Abstract
The changes in chemical composition, antioxidant activity and minerals content of horse gram seed after dehulling and germination of 12 advance lines were investigated. Dehulled samples had a higher protein content compared with the raw and germinated. Total soluble sugars (TSS) content increased significantly (p ≤ 0.05) after dehulling (29.31 %) and germination (98.73 %) whereas, the total lipids increased (10.98 %) significantly (p ≤ 0.05) after dehulling and decreased (36.41 %) significantly (p ≤ 0.05) after germination. Dehulling and germination significantly decreased the amount of phytic acid (PA), tannin (TN) and oxalic acid (OA). Trypsin inhibitor units decreased (26.79 %) significantly (p ≤ 0.05) after germination. The minerals (Ca, Fe and Cu) composition of the germinated horsegram flour samples was significantly higher than the raw and dehulled flour. The functional properties of flours were studied and found that the bulk density (11.85 %) and oil absorption capacity (18.92 %) significantly increased after germination. Raw samples followed by germinated samples showed the highest concentrations of phytochemicals responsible for the antioxidant activity and also the antioxidant capacities. principal component analysis revealed that in case of dehulled samples; TN, polyphenols, DPPH and ABTS radical inhibition, TSS, total antioxidant, OA, protein, FRAP value, Ca and Zn had positive correlation among themselves while in case of germinated samples, protein, oil absorption capacity, FRAP value, OA, total flavonoids, DPPH radical inhibition, Ca and Cu had positive correlation among themselves. Present study suggest that germination combined with dehulling process improved quality of horsegram by enhancing the nutritive value and reducing the antinutrients.
Keywords: Horsegram, Dehulling, Germination, Nutrients, Antinutrients, Antioxidant properties
Introduction
Legumes play an important role in sustaining the soil fertility and therefore, are an integral part of sustainable agriculture. These are a major source of dietary nutrients for many people, particularly vegetarians, in many developing countries. Among legumes, horsegram [Macrotyloma uniflorum (Lam.) Verdc.] is a minor legume used as a pulse crop in India and has been found good in nutritional quality (Bravo et al. 1999). Horsegram is normally consider as a poor man’s pulse as it offers a relatively cheap source of proteins for human consumption and livestock production. Horsegram is relatively high in iron, but the availability of the iron is reduced by the phytates, tannins, and oxalic acid it contains. Horsegram is also a good source of protein and appears to be a good source of calcium too. However, the oxalic acid content is high in horsegram which combines with calcium and iron to form an insoluble salt, rendering the calcium and iron unavailable for absorption (Borhade et al. 1984).
Traditional processing methods of legumes such as germinating, soaking and dehulling are sometimes used to reduce or eliminate the antinutrients that affect protein utilization. Dehulling decreased the levels of condensed tannins. Tannins are located mainly in the seed coats, which could explain their reduction after dehulling (Ghavidel and Prakash 2007). Germination is one of the most simple, common and effective processes for improving the nutritional quality of pulses by the reduction of anti-nutritive compounds and augmenting the levels of free amino acids, available carbohydrates, dietary fiber, and other components (Vidal-Valverde et al. 2002); as well as, increasing the functionality of the seeds due to the subsequent increase in the bio-active compounds (Frias et al. 2002). It decreases the phytin phosphorus level and increases the availability of iron and calcium. Further, it has been reported that protein and mineral bioavailability increased, whereas, phytic acid and tannin decreased during germination of legumes. The presence of anti-nutritional factors in horsegram is a matter of concern. Antioxidant activities and α-amylase as well as angiotensin-I-converting enzymes’ inhibitory potentials were reported recently in dehulled seed flours of horsegram (Sreerama et al. 2012a). Most researchers have studied the effect of soaking and germination on nutritional quality of legumes, but information on effect of processes such as germination and dehulling on improvement of nutritional quality of horsegram is scarce. The changes occurring in the total phenolic content and antioxidant activity as a result of germination and dehulling in horsegram need to be investigated. Therefore, the present study was undertaken to find out the effect of dehulling as well as germination in little-known legume, horsegram on proximate composition (total protein, total soluble sugars, total lipids, bulk density, water absorption capacity, and oil absorption capacity); antinutrients such as tannins, oxalic acid, phytic acid and trypsin inhibitor; minerals, viz., iron, calcium, zinc and cupper; and antioxidant activities by various in-vitro methods (free radical scavenging activities on DPPH and ABTS, total antioxidant activity, ferric iron reducing potential). These findings are expected to give insight into the possible utilization of horsegram flour as partial substitutes of well known legume flour in snack, confectionary and other traditional food products.
Materials and methods
Plant materials
The experimental materials consisted of 12 elite advance lines of horsegram [Macrotyloma uniflorum (Lam.) Verdc] seeds were cleaned, washed and 100 g seeds were soaked 500 ml of water at 25–30 °C for 12 h. At the end of the period, a portion of samples was dehulled manually and the another portion of sample was allowed to germinate under a wet muslin cloth for 48 h and then dried in a oven at 50 ± 2 °C for 16–18 h. Raw seeds (not soaked, dehulled and germinated) served as control. All the three samples, (1) raw (control), (2) dehulled and (3) germinated were milled to flour by using Newport scientific super mill grinder with a 0.25 mm sieve. The processing of samples was done in one batch and processed samples were stored in airtight containers for further analysis.
Chemical analysis
The nitrogen content was estimated by Kjeldhal method (AOAC 2005) based on the assumption that plant proteins contain 16 g/100 g nitrogen, protein content was calculated using the formula, protein = nitrogen × 6.25. Total sugar content (TSS) was determined calorimetrically by the anthrone method. Gravimetric method by Bligh and Dyer (1959) was used for determination of total fat content. Tannin was determined calorimetrically by following the AOAC method (AOAC 2005). Oxalic acid (OA) was determined titrimetrically by being precipitated as calcium oxalate and titrated against standard potassium permanganate (AOAC 2005). Phytic acid (PA) contents of defatted legume flours were determined by the method of Haug and Lantzsch (1983). The phytic acid content was calculated from a calibration curve using phytate phosphorus salt in the range of 10–50 μg. Trypsin inhibitor activity (TI) was determined by the method of Kakade et al. (1974), using benzoyl-DL-arginine-p-nitroanilide (BAPNA) as substrate. Results were expressed as trypsin inhibitor units (TIU). One TIU was defined as an increase of 0.01 in absorbance units under conditions of assay. Trypsin inhibitory activity was defined as the number of TIU. For the preparation of extract for total phenolics, and antioxidant activities determination, Fine powders of clean dry raw, dehulled and germinated samples (1.0 g) was extracted by stirring with 20 ml of 85 % methanol at 35 °C, 150 rpm/min for 12 h and filtered through Whatman filter paper No. 1. The extraction was repeated again as described earlier. The extracts were mixed, filtrated and diluted to 100 ml with 85 % methanol. The extract solution stored in amber bottles at 4 °C served as the working solution (10 mg/ml) for determination of total phenolics, and antioxidant activities. The total polyphenolic (TPP) compounds were determined by Folin Ciocalteu reagent (Singleton and Rossi 1965) and calculated from a standard calibration curve based on tannic acid (0–0.1 mg/ml) and the results were expressed as tannic acid equivalents in mg per g dry weight (mg TAE/g DW). Total flavonoid content in extract was measured by spectrophotometrically method (Tiwari et al. 2013). Results were expressed as catechins equivalents in mg per g dry weight (mg CE/g DW).
Mineral analysis
The oven-dried grinded horsegram samples were passed through a 0.2 mm sieve for estimation of nitrogen (N) content in Kjeltec 2300 auto-analyzer (Foss Pvt. Ltd). For estimation of Calcium (Ca), Zinc (Zn), Copper (Cu) and Iron (Fe) sieved samples were digested with a mixture of nitric acid and perchloric acid in the ratio of 10:4 (v/v) on hot plates sand bath. After complete digestion, samples were cooled at room temperature and appropriately diluted. Total Ca, Zn, Cu and Fe were analyzed by Atomic Absorption Spectrometry (AAS vario 6, Perkin Elmer).
Determination of functional properties
Bulk density (BD) was determined by the method of Wang and Kinsella (1976). Ten grams of the tested flour were placed in a 25 ml graduated cylinder and packed by gentle tapping of the cylinder on a bench top, ten times, from a height of 5–8 cm. The final volume of the test flour was measured and expressed as g/ml. Water absorption capacity (WAC) was determined according to the method described by Anderson et al. (1969) and oil absorption capacity (OAC) was estimated according to the procedure of Sosulski (1962). Briefly 1 g of each flour sample was weighed into a pre-weighed centrifuge tube and 10 ml of distilled water were added. Samples were vortexed for 1 min and allowed to stand for 30 min at 25 ± 2 °C before being centrifuged at 4000 g for 25 min. Excess water was decanted by inverting the tubes over absorbent paper and samples were allowed to drain. For oil absorption, 10 ml refined peanut oil were used in place of water. The weights of water and oil retained were calculated by measurement of difference in the weights of the sample before and after equilibration with water and oil.
Determination of antioxidative properties
Scavenging effects on 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azobis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) free radicals by horsegram methanolic extract was measured following standard methods of Brand-Williams et al. (1995) and Arnao et al. (2001), respectively. DPPH and ABTS radical scavenging activity was expressed in percent inhibition. The total antioxidant activity (TA) of horsegram methanolic extract was estimated using the phosphomolybdenum method of Prieto et al. (1999) based on the reduction of Mo (VI) to Mo (V) by the sample analyte and subsequent formation of specific green phosphate /Mo (V) compounds. The ferric reducing antioxidant power (FRAP) assayed following the method of Benzie and Strain (1996). A standard curve of trolox (10–100 μM) was prepared. Total antioxidant activity and ferric reducing antioxidant power were expressed as μM trolox equivalent/gram dry weight (μM TE/g DW).
Statistical analysis
The analysis was carried out in three replicates for all determinations. The statistical analyses were performed using the statistical package SPSS (Statistical Package for Social Science, SPSS Inc., Chicago, IL). Analyses of variance (ANOVA) were performed and significance of each group was verified with one-way analysis of variance followed by Duncan’s multiple range test (P < 0.05). For multifactorial comparison, principal component analyses (PCA) was used to display the correlation between the various parameters and their relationship with the different horsegram genotypes. Varimax rotation was performed to produce orthogonal transformations to the reduced factors to identify the high and low correlations better. Multifactorial analysis was carried out using the XLStat-Pro 7.5 (Addinsoft, New York, USA).
Results and discussion
Effect of dehulling and germination on nutritive properties
The results in Table 1 showed that total protein in raw samples ranged from 21.77 (HPK 2) to 23.06 (VL Gahat 1) g/100 g. Significant increase in protein was found after dehulling, whereas germination non-significantly affected the protein content. After dehulling the total protein content ranged from 22.58 (VL Gahat 1) to 25.36 (VL Gahat 10) g/100 g. Decrease in total protein content after germination could be due to the increased level of protease activity during germination (Torres et al. 2007). Protein level improved significantly after dehulling which could be due to removal of hull portion and concentration of endosperm. These results are comparable with findings of Sudha et al. (1995) for horsegram. Total soluble sugars (TSS) content increased significantly (p ≤ 0.05) after dehulling and germination (Table 1). Germinated samples showed the highest content of total soluble sugar as compare to raw and dehulled samples. TSS content of raw samples ranged from 5.40 g/100 g in advance line VLG 31 to 12.11 g/100 g in HG-3. In dehulled samples, it ranged from 7.79 mg/g (VL Gahat 1)-16.39 mg/g (HG-1). In germinated samples, highest content of TSS (24.19 mg/100 mg) was found in advance line HG-9 whereas, the lowest (8.45 mg/100 mg) was recorded in VL Gahat 15. Previous study with soybean showed a significant increase in sugars content with germination (Ramadan 2012).
Table 1.
Effect of germination and dehulling Total protein, Total soluble sugars, and Total lipids contents of horsegram flours (on dry weight basis/100 g)
| Genotype | Total protein (g) | Total soluble sugars (g) | Total lipids (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | R | DH | G | R | DH | G | |
| HG-6 | 22.51 | 23.93abc | 23.63ab | 8.63d | 9.42de | 18.50c | 0.91d | 1.67def | 0.88e |
| HG-1 | 22.29ab | 23.96abc | 23.06ab | 10.08bc | 16.39a | 20.87b | 1.39c | 1.64ef | 0.64f |
| HG-9 | 22.57ab | 25.07ab | 21.88abc | 9.71cd | 13.28bc | 24.19a | 2.11a | 2.40a | 1.25bc |
| HG-3 | 22.62ab | 23.59abc | 21.84abc | 12.11a | 13.89b | 23.11ab | 1.96ab | 2.04bc | 1.34b |
| VLGahat 19 | 22.28ab | 23.60abc | 20.14c | 9.37cd | 13.15bc | 21.57b | 2.29a | 2.42a | 1.73a |
| HPK 4 | 22.56ab | 22.61c | 21.91abc | 9.21cd | 14.31b | 18.46c | 2.22a | 1.42f | 1.12cd |
| VLG-31 | 22.94ab | 23.11bc | 22.54abc | 5.40e | 8.34e | 14.60d | 1.60c | 1.88cde | 0.93de |
| VLGahat1 | 23.06a | 22.58c | 23.76a | 5.68e | 7.79e | 11.67e | 1.37c | 1.77cde | 1.23bc |
| VLGahat 10 | 22.04ab | 25.36a | 21.05bc | 8.46d | 9.21e | 13.03de | 1.65bc | 1.85cde | 0.93de |
| VLGahat 15 | 22.61ab | 23.08bc | 22.85ab | 5.48e | 8.25e | 8.45f | 2.02a | 2.23ab | 0.87e |
| HPK 2 | 21.77b | 23.13abc | 21.08bc | 9.04cd | 9.57de | 14.48d | 1.63bc | 1.97bcd | 0.97de |
| VLGahat 8 | 22.30ab | 23.40abc | 22.46abc | 11.24ab | 11.42cd | 18.50c | 1.56c | 1.75cde | 1.33bc |
| Average | 22.46B | 23.62A | 22.18B | 8.70C | 11.25B | 17.29A | 1.73B | 1.92A | 1.10C |
Data expressed as mean (Mean; n = 3) and standard deviation (SD)
a, b, c and d superscript are significantly (p ≤ 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p ≤ 0.05) different treatment within a row
R Raw, DH Dehulled, G Germinated
Total lipid in horsegram was affected by the dehulling and germination (Table 1). Total lipids increased significantly (p ≤ 0.05) after dehulling whereas, it decreased significantly (p ≤ 0.05) after germination, could be due to total solid loss during soaking prior to germination or use of fat as an energy source in sprouting process (Wang et al. 1997). The total lipids in germinated samples varied from 0.64 (HG-1) to 1.73 (VL Gahat 19) g/100 g whereas after dehulling, recorded lowest (1.42 g/100 g) in genotype HPK 4 and highest (2.42 g/100 g) in VL Gahat 19. Total lipid levels improved significantly due to removal of hull portion and concentration of endosperm. In raw samples, highest content of total lipids (2.29 g/100 g) was recorded in VL Gahat 19 whereas the lowest (0.91 g/100 g) was recorded in HG-6. The results are comparable with findings of Sreerama et al. (2012b) for horsegram.
Effect of dehulling and germination on anti-nutritive properties
The results on antinutritional quantitative analysis for the raw, dehulled and germinated horsegram samples were presented in Table 2. The concentration of tannins in raw, dehulled and germinated samples obtained ranged 716.73–940.16, 245.97–398.60 and 532.90–689.65 mg/100 g, respectively. Overall statistically significant differences among the genotypes and treatments viz., control, dehulled and germinated seeds were observed. Germination and dehulling significantly (p ≤ 0.05) reduced the tannins content of horsegram as previously observed by Ghavidel and Prakash (2007) in cowpea, chickpea, green gram and lentil. After dehulling, there was little phytic acid and tannin detectable in cotyledons, indicating that most of the phytic acid and tannin are present in seed coat. Rao and Prabhavathi (1982) also reported similar results for some decorticated legumes.
Table 2.
Effect of germination and dehulling on anti-nutritive (tannins, oxalic acid, phytic acid, trypsin inhibitors) contents of horsegram flours (on dry weight basis)
| Genotype | Tannins (mg/100 g) | Oxalic acid (mg/100 g) | Phytic acid (mg/g) | Trypsin Inhibitor (Units/mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | R | DH | G | R | DH | G | R | DH | G | |
| HG-6 | 903.46abc | 384.32a | 642.91b | 587.84ab | 373.75d | 515.57a | 12.03a | 4.63cd | 7.68a | 9.25gh | 9.84f | 8.03de |
| HG-1 | 927.45ab | 311.95b | 588.44d | 575.60abc | 346.23f | 532.35a | 11.22b | 4.92bc | 7.56ab | 8.97h | 9.54f | 7.88de |
| HG-9 | 880.43cd | 269.16de | 681.48a | 547.30cd | 437.90a | 457.34bc | 11.01b | 5.29ab | 7.21abc | 9.87g | 10.22f | 8.43cd |
| HG-3 | 940.16a | 265.30ef | 537.96e | 485.02fg | 387.40cd | 387.90e | 11.34ab | 4.37d | 7.64ab | 8.78h | 9.87f | 7.09f |
| VLGahat 19 | 898.99bc | 299.11bc | 627.18c | 560.53bc | 449.47a | 477.04b | 9.42cd | 5.32ab | 7.16bc | 10.78ef | 12.82cd | 7.56ef |
| HPK 4 | 857.19de | 398.60a | 588.42d | 596.44a | 411.88b | 423.04d | 9.07cde | 4.87bc | 6.94cd | 10.05fg | 11.66e | 7.95de |
| VLG-31 | 827.87ef | 245.97f | 623.25c | 524.07de | 451.20a | 441.42cd | 9.76c | 4.35d | 6.63d | 11.09e | 13.31bcd | 8.20cde |
| VLGahat1 | 707.52h | 286.86cd | 628.30c | 516.43de | 351.15ef | 475.60b | 8.37ef | 5.67a | 5.73e | 13.79c | 12.48de | 9.33ab |
| VLGahat 10 | 796.00fg | 287.61cd | 689.65a | 462.69g | 398.09bc | 316.51g | 7.78f | 4.94bc | 5.80e | 13.92bc | 14.13b | 8.89bc |
| VLGahat 15 | 722.81h | 275.06de | 575.35d | 498.95ef | 325.95g | 342.38f | 8.46ef | 4.75cd | 5.08f | 14.77ab | 13.26bcd | 9.96a |
| HPK 2 | 761.31g | 299.49bc | 621.09c | 466.20g | 318.56g | 357.60f | 8.73de | 5.09bc | 5.47ef | 12.72d | 13.64bc | 8.29cde |
| VLGahat 8 | 716.73h | 290.51bcd | 532.90e | 456.69g | 369.23de | 388.34e | 8.94de | 5.03bc | 5.72e | 14.88a | 15.26a | 10.00a |
| Average | 828.33A | 301.16C | 611.40B | 523.14A | 385.06C | 426.25B | 9.67A | 4.94C | 6.55B | 11.57B | 12.17A | 8.47C |
Data expressed as mean (Mean; n = 3) and standard deviation (SD)
a, b, c and d superscript are significantly (p ≤ 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p ≤ 0.05) different treatment within a row
R Raw, DH Dehulled, G Germinated
The oxalic acid content in raw samples varied from 456.69 (VL Gahat 8) to 596.44 (HPK4) mg/100 g whereas after dehulling, a highly significant decrease in amount of oxalic acid content was found. Lowest oxalic acid content (318.56 mg/100 g) was recorded in genotype HPK 2 and highest (451.20 mg/100 g) in VLG-31. Germinated samples showed a significant (P ≤ 0.05) decrease in the oxalic acid content over the raw and dehulled samples. In germinated samples, highest content of oxalic acid (532.35 mg/100 g) was found in genotype HG-1 whereas the lowest (316.51 mg/100 g) was recorded in VL Gahat 10. These findings indicate that dehulling and germination could remove a large portion of oxalic acid. Similar findings have been reported by Murugkar et al. (2013). During germination, oxalate oxidase gets activated which breaks down oxalic acid into carbon dioxide and hydrogen peroxide consequently releases calcium (Murugkar et al. 2013).
The amount of phytic acid in raw sample was found significantly higher than dehulled and germinated samples (Table 2). In raw samples, phytic acid varied from 7.78 (VL Gahat 10) to12.03 (HG-6) mg/g whereas in dehulled samples it varies from 4.35 (VLG-31) to 5.67 (VL Gahat 1) mg/g. This result correlate well with an earlier report on wheat, that dehulling to get refined flours considerably reduced the phytate content (Guansheng et al. 2005). In germinated samples, genotype HG-6 contained the highest (7.68 g/100 g) whereas, VL Gahat 15 contained the lowest (5.08 g/100 g) amount of phytic acid. Overall 34 % significant (P ≤ 0.05) decrease in phytic acid in germinated horsegram samples were comparable to the results reported for other germinated legumes including green gram, cow pea, chick pea and lentil (Ghavidel and Prakash 2007). Decrease in phytic acid content during germination could be due to increase in phytase activity as reported in horsegram (Borhade et al. 1984). Previous report showed that phytate phosphorus significantly decreased with germination and it accounted for only 20 % in horsegram and 26 % in moth bean, of the total phosphorus of the 48 h germinated seeds (Borhade et al. 1984).
Trypsin inhibitors from horsegram seeds in the raw, dehulled and germinated samples had an average content of 11.57, 12.17 and 8.47 units/mg, respectively; raw and germinated samples contained significantly higher amount of trypsin inhibitors than the dehulled sample. In raw samples, trypsin inhibitor varies from 8.78 (HG-3) to 14.88 (VL Gahat 8) units/mg, whereas in dehulled samples it varied from 9.54 (HG-1) to 15.26 (VL Gahat 8) units/mg. In germinated samples, genotype VL Gahat 8 contained the highest (10.00 units/mg) whereas, genotype HG-3 contained the lowest (7.09 units/mg) amount of trypsin inhibitors. Wang et al. (1997) reported that trypsin inhibitors decreased during germination and increased slightly as the length of germination increased. Sangronis and Machado (2007) found a significant decrease of TIA in pigeon pea, white beans and black beans after 5 days of germination.
Effect of dehulling and germination on functional properties
The results in Table 3 showed that germination significantly (P < 0.05) decreased the bulk density (BD) of horsegram. Bulk density in raw samples varied from 0.72 g/ml (VL Gahat 1) to 0.78 g/ml (HPK 2, HPK 4) whereas after germination, genotypes HG-6 and VLG-31 recorded lowest (0.62 g/ml) and genotype HPK 2 recorded highest (0.69 g/ml) BD. It may be expected that decreased BD would be advantageous in the preparation of weaning food formulations. Malleshi et al. (1989) have shown that the BD of a weaning food formulation prepared from a blend of sorghum and cowpea flours was reduced by 12 % compared to the ungerminated materials. Dehulling significantly (P < 0.05) increased the BD of horsegram samples. In dehulled samples, genotype HPK 2 and VL Gahat 19 recorded highest (0.89) BD and genotype HPK 4 and VL Gahat 1 recorded lowest (0.81) BD. Similar results of increased BD on dehulling was reported by Ghavidel and Prakash (2007) for green gram, cowpea, lentil and bengal gram.
Table 3.
Effect of germination and dehulling on functional properties (Bulk density, water absorption capacity, oil absorption capacity) of horsegram flours
| Genotype | Bulk density (g/ml) | Water absorption capacity (%) | Oil absorption capacity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | R | DH | G | R | DH | G | |
| HG-6 | 0.76c | 0.65bcd | 0.86b | 127.51b | 66.02g | 134.05f | 85.92e | 55.20e | 106.58d |
| HG-1 | 0.76cd | 0.62e | 0.83de | 128.48b | 67.63f | 139.35cdef | 94.48a | 70.52a | 113.66a |
| HG-9 | 0.73e | 0.64cde | 0.85bc | 134.69b | 81.37b | 143.04cd | 91.47bc | 57.16d | 110.60b |
| HG-3 | 0.75d | 0.64cd | 0.86b | 125.20b | 62.44h | 134.32f | 81.82f | 53.41f | 95.07h |
| VLGahat 19 | 0.77bc | 0.66cd | 0.89a | 134.47b | 71.57d | 142.85cd | 82.61f | 56.51de | 102.64f |
| HPK 4 | 0.78ab | 0.66b | 0.81e | 145.23ab | 83.19a | 155.67a | 92.26b | 60.12c | 109.25bc |
| VLG-31 | 0.76cs | 0.62e | 0.84cd | 129.78b | 67.62f | 145.06bc | 89.82cd | 53.80f | 102.57f |
| VLGahat1 | 0.72e | 0.65bcd | 0.81e | 131.57b | 75.59c | 142.11cd | 90.59cd | 60.54c | 104.12ef |
| VLGahat 10 | 0.77abc | 0.63de | 0.84cd | 227.70a | 72.26d | 140.46cde | 85.76e | 56.18de | 99.50g |
| VLGahat 15 | 0.76cd | 0.65bcd | 0.83de | 122.56b | 69.24e | 135.37ef | 92.47ab | 64.49b | 107.17cd |
| HPK 2 | 0.78a | 0.69a | 0.89a | 139.58b | 80.62b | 149.99b | 88.92d | 56.22de | 105.28de |
| VLGahat 8 | 0.76cd | 0.64cde | 0.88a | 125.52b | 70.15e | 137.88def | 86.79e | 56.52de | 107.49cd |
| Average | 0.76B | 0.65C | 0.85A | 139.35A | 72.31B | 141.68A | 88.57B | 58.39C | 105.33A |
Data expressed as mean (Mean; n = 3) and standard deviation (SD)
a, b, c and d superscript are significantly (p ≤ 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p ≤ 0.05) different treatment within a row
R Raw, DH Dehulled, G Germinated
Germination non-significantly (P < 0.05) increased the water absorption capacity (WAC) of horsegram flour whereas dehulling significantly decreased the water absorption capacity (Table 3). WAC in raw samples varied from 122.56 % (VL Gahat 15) to 227.70 % (VL Gahat 10) whereas after dehulling, genotype HG-3 recorded lowest (62.44 %) and genotype HPK 4 recorded highest (83.19 %) for WAC. The WAC in germinated samples varied from 134.05 (HG-6) to 155.67 (HPK 4). Other studies also reported that the WAC of cowpea, green gram, lentil and bengal gram were improved by germination and decreased by dehulling (Ghavidel and Prakash 2007). An increase of WAC on germination could be attributed to breakdown of polysaccharide molecules; hence, the sites for interaction with water and holding water would be increased. Germination significantly (P < 0.05) increased the oil absorption capacity (OAC) of horsegram flour whereas dehulling significantly decreased the oil absorption capacity (Table 3). OAC in raw samples varied from 81.82 % (HG-3) to 94.48 % (HG-1) whereas after dehulling, genotype HG-3 recorded lowest (53.41 %) and genotype HG-1 recorded highest (70.52 %) for OAC. The oil absorption capacity in germinated samples varied from 95.07 % (HG-3) to 113.66 % (HG-1). Germination increased the capacities of cowpea, green gram, lentil and bengal gram to bind oil as observed by Ghavidel and Prakash (2007). Since the binding of the oil depends on the surface availability of hydrophobic amino acids, the enhancement in oil-absorption capacities of germinated samples could be attributed to an increase in the availability of these amino acids by unmasking the non-polar residues from the interior protein molecules (Sosulski 1962). The higher oil-binding capacity of horsegram flour suggests that this flour would be useful in formulation of foods where an oil holding property is an important consideration.
Effect of dehulling and germination on antioxidant properties
The results showed that there was significant differences (p ≤ 0.05) in the amounts of polyphenolic compounds among different treatments (Table 4). The total polyphenols (TPP) content in raw samples varied from 1.79 (VL Gahat 1) to 3.69 (HG-9) mg TAE/g DW whereas after dehulling, a significant decrease in amount of polyphenols was found and recorded lowest (1.04 mg TAE/g DW) in the VL Gahat 1 and highest (2.14 mg TAE/g DW) in VL Gahat 15. In germinated samples, highest content of polyphenols (1.21 mg TAE/g DW) was found in genotype HG-9 whereas the lowest (0.77 mg TAE/g DW) was recorded in VLGahat 10. From the results, it was observed that horsegram is a rich natural source of polyphenols and the results were in accordance of Kawsar et al. (2008). Germination, significantly (P ≤ 0.05) decreases the polyphenols content which was in accordance with an earlier report on horsegram (Satwadhar et al. 1981). Total flavonoids content ranged from 0.57 to 0.89 mg CE/g DW in raw horsegram and genotype ‘HG-1’ represents the highest value (Table 4). Total flavonoids contents were decreased after dehulling and germination. Results are in agreement with Tiwari et al. (2013) for horsegram. Germinated samples found the lowest total flavonoids among all treatments. In germinated samples, highest content of total flavonoids (0.48 mg CE/g DW) was found in HG-6 whereas the lowest (0.20 mg CE/g DW) was recorded in VL Gahat 8.
Table 4.
Effect of germination and dehulling on antioxidative compound (TPP and TF) and antioxidant capacities (Total antioxidant and FRAP value) in flours of different horsegram genotypes
| Genotype | Total polyphenols (mg TAE/g DW) | Total Flavonoids (mg CE/g DW) | Total Antioxidant Activity (μM TE/g DW) | FRAP value (μM TE/g DW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | R | DH | G | R | DH | G | R | DH | G | |
| HG-6 | 3.57b | 2.03b | 1.05b | 0.85ab | 0.50ab | 0.48a | 643.94b | 668.56a | 569.52de | 430.17e | 256.14c | 283.59cde |
| HG-1 | 3.68ab | 1.28e | 1.03bc | 0.89a | 0.34e | 0.34d | 611.50c | 542.12ef | 572.89de | 513.50cde | 202.46e | 302.95bc |
| HG-9 | 3.69a | 1.59c | 1.21a | 0.86a | 0.37de | 0.33d | 604.80c | 576.81c | 644.87ab | 725.28a | 263.49c | 332.61b |
| HG-3 | 3.58ab | 1.31e | 1.18a | 0.80abc | 0.23fg | 0.26e | 676.39a | 557.79d | 654.76a | 753.71a | 238.49d | 314.23bc |
| VLGahat 19 | 2.71d | 2.02b | 1.08b | 0.66ef | 0.41cd | 0.41b | 614.85c | 646.19b | 568.40de | 554.19bcd | 181.87f | 256.14e |
| HPK 4 | 2.85c | 1.98b | 1.07b | 0.66ef | 0.48ab | 0.36cd | 583.53d | 532.05f | 558.31e | 651.50ab | 182.11f | 265.70de |
| VLG-31 | 2.36f | 1.59c | 1.07b | 0.70de | 0.41cd | 0.39bc | 552.19e | 575.69c | 610.09c | 562.53bc | 295.35a | 295.60cd |
| VLGahat1 | 1.79g | 1.04g | 0.92d | 0.70de | 0.46bc | 0.31d | 534.29f | 418.06i | 577.38d | 434.58de | 148.53d | 371.10a |
| VLGahat 10 | 2.53e | 1.15f | 0.77e | 0.74cde | 0.27f | 0.22ef | 617.09c | 439.18h | 493.52h | 555.18bcd | 128.68h | 176.23g |
| VLGahat 15 | 2.81cd | 2.14a | 0.97cd | 0.77bcd | 0.54a | 0.33d | 605.92c | 549.98de | 542.60f | 588.02bc | 182.25f | 264.23de |
| HPK 2 | 2.35f | 1.26e | 0.96d | 0.66ef | 0.45bc | 0.41bc | 534.28f | 464.91g | 634.59b | 539.50bcde | 175.99f | 206.87f |
| VLGahat 8 | 2.52e | 1.39d | 0.94d | 0.57f | 0.21g | 0.20f | 526.46f | 671.94a | 510.36g | 548.07bcde | 276.24b | 221.11f |
| Average | 2.87A | 1.56B | 1.02C | 0.73A | 0.39B | 0.34C | 592.10A | 553.60B | 578.11A | 571.35A | 210.97C | 274.19B |
Data expressed as mean (Mean; n = 3) and standard deviation (SD)
a, b, c and d superscript are significantly (p ≤ 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p ≤ 0.05) different treatment within a row
R Raw, DH Dehulled, G Germinated
As shown in Table 4, the total antioxidant activity (TA) by phosphomolybdate method of horsegram was affected by the dehulling and germination. The phosphomolybdenum method usually detects antioxidants such as ascorbic acid, some phenolics, α-tocopherol, and carotenoids (Prieto et al. 1999). The results showed that the TA decreased significantly (p ≤ 0.05) after dehulling and germination. Dehulled samples found the lowest TA among all treatments. Total antioxidant activity in raw samples varied from 526.46 (VL Gahat 8) to 676.39 (HG-3) μM TE/g DW whereas after dehulling, a significant decrease in amount of antioxidant activity was found and recorded lowest (418.06 μM TE/g DW) in VL Gahat 1 and highest (671.94 μM TE/g DW) in VL Gahat 8. In germinated samples, highest TA (654.76 μM μM TE/g DW) was found in genotype HG-3 whereas the lowest (493.52 μM TE/g DW) was recorded in VL Gahat 10.
Ferric reducing antioxidant power (FRAP) assay is a colorimetric method based on the reduction of a ferrictripyridyltriazine (TPTZ) complex to its ferrous form. This reduction originates an intense blue complex with an absorption maximum at 593 nm (Benzie and Strain 1996). FRAP value of the studied horsegram samples as affected by processing (dehulling and germination). The results showed that the FRAP value decreased significantly (p ≤ 0.05) after dehulling and germination. Dehulled samples found the lowest FRAP value among all treatments. In raw samples the FRAP value varied from 430.17 (HG-6) to 753.71 (HG-3) μM TE/g DW whereas after dehulling, a significant decrease in amount of FRAP value was found and recorded lowest (128.68 μM TE/g DW) in VL Gahat-10 and highest (295.35 μM TE/g DW) in VLG-31. In germinated samples, highest content of FRAP value (371.10 μM TE/g DW) was found in VL Gahat 1 whereas the lowest (176.23 μM TE/g DW) in VL Gahat 10.
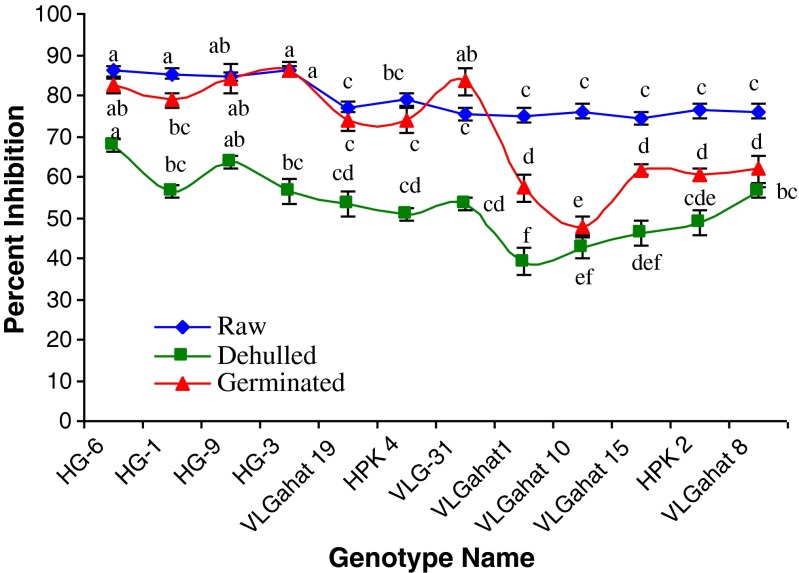
DPPH, a stable organic free radical has a maximum absorption at 517 nm, but upon reduction by an antioxidant the absorption disappears. This method is based on the reduction of alcoholic DPPH solution in the presence of hydrogen donating antioxidant compound due to the formation of a non-radical form (DPPH-H). The DPPH radical scavenging activity of methanolic extract obtained from raw horsegram samples were found in the range of 74.38 to 85.97 % inhibition (Fig. 1). In most of the genotypes, the germinated samples showed the lesser DPPH radical scavenging activity than raw samples. This may be due to leaching of antioxidant compound from horsegram into soaking during the prolonged exposure to water (Sreerama et al. 2012a). Dehulled samples showed the lowest DPPH radical scavenging activity among all three treatments. In germinated samples, DPPH radical scavenging activity varied from 47.65 (VL Gahat 10) to 86.37 (HG-3) percent inhibition whereas in dehulled samples it varied from 39.22 (VLG-31) to 67.64 (HG-6) percent inhibition.
Fig. 1.
Effect of dehulling and germination on DPPH free radical scavenging activity
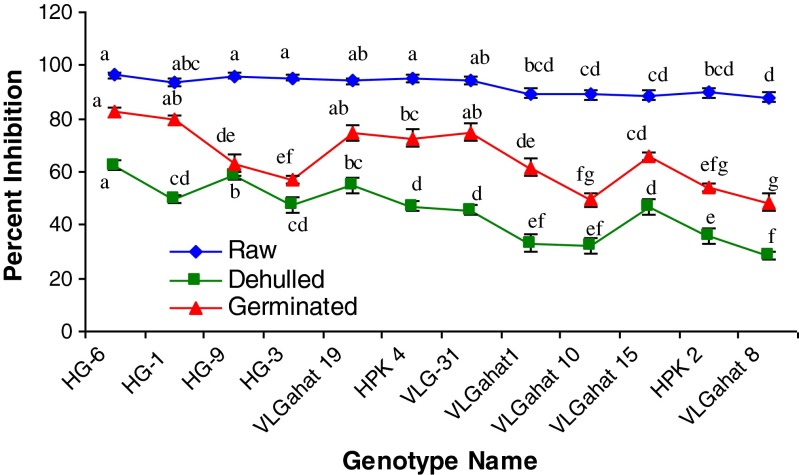
ABTS free radical scavenging activity of horsegram before and after treatments presented in Fig. 2. Activity ranged from 88.01 to 96.37 % inhibition in raw horsegram and ‘HG-6’ genotypes represents the highest free radical inhibition. Activity decreased after dehulling and germination. Results are in agreement with previous study by Tiwari et al. (2013) for the horsegram. Dehulled samples found the lowest scavenging activity among all treatments. In dehulled samples, lowest content of scavenging activity (28.21 % inhibition) was found in VL Gahat 8 whereas the highest (62.51 % inhibition) was recorded in genotype HG-6. The results are comparable with findings of Petchiammal and Hopper (2014) for horsegram, which showed that removal of seed coats (dehulling) significantly decreased concentrations of phytochemicals responsible for the antioxidant activity.
Fig. 2.
Effect of dehulling and germination on ABTS free radical scavenging activity
Effect of dehulling and germination on mineral content
Table 5 depicts the mineral content of raw and processed horsegram varieties. The total iron content in raw samples varied from 51.75 (VL Gahat 8) to 97.71 (HG-1) mg/100 g whereas after dehulling, a significant decrease in amount of iron was found and recorded lowest (25.70 mg/100 g) in genotype HG-9 and highest (44.82 mg/100 g) in VL Gahat 1. It was found that in horsegram samples after subjecting to germination, significantly (P ≤ 0.05) increased the iron content which was in accordance with an earlier report on pearl millet (Sushma et al. 2008). This increase could be attributed to the mineral contamination in the water used for soaking prior to germination. In germinated samples, highest content of iron (99.40 mg/100 g) was found in VLG-31 whereas the lowest (55.85 mg/100 g) was recorded in genotype HPK 2. The total calcium content in raw samples varied from 136.83 (VLG-31) to 652.02 (HG-1) mg/100 g whereas after dehulling, a highly significant decrease in amount of calcium was found and recorded lowest (36.56 mg/100 g) in VLG-31 and highest (68.85 mg/100 g) in the genotype HG-9. Germinated samples showed a significant (P ≤ 0.05) increase in the calcium content over the raw and dehulled samples. In germinated samples, highest content of calcium (737.17 mg/100 g) was found in HG-1 whereas the lowest (227.52 mg/100 g) was recorded in HPK 4. Decline in iron and calcium levels after dehulling was observed, which may be contributed to presence of these minerals in hull portion. The results are in the accordance with an earlier report on pearl millet (Sushma et al. 2008).
Table 5.
Effect of germination and dehulling on Mineral content (Ca, Fe, Zn, Cu) of horsegram flours (mg/100 g on dry weight basis)
| Genotype | Ca | Fe | Zn | Cu | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | R | DH | G | R | DH | G | R | DH | G | |
| HG-6 | 529.25b | 67.41ab | 574.20b | 52.73fg | 26.90gh | 75.32bc | 44.68b | 40.78ab | 44.27a | 9.35a | 9.67d | 11.21bcde |
| HG-1 | 652.02a | 58.35d | 737.17a | 97.71a | 35.78de | 78.44bc | 33.38f | 35.56cd | 32.02f | 8.09bcd | 9.79cd | 11.47bcd |
| HG-9 | 343.95d | 68.85a | 425.07c | 54.95fg | 25.70h | 75.78bc | 39.99bcde | 42.67a | 41.05ab | 9.26a | 12.23a | 13.13a |
| HG-3 | 370.65c | 67.20ab | 429.41c | 88.00b | 30.29fg | 98.14a | 63.81a | 34.33d | 34.25def | 7.96bcd | 10.16bcd | 11.63bc |
| VLGahat 19 | 342.70d | 63.30bc | 358.72d | 95.42a | 33.23ef | 67.47d | 42.07bcd | 38.28abcd | 33.64ef | 7.51cdef | 11.20b | 11.93b |
| HPK 4 | 139.00g | 48.80e | 227.52h | 61.80de | 32.78ef | 74.13c | 40.80bcde | 38.55abcd | 40.18ab | 6.85ef | 11.14b | 11.57bc |
| VLG-31 | 136.83g | 36.56g | 235.96gh | 57.83ef | 27.50gh | 99.40a | 41.53bcde | 40.47ab | 39.62abc | 7.87bcde | 9.40d | 10.65ef |
| VLGahat1 | 167.03ef | 47.78ef | 258.96f | 57.69ef | 44.82a | 64.96d | 35.38ef | 40.06abc | 34.37def | 8.54abc | 12.33a | 13.13a |
| VLGahat 10 | 175.33e | 45.31ef | 249.09fg | 77.24c | 43.66ab | 81.13b | 37.48cdef | 38.35abcd | 38.96bcd | 6.75f | 10.75bc | 11.08cde |
| VLGahat 15 | 149.75fg | 43.50f | 245.14fg | 55.19fg | 40.66bc | 57.01e | 43.02bc | 37.08bcd | 38.72bcde | 8.71ab | 10.36bcd | 11.87b |
| HPK 2 | 327.52d | 48.12ef | 424.98c | 65.46d | 38.92cd | 55.85e | 39.33bcdef | 35.16d | 34.48cdef | 7.13def | 9.89cd | 10.84def |
| VLGahat 8 | 145.21fg | 61.81cd | 273.63e | 51.75g | 41.19abc | 56.90e | 35.82def | 40.57ab | 38.11bcde | 6.57f | 9.32d | 10.25f |
| Average | 289.94B | 54.75C | 369.98A | 67.98B | 35.12C | 73.71A | 41.44A | 38.49B | 37.47B | 7.88C | 10.52B | 11.56A |
Data expressed as mean (Mean; n = 3) and standard deviation (SD)
a, b, c and d superscript are significantly (p ≤ 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p ≤ 0.05) different treatment within a row
R Raw, DH Dehulled, G Germinated
The zinc content varied from 33.38 to 63.81 mg/100 g in the different raw samples of horsegram. Genotype HG-3 showed the highest content and HG-1 showed the least (Table 5). The zinc content decreased significantly after the dehulling and germination, however, a non-significant difference recorded in dehulled and germinated samples. In germinated samples, highest content of zinc (44.27 mg/100 g) found in HG-6 whereas the lowest (32.02 mg/100 g) recorded in genotype HG-1. The total copper content was significantly (P ≤ 0.05) higher in germinated samples compared to the raw and dehulled samples. Horsegram appears to be a good source of copper. Statistically significant differences among the varieties of control, dehulled and germinated seeds were observed. In raw samples, copper content varied from 6.57 (VL Gahat 8) to 9.35 (HG-6) mg/100 g whereas after dehulling, lowest (9.32 mg/100 g) in VL Gahat 8 and highest (12.33 mg/100 g) in VL Gahat 1 were recorded. In germinated samples, highest content of copper (13.13 mg/100 g) was found in varieties HG-9 and VL Gahat 1 whereas the lowest (10.25 mg/100 g) was recorded in VL Gahat 8. The minerals (Ca, Fe and Cu) composition of the germinated horsegram flour samples was significantly higher than the raw and dehulled flour. This observation is similar to other investigators who have reported that germination increases retention of all minerals and B-complex vitamins compared to other processing methods (El-Adawy 2002).
Principal component analysis (PCA)
Principal Component Analysis (PCA) is a useful statistical technique, which has found application to find out interrelationships between the different variables (Mishra et al. 2013). The projections of genotypes and traits are shown in PC1 and PC2 biplot. (Fig. 3a, b, c). In PCA the length, direction and the angles between the lines indicate correlation between the variables or between variables and principal component axes (eg., α = 00 and/or 1800 and r = 1; α = 900 and r = 0). The longer the line, the higher is the variance. The cosine of the angle between the lines approximates the correlation between the variables they represent. The closer the angle is to 90 or 270 degrees, the smaller the correlation. An angle of 0 or 180 degrees reflects a correlation of 1 or −1, respectively (Lopez et al. 2006).
Fig. 3.
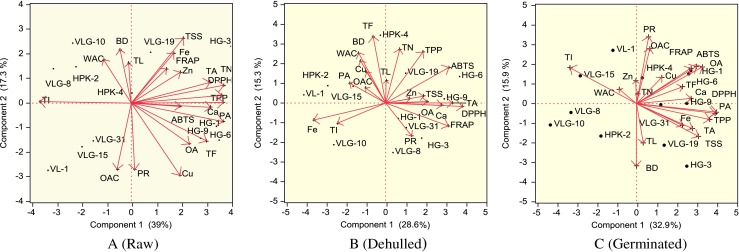
Multifactorial comparison and correlation matrix of studied parameters obtained from horsegram varieties using Principal component analysis (PCA)
In present study, multifactorial comparisons using principal component analysis clearly indicated correlation between various nutritive, anti-nutritive, functional, antioxidant and micronutrient parameters and their relationship under raw, dehulled and germinated horsegram samples. The principal component analysis (PCA) and their correlation are shown in raw, dehulled and germinated samples (Fig. 3a, b, c). Among the data, first component 1 represented 39.00 % of variability, whereas the component 2 represented 17.30 % of variability in case of raw samples. In case of dehulled and germinated samples component 1 represented 28.60 and 32.90 % of variability and the component 2 represented of 15.30 and 15.90 % variability, respectively. In case of raw samples, almost all the parameters (TN-Tannins, OA- oxalic acid, PA- phytic acid, PR- protein, TSS- total soluble sugars, TPP- Total polyphenols, TF- total flavonoids, DPPH radical inhibition, ABTS radical inhibition, TA- total antioxidant, FRAP value, Ca, Fe, Zn and Cu) were occupied on the right side of the biplot whereas the parameters, TL-Total lipid, BD-bulk density, WAC-water absorption capacity, TI-trypsin inhibitors and OAC-oil absorption capacity were found occupied at left side of biplot. This suggested that TPP-Total polyphenols, TF-total flavonoids, DPPH radical inhibition, ABTS radical inhibition, TA-total antioxidant, FRAP value had positive correlation among themselves. WAC and BD were observed on the left upper side of the biplot and TL and protein were found in middle portion of biplot. Based on this mathematical rule, uncorrelated variables occur at right angles to one another because the cosine of the angle between them is cosine 90° = 0, or not correlated. Similarly, the cosine of 0 is 1, which denotes a positive correlation between the variables (Lopez et al. 2006). The WAC and BD showed negative correlation with Cu, TF, OA, ABTS and protein content. Similarity, OAC have negative correlation with TSS, Fe, Fe, Zn, Total antioxidant, DPPH and Tannin content.
In case of dehulled samples, the parameters (TN-tannins, TPP-total polyphenols, ABTS radical inhibition, TSS-total soluble sugars, TA-total antioxidant, OA-oxalic acid, PR- protein, DPPH radical inhibition, FRAP value, Ca and Zn) were occupied on the right side of the biplot whereas the parameters, PA- phytic acid, TL-total lipid, BD- bulk density, WAC- water absorption capacity, TF- total flavonoids TI- trypsin inhibitors and OAC- oil absorption capacity, Fe and Cu were occupied at left side of biplot. This suggested that TN, TPP, ABTS, TSS, TA, OA, PR, DPPH, FRAP value, Ca and Zn had positive correlation among themselves. TF, PA, WAC, OAC and BD were observed on the left upper side of the biplot and TL was found in middle portion of biplot. The TF, PA, WAC, OAC and BD showed negative correlation with FRAP value and protein content. Similarity, TI and Fe have negative correlation with TPP, Tannins, ABTS, Zn and TSS content. In case of germinated samples, all the parameters except WAC and TI were occupied on the right side of the biplot while the parameters. This suggested that Protein, OAC, FRAP, OA, TF, DPPH, Ca and Cu had positive correlation among themselves. TI and WAC were observed on the left upper side of the biplot and BD and Zn were found in middle portion of biplot. The total antioxidant (TA), TSS, TPP, PA and Fe showed negative correlation with TI and WAC.
Conclusion
Unprocessed horsegram seeds have been used traditionally by rural people for preparation of different food items. The present study showed that germination improved total soluble sugars, bulk density, oil absorption capacity, water absorption capacity, iron, calcium, and cupper content in horsegram, significantly (P ≤ 0.05). On dehulling the content of total protein, total soluble sugars, total lipids and copper were improved significantly. Anti-nutritive compound like phytic acid, oxalic acid, tannins and trypsin inhibitors were significantly decreased after germination. The antioxidant compound (total polyphenols, flavonoids), antioxidant capacities (total antioxidant activity and ferric reducing antioxidant power) and free radical scavenging activity against DPPH and ABTS were significantly decreased after dehulling and germination. The improvement of functional properties (bulk density, oil and water absorption capacity) suggests that flour of germinated horsegram would be useful in formulation of foods where an oil and water holding property is an important consideration. Germination combined with dehulling process improved quality of horsegram by enhancing the nutritive value and reducing the antinutrients. The cost of germinating horse gram is minimal as expensive equipment and specialised facilities are not necessary so it may recommended that farmers in rural areas may apply a germination period of 3 days for horse gram when properly controlled heat treatment is not possible.
Acknowledgments
The authors are grateful to Indian Council of Agricultural Research (ICAR), for financial support to carry out this work at Vivekanand Parvatiya Krishi Anusandhan Sansthan (VPKAS), Almora (Uttarakhand) 263601.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Highlights
➢ Dehulling improves the protein, sugars and lipids content, significantly.
➢ Tannin, oxalic acid and phytic acid reduced significantly in dehulled samples over raw.
➢ Germination improved the nutrients content in horsegram, significantly.
➢ Anti-nutritive compounds were significantly decreased after germination.
➢ Antioxidant capacities decreased significantly after dehulling and germination.
References
- Anderson RA, Conway HF, Pfeifer VF, Griffin EL. Roll and extrusion – cooking of grain sorghum grits. Cereal Sci Today. 1969;14:372–380. [Google Scholar]
- AOAC . Official methods of analysis. 18. Washington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Phys. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Borhade VP, Kadam SS, Salunke DK. Solubilization and functional properties of mothbean (Vigna aconitifolia marechal) and horsegram (Macrotyloma uniflorum L. Verdc.) J Food Biochem. 1984;8:229–35. doi: 10.1111/j.1745-4514.1984.tb00326.x. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bravo L, Siddhuraju P, Saura-Calixto F. Composition of underexploited Indian pulses. Comparison with common legumes. Food Chem. 1999;64:185–192. doi: 10.1016/S0308-8146(98)00140-X. [DOI] [Google Scholar]
- El-Adawy TA. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Food Hum Nutr. 2002;57:83–97. doi: 10.1023/A:1013189620528. [DOI] [PubMed] [Google Scholar]
- Frias J, Fernandez-Orozco R, Zielinski H, Piskula M, Kozlowska H, Vidal-Valverde C. Effect of germination on the content of vitamin C and E of lentils. Polish J Food Nutr Sci. 2002;52:76–82. [Google Scholar]
- Ghavidel RA, Prakash J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci Technol. 2007;40:1292–1299. doi: 10.1016/j.lwt.2006.08.002. [DOI] [Google Scholar]
- Guansheng M, Ying J, Jianhua P, Frans K. Phytate, calcium, iron and zinc contents and their molar ratios in foods commonly consumed in China. J Agr Food Chem. 2005;53:10285–10290. doi: 10.1021/jf052051r. [DOI] [PubMed] [Google Scholar]
- Haug W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereal products. J Sci Food Agr. 1983;34:1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Kakade ML, Rackis JJ, Mcghee JE, Puski G. Determination of trypsin inhibitor activity of soy products: a collaborative analysis of an improved procedure. Cereal Chem. 1974;51:376–382. [Google Scholar]
- Kawsar SMA, Huq E, Nahar N, Ozeki Y. Identification and quantification of phenolic acids in Macrotyloma uniflorum by reversed phase HPLC. Am J Plant Physiol. 2008;3:165–172. doi: 10.3923/ajpp.2008.165.172. [DOI] [Google Scholar]
- Lopez A, Montano A, Garcia P, Garrido A. Fatty acid profile of table olives and its multivariate characterization using unsupervised (PCA) and supervised (DA) chemometrics. J Agric Food Chem. 2006;54:6747–6753. doi: 10.1021/jf0612474. [DOI] [PubMed] [Google Scholar]
- Malleshi NG, Daodu MA, Chandrasekhar A. Development of weaning food formulations based on malting and roller drying of sorghum and cowpea. Int J Food Sci Technol. 1989;24(5):519. [Google Scholar]
- Mishra KK, Pal RS, Arun KR, Chandrashekara C, Jain SK, Bhatt JC. Antioxidant properties of different edible mushroom species and increased bioconversion efficiency of Pleurotus eryngii using locally available casing materials. Food Chem. 2013;138:1557–1563. doi: 10.1016/j.foodchem.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Murugkar DA, Gulati P, Gupta C. Effect of sprouting on physical properties and functional and nutritional components of multi-nutrient mixes. Int J Food Nutr Sci. 2013;2(2):2–15. [Google Scholar]
- Petchiammal C, Hopper W. Antioxidant activity of proteins from fifteen varieties of legume seeds commonly consumed in India. Int J Pharm Pharm Sci. 2014;6(l 2):476–479. [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Ramadan EA. Effect of processing and cooking methods on the chemical composition, sugars and phytic acid of soybeans. Food Public Health. 2012;2(1):11–15. doi: 10.5923/j.fph.20120201.03. [DOI] [Google Scholar]
- Rao BSN, Prabhavathi T. Tannin content of foods commonly consumed in India and its influence on ionisable iron. J Sci Food Agr. 1982;33:89–96. doi: 10.1002/jsfa.2740330116. [DOI] [PubMed] [Google Scholar]
- Sangronis E, Machado CJ. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. J Food Sci Agri Technol. 2007;40(1):116–120. [Google Scholar]
- Satwadhar PN, Kadam SS, Salunkhe DK. Effects of germination and cooking on polyphenols and in vitro digestibility of horsegram and moth bean. Plant Foods Hum Nutr. 1981;31:71–76. doi: 10.1007/BF01093890. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sosulski FW. The centrifuge method for determining flour absorption in hard red spring wheat. Cereal Chem. 1962;39:344–350. [Google Scholar]
- Sreerama YN, Sashikala VB, Pratape VM. Phenolic compounds in cowpea and horsegram flours in comparison to chickpea flour: evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012;133:156–162. doi: 10.1016/j.foodchem.2012.01.011. [DOI] [Google Scholar]
- Sreerama YN, Sashikala VB, Pratape VM, Singh V. Nutrients and antinutrients in cowpea and horsegram flours in comparison to chickpea flour: evaluation of their flour functionality. Food Chem. 2012;131:462–468. doi: 10.1016/j.foodchem.2011.09.008. [DOI] [Google Scholar]
- Sudha N, Mushtari Begum J, Shambulingappa KG, Babu CK. Nutrients and some anti-nutrients in horsegram (Macrotyloma uniflorum (Lam.) Verdc.) Food Nutr Bull. 1995;16(1):81–83. [Google Scholar]
- Sushma D, Yadav BK, Tarafdar JC. Phytate phosphorus and mineral changes during soaking, boiling and germination of legumes and pearl millet. J Food Sci Technol. 2008;45(4):344–348. [Google Scholar]
- Tiwari AK, Manasa K, Kumar DA, Zehra A. Raw horsegram seeds possess more in vitro antihyperglycaemic activities and antioxidant properties than their sprouts. Nutrafoods. 2013;12:47–54. doi: 10.1007/s13749-013-0012-z. [DOI] [Google Scholar]
- Torres A, Frias J, Granito M, Vidal-Valverde C. Germinated Cajanus cajan seeds as ingredients in pasta products: chemical, biological and sensory evaluation. Food Chem. 2007;101(1):202–211. doi: 10.1016/j.foodchem.2006.01.018. [DOI] [Google Scholar]
- Vidal-Valverde C, Frias J, Sierra I, Blazquez I, Lambien F, Kuo YH. New functional legume food by germination: effect on the nutritive value of beans, lentils and peas. Eur Food Res Technol. 2002;215:472–476. doi: 10.1007/s00217-002-0602-2. [DOI] [Google Scholar]
- Wang C, Kinsella JE. Functional properties of novel proteins, AIfaIfa leaf protein. J Food Sci. 1976;41:286–292. doi: 10.1111/j.1365-2621.1976.tb00602.x. [DOI] [Google Scholar]
- Wang N, Lewis MJ, Brennan JG, Westby A. Effect of processing methods on nutrients and antinutritional factors in cowpea. Food Chem. 1997;58:59–68. doi: 10.1016/S0308-8146(96)00212-9. [DOI] [Google Scholar]