-marched calibration curves. Ion chromatography was employed to analyze organic acids, and six volatile acids and four non-volatile acids were quantified. The OAVs were calculated, and 19 volatile compounds were considered as aroma-active compounds in SAV. Among them, propanoic acid, acetic acid, trimethyl-oxazole, butanoic acid, acetoin, 3-methylbutanoic acid and furfural were most powerful odorants. Main characteristic aromatic feature of SAV was identified for the first time, and it is associated with fatty, roasty, woody, nutty, fruity and floral. Finally, the PCA separated unique SAV samples and clustered similar SAV samples on basis of volatile profile, OAVs, the generalized OAVs and organic acid profile.
Acknowledgments
This research was funded by the Chinese Agricultural Research System (grant number: CARS-08-D-3).
Footnotes
Research highlights
1. A SPME-GC-MS method was developed to identify and quantify volatile compounds of Shanxi aged vinegar.
2. Aroma profile of Shanxi aged vinegar was evaluated by odor activity value calculation and aroma wheel.
3. The sensory quality of Shanxi aged vinegar was defined as fatty, roasty, woody, nutty, fruity, and floral by analysis chemical date.
References
- Andujar-Ortiz I, Moreno-Arribas M, Martín-Álvarez P, Pozo-Bayón M. Analytical performance of three commonly used extraction methods for the gas chromatography–mass spectrometry analysis of wine volatile compounds. J Chromatogr A. 2009;1216:7351–7357. doi: 10.1016/j.chroma.2009.08.055. [DOI] [PubMed] [Google Scholar]
- Antalick G, Perello M-C, de Revel G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010;121:1236–1245. doi: 10.1016/j.foodchem.2010.01.011. [DOI] [Google Scholar]
- Burdock GA. Fenaroli's handbook of flavor ingredients. 5th. Boca Raton: CRC press; 2004. [Google Scholar]
- Burin VM, Marchand S, de Revel G, Bordignon-Luiz MT. Development and validation of method for heterocyclic compounds in wine: optimization of HS-SPME conditions applying a response surface methodology. Talanta. 2013;117:87–93. doi: 10.1016/j.talanta.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Callejon RM, Morales ML, Ferreira ACS, Troncoso AM. Defining the typical aroma of sherry vinegar: sensory and chemical approach. J Agric Food Chem. 2008;56:8086–8095. doi: 10.1021/jf800903n. [DOI] [PubMed] [Google Scholar]
- Capone S, Tufariello M, Siciliano P. Analytical characterisation of negroamaro red wines by “aroma wheels”. Food Chem. 2013;141:2906–2915. doi: 10.1016/j.foodchem.2013.05.105. [DOI] [PubMed] [Google Scholar]
- Charles M, Martin B, Ginies C, Etievant P, Coste G, Guichard E. Potent aroma compounds of two red wine vinegars. J Agric Food Chem. 2000;48:70–77. doi: 10.1021/jf9905424. [DOI] [PubMed] [Google Scholar]
- Francis I, Newton J. Determining wine aroma from compositional data. Aust J Grape Wine R. 2005;11:114–126. doi: 10.1111/j.1755-0238.2005.tb00283.x. [DOI] [Google Scholar]
- Gómez-Míguez MJ, Gómez-Míguez M, Vicario IM, Heredia FJ. Assessment of colour and aroma in white wines vinifications: effects of grape maturity and soil type. J Food Eng. 2007;79:758–764. doi: 10.1016/j.jfoodeng.2006.02.038. [DOI] [Google Scholar]
- Guerrero ED, Marín RN, Mejías RC, Barroso CG. Stir bar sorptive extraction of volatile compounds in vinegar: validation study and comparison with solid phase microextraction. J Chromatogr A. 2007;1167:18–26. doi: 10.1016/j.chroma.2007.08.039. [DOI] [PubMed] [Google Scholar]
- Heaven MW, Nash D. Recent analyses using solid phase microextraction in industries related to food made into or from liquids. Food Control. 2012;27:214–227. doi: 10.1016/j.foodcont.2012.03.018. [DOI] [Google Scholar]
- Lan-Phi NT, Shimamura T, Ukeda H, Sawamura M. Chemical and aroma profiles of yuzu (citrus junos) peel oils of different cultivars. Food Chem. 2009;115:1042–1047. doi: 10.1016/j.foodchem.2008.12.024. [DOI] [Google Scholar]
- Lattey K, Bramley B, Francis I. Consumer acceptability, sensory properties and expert quality judgements of Australian cabernet sauvignon and shiraz wines. Aust J Grape Wine Res. 2010;16:189–202. doi: 10.1111/j.1755-0238.2009.00069.x. [DOI] [Google Scholar]
- Lu ZM, Xu W, Yu NH, Zhou T, Li GQ, Shi JS, Xu ZH. Recovery of aroma compounds from Zhenjiang aromatic vinegar by supercritical fluid extraction. Int J Food Sci Tech. 2011;46:1508–1514. doi: 10.1111/j.1365-2621.2011.02649.x. [DOI] [Google Scholar]
- Mahattanatawee K, Rouseff RL. Comparison of aroma active and sulfur volatiles in three fragrant rice cultivars using GC–olfactometry and GC–PFPD. Food Chem. 2014;154:1–6. doi: 10.1016/j.foodchem.2013.12.105. [DOI] [PubMed] [Google Scholar]
- Marchand S, de Revel G, Bertrand A. Approaches to wine aroma: release of aroma compounds from reactions between cysteine and carbonyl compounds in wine. J Agric Food Chem. 2000;48:4890–4895. doi: 10.1021/jf000149u. [DOI] [PubMed] [Google Scholar]
- Metafa M, Economou A. Chemometrical development and comprehensive validation of a solid phase microextraction/gas chromatography–mass spectrometry methodology for the determination of important free and bound primary aromatics in Greek wines. J Chromatogr A. 2013;1305:244–258. doi: 10.1016/j.chroma.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Mitropoulou A, Hatzidimitriou E, Paraskevopoulou A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res Int. 2011;44:1561–1570. doi: 10.1016/j.foodres.2011.04.023. [DOI] [Google Scholar]
- Muñoz D, Peinado RA, Medina M, Moreno J. Biological aging of sherry wines under periodic and controlled microaerations with Saccharomyces cerevisiae var. capensis: effect on odorant series. Food Chem. 2007;100:1188–1195. doi: 10.1016/j.foodchem.2005.10.065. [DOI] [Google Scholar]
- Murray J, Delahunty C, Baxter I. Descriptive sensory analysis: past, present and future. Food Res Int. 2001;34:461–471. doi: 10.1016/S0963-9969(01)00070-9. [DOI] [Google Scholar]
- Natera Marín R, Castro Mejías R, de Valme García Moreno M, García Rowe F, García Barroso C. Headspace solid-phase microextraction analysis of aroma compounds in vinegar: validation study. J Chromatogr A. 2002;967:261–267. doi: 10.1016/S0021-9673(02)00786-0. [DOI] [PubMed] [Google Scholar]
- Palacios V, Valcárcel M, Caro I, Pérez L. Chemical and biochemical transformations during the industrial process of sherry vinegar aging. J Agric Food Chem. 2002;50:4221–4225. doi: 10.1021/jf020093z. [DOI] [PubMed] [Google Scholar]
- Pang X, Guo X, Qin Z, Yao Y, Hu X, Wu J. Identification of aroma-active compounds in jiashi muskmelon juice by GC-O-MS and OAV calculation. J Agric Food Chem. 2012;60:4179–4185. doi: 10.1021/jf300149m. [DOI] [PubMed] [Google Scholar]
- Paula Barros E, Moreira N, Elias Pereira G, Leite SGF, Moraes Rezende C, Guedes de Pinho P. Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta. 2012;101:177–186. doi: 10.1016/j.talanta.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Peinado RA, Moreno J, Bueno JE, Moreno JA, Mauricio JC. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004;84:585–590. doi: 10.1016/S0308-8146(03)00282-6. [DOI] [Google Scholar]
- Piccino S, Boulanger R, Descroix F, Shum Cheong Sing A. Aromatic composition and potent odorants of the "specialty coffee" brew "bourbon pointu" correlated to its three trade classifications. Food Res Int. 2013;61:264–271. doi: 10.1016/j.foodres.2013.07.034. [DOI] [Google Scholar]
- Rothe M, Thomas B. Aromastoffe des brotes. Z Lebensm Unters Forsch. 1963;119:302–310. doi: 10.1007/BF01891082. [DOI] [Google Scholar]
- Taylor K, Wick C, Castada H, Kent K, Harper WJ. Discrimination of Swiss cheese from 5 different factories by high impact volatile organic compound profiles determined by odor activity value using selected ion flow tube mass spectrometry and odor threshold. J Food Sci. 2013;78:C1509–C1515. doi: 10.1111/1750-3841.12249. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Callejón R, Hidalgo C, Torija M, Mas A, Troncoso A, Morales M. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography–mass spectrometry method. Food Res Int. 2011;44:259–268. doi: 10.1016/j.foodres.2010.10.025. [DOI] [Google Scholar]
- Vilanova M, Martínez C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castanal grown in Galicia (NW Spain) Eur Food Res Technol. 2007;224:431–436. doi: 10.1007/s00217-006-0322-0. [DOI] [Google Scholar]
- Wang A, Song H, Ren C, Li Z. Key aroma compounds in Shanxi aged tartary buckwheat vinegar and changes during its thermal processing. Flavour Fragr J. 2012;27:47–53. doi: 10.1002/ffj.2094. [DOI] [Google Scholar]
- Wang A, Zhang J, Li Z. Correlation of volatile and nonvolatile components with the total antioxidant capacity of Tartary buckwheat vinegar: influence of the thermal processing. Food Res Int. 2012;49:65–71. doi: 10.1016/j.foodres.2012.07.020. [DOI] [Google Scholar]
- Xiao Z, Dai S, Niu Y, Yu H, Zhu J, Tian H, Gu Y. Discrimination of Chinese vinegars based on headspace solid-phase microextraction-gas chromatography mass spectrometry of volatile compounds and multivariate analysis. J Food Sci. 2011;76:C1125–C1135. doi: 10.1111/j.1750-3841.2011.02356.x. [DOI] [PubMed] [Google Scholar]