Abstract
Histamine formation and bacteriological changes caused by temperature abuse commonly occurring in the manufacturing process of standard canned tuna was assessed in microbiologically challenged tonggol (Thunnus tonggol). The in situ challenge was performed by water-soaking at 26–28 °C for 7 h to ensure the multiplication and active phase of fish microflora. Right after pre-cooking to back-bone temperature (BBT) of 50–52 °C, histamine dropped to 5.17 ± 2.71 ppm, and slowly reached 6.84 ± 1.69 ppm at 16 h abuse. On the contrary, histamine was reduced to 2.87 ± 1.23 ppm and eventually reached 5.01 ± 1.32 ppm at 24 h abuse in the pre-cooked fish previously frozen. The numbers of total aerobic bacteria, Enterobactericeae, psychrotroph, histamine forming bacteria (HFB) and diversity of fish microflora were revealed by cultural and nested PCR-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) techniques. Interestingly, frozen storage effectively halted histamine formation in raw fish throughout 16 h abuse despite the presence of HFB. These included the prolific strains of Morganella morganii, Proteus penneri, Proteus mirabilin, Citrobacter spp. The nested PCR-DGGE profile confirmed the presence of M. morganii and Citrobacter spp. in raw fish. These prolific strains were hardly observed in the precooked fish previously frozen. Frozen storage did not only promote even histamine distribution throughout fish muscle but also enhanced histamine loss during thawing and pre-cooking. Therefore, pre-cooking and frozen storage were proven to be the effective combined hurdles not only to reduce but also prolong histamine formation of the challenged toggol throughout 24 h of temperature abuse during canning process.
Keywords: Histamine, Thunnus tonggol, Precooking, Freezing, Histamine-forming Bacteria, PCR-DGGE
Introduction
Histamine is heterocyclic biologically active primary amine formed post-mortem in the muscle of scrombriod and non-scrombroid fish rich in free histidine by the catalysis of bacterial histidine decarboxylase (HDC) (Yesudhason et al. 2013). Apart from the abundant presence of free histidine in tuna muscle, histamine accumulation also depends on the contamination of HDC-producing microorganisms as well as time and temperature exposures during fish capturing, handling and processing (Economou et al. 2007; Guizani et al. 2005; Oliveira et al. 2012). Histamine intoxication is likely to occur with fish consumption because the presence of histamine is hardly detected by consumers as fish appearance and odor appear normal. Although the enzyme HDC is inactivated by cooking, histamine which is heat resistant cannot be destroyed by cooking, canning, smoking and freezing (Korashy and Farag 2005). Histamine in spoiled fish is more toxic than the pure form taken orally due to a missing factor as immidazole compound, and histamine-like compound derived from histidine (Lehane and Olley 2000). Apart from differences in sensitivity between individuals, the uneven distribution of histamine in the fish loin was suggested to explain why some people got ill, whereas others who had the same meal did not (Lόpez-Sabater et al. 1996).
In 1995, the US Food and Drug Administration had reduced maximum histamine limit to 5 mg per 100 g (50 ppm) in fish, and proclaimed new food safety regulation based on Hazard Analysis Critical Control Point (HACCP) approach (Yesudhason et al. 2013). Any fish containing histamine above this level have to be discarded for human consumption. However, the maximum histamine limit of 10 mg/100 g has been applied in Canada, Switzerland and Brazil (Oliveira et al. 2012). The European Commission (Commission Regulation EC No. 1441/2007 2007) has proposed that the average content of histamine in fish should not exceed 10 mg/100 g, and no single sample may contain more than 20 mg/100 g (Guizani et al. 2005).
Immediately after capturing, no histamine develops in fresh muscle of tuna (Lόpez-Sabater et al. 1996; Silva et al. 1998). However, histamine level increases as fish decomposition progresses. Both mishandling and high temperature abuse are common causes, which significantly enhance histamine concentration. The inactivation treatments such as heating, refrigeration and freezing have been well recognized to effectively prevent histamine formation (Baranowski et al. 1990; Kim et al. 1999). Nevertheless, histamine can subsequently develop once the abuse condition occurs. In fact, USFDA has suggested total of 4 and 12 h exposures for processing the whole fresh and previously frozen tuna (6 months storage), respectively, when the fish is abused at 21.1 °C (40 °F) or higher (USFDA 2011). This strict limitation does not practically accommodate tuna canning process of whole fish, particularly the large size. Since temperature/time abuse can hardly be avoided in a canned tuna process, in which whole frozen fish must be washed, thawed, eviscerated, pre-cooked/heated, skinned, deboned, cleaned, packed and sterilized. The pre-cooking and freezing could possibly prolong the subsequent histamine development. Therefore, maximum abuse period of tuna fish during processing and handling must be appropriately established to ensure product safety. Unfortunately, histamine formation has never been investigated in the microbially-challenged tuna in parallel to the diversity of fish microflora.
This study is aiming at evaluating the effectiveness of pre-cooking, frozen storage and their combination commonly practiced in most canned tuna industry on the subsequent histamine formation in relation to HFB profile. The whole fish challenged with in situ microflora originally existing in raw toggol were used to ensure the effectiveness of these hurdles. The alteration of bacterial number and diversity in fish muscle throughout the canning process was revealed using the nested PCR-DGGE technique.
Materials and methods
Fish and fish processing
Tonggol tuna (Thunnus tonggol) fish (weight range of 0.8–1.2 kg/fish), caught from the same shoaling group, were obtained fresh from suppliers (200 fish) in isothermal tanks containing abundant ice (0–4 °C). Ten of fresh fish were taken immediately upon arrival for histamine analysis. One hundred and ninety fish were submerged in water at ambient temperature (25–28 °C) for 7 h to naturally raise the microbial load. According to the sampling scheme expressed in Fig. 1, ten challenged fresh fish ([1]) were taken for histamine analysis. One hundred and ten fish were subjected to freezing at temperature of −30 °C (air blast freezer). They were stored at −18 to −25 °C for 5 weeks in a freezing storage facility of the canned tuna factory. Another seventy of the challenged fresh fish were eviscerated and ten of them were then left at temperature of 25–28 °C for 16 h (positive control, [2]). Ten eviscerated fish were taken for the analysis at 1.5 h cumulative abuse period ([3]). The rest (50 fish) was processed through pre-cooking and cooling and ten of them ([4]) were taken for the analysis right afterward. The rest of 40 precooked ones were left under manufacturing condition (24.8 – 32.7 °C) until the exposure periods reached 8.5, 12, 14 and 16 h ([5], [6], [7], [8], respectively). At each exposure period ([1] to [8]), ten fish were taken for histamine determination and microbiological analysis.
Fig. 1.
Sampling schemes for histamine and bacteriological analyses ([1] to [8] and [A] to [J], were sampling points for fresh and frozen fish, respectively). Air and water temperatures (at which fish were exposed) were monitored and recorded throughout the process
After 5-week frozen storage (110 fish), the first 10 fish ([A]) were subjected to histamine analysis. The rest of 100 frozen fish were subjected to through thawing, evisceration and pre-cooking, and cooling under the tuna manufacturing condition (Fig. 1). After thawing, twenty fish ([B]) were eviscerated and left under controlled temperature of 25–28 °C for 16 and 24 h. At each processing step, ten fish were taken after thawing ([C]), eviscerating ([D]), pre-cooking and cooling ([E]). Another 50 pre-cooked thawed fish were then left under manufacturing condition (24.8–32.7 °C) with cumulative abuse periods of 11, 12, 14, 16 and 24 h ([F], [G], [H], [I], [J], respectively). Ten fish were drawn at each cumulative period for histamine determination and microbiological analysis. Exposure temperatures and fish BBT throughout the experiment were monitored and recorded using a data logger set (Technical, USA). Both sets of experiments were conducted under an actual manufacturing condition of a GMP and HACCP certified canned tuna facility.
Fish thawing, precooking and temperature monitoring
After 5 week storage, the frozen fish were thawed by submerging in a running water containing tank for approximately 3 h until BBT reached 3 °C according to the processor’s thawing procedure and thawing facility. The water temperature was monitored and recorded using a data logger set (Technical, USA).
After thawing, tonggol fish were eviscerated, and pre-cooked thereafter using a pre-cooking facility of the tuna factory. The condensed steam (96–98 °C) was applied until the BBTs of three biggest fish (1.2 kg) in the lot approached 51–52 °C. The steam was off once the lowest BBT among these fish reached ≥ 50 °C.
To monitor BBT at freezing, thawing and pre-cooking; thermocouples (Ecklun-Harrison Technologies Inc., USA) were inserted from the upper dorsal part of the whole fish. The temperature monitoring during pre-cooking were recorded using a computerized temperature data logger set (Technical, USA).
Histamine analysis
The fish samples taken from each fish were cut from each side of lower and upper anterior loins, lower and upper mid sections about 50–100 g/portion for histamine analysis as shown in Fig. 2. Histamine contents in fish samples were determined based on the standard fluorometric method (AOAC Method 977.13 2012). Histamine was extracted from 10 g of fish muscle with 50 ml of 75 % methanol (LabScan, Thailand) using a homogenizer Model T25D (IKA, Germany). The interfering substances were removed by passing 1 ml of the extract through ion exchange resin Bio-Rad AG1-X8 (Bio-Rad, USA). The eluted extract and histamine standards (histamine dihydrochloride (Sigma-Aldrich, USA) were derivertized to allow condensation between histamine and orthophtalic aldehydes (Scharlau, Spain). The fluorescence intensity of the derivertized histamine was measured by a fluorometer Model FM109510-33 (Turner, USA) at 350 nm excitation and 444 nm emission wavelengths. From each section of fish, four determinations were done in duplicate samples for histamine analysis. The mean value of histamine concentration was taken from each sampled section. The histamine content reported for each section of fish representing the mean value of four estimations taken from duplicate determinations for both sides of the section.
Fig. 2.
Numbers representing fish sections taken for histamine analysis
Bacteriological enumeration
Total viable, psychrotroph and Enterobactericeae counts of tuna samples were performed using 25 g/fish of blended fish muscle from all sections. The flesh was homogenized using stomacher and serially diluted in 10 fold manner with sterile 0.1 % peptone. The appropriate dilutions were inoculated on duplicate plates of Trypticase Soy Agar (TSA) and Violet Red Bile Agar (VRBA) (BBL Microbiology Systems). TSA plates were incubated at 37 °C and 7 °C to enumerate total viable and psychrotrophic bacteria, respectively. Enterobactericeae count was determined from VRBA plates. All types of bacterial counts were expressed as CFU/g of fish.
Enumeration, isolation and identification of HFB
The appropriate dilutions prepared above were spread onto sterile petridish of Niven agar medium (0.5 % tryptone (Difco), 0.5 % yeast extract (Difco), 2 % L-histidine (free base), 0.5 % NaCl, 2.0 % agar, 0.006 % bromocresol purple and the final pH of the medium was adjusted to 5.3 with 5 N NaOH) for determination of presumptive HFB according to Fletcher et al. (1998). The purple or slight-purple colonies were counted and picked out from an appropriate dilution on the Niven agar plate. The number of such colony was reported as presumptive HFB count. The histamine production was confirmed using thin-layer chromatography (TLC) as described below. The positive supernatant was then quantitatively determined for histamine level using AOAC standard fluorometric method. The 16S rDNA of the positive isolates were amplified by PCR using universal primers (Altschul et al. 1990) as briefly described below. The nucleotide sequences were analyzed by BioDesign Co., Ltd. (Bangkok, Thailand). The sequences of full-length 16S rDNA were compared with those deposited in GenBank using the BLAST search program (available at http://www.ncbi.nlm.nih.gov/) for bacterial identification.
Detection of histamine production by thin layer chromatography
Each presumptive HFB was cultivated in TSB supplement with 2 % L-histidine using a 96 Deep-Well Plate, 2.2 ml Square Wells (Axygen Scientific, USA). After incubation at 35 °C for 24 h, the medium broth was centrifuged at 8000 rpm for 15 min (Sorvall, Legend XTR, Thermo, Germany). The clear supernatants was analyzed for histamine by TLC described by Lin et al. (1977) and modified by Barancin et al. (1998). The supernatant (0.5 μl) was loaded onto pre-coated silica gel 60 F254 TLC plates (Merck, Germany). Histamine dihydrochloride (100 mM) and histidine dihydrochloride (100 mM) were used as standards. The TLC plate was developed in a mobile phase consisting of methanol:ammonium hydroxide (20:1). The TLC plate was then air-dried and sprayed with 0.5 % ninhydrin in n-butanol, Tokyo Kasei Industry Co.) before heating at 105 °C for 1 min to visualize the presences of histamine and histidine.
Determination of bacterial diversity in fish muscle by PCR-DGGE technique
Each fish sample was incubated in 5 ml of TSB at 35 °C overnight. Total DNA from fish bacteria were extracted using phenol-chloroform method. The nested PCR was performed to amplify target DNA. The first round PCR was done with 27-f (5′-AGAGTTTGATCC TGGCTCAG-3′) and 1492-r (5′-GGTTACCTTGTTACGACTT-3′) primers to amplify the target 16S rDNA. PCR mixture (25 μl) consisted of 12.5 μl red dye PCR master mix (GeNei, Bangalore, India), 2.5 μl of each primer (2 mM), 0.5 μl DNA templates and 7 μl of sterile Milli-Q water. The first round PCR products were then used as templates for the second amplification. The second round PCR was performed to target the target V3-region with the primers of 338-357BA338f (5′-ACTCCTACGGGAGGCAGCAG-3′) and 518-534UN518-r (5′- GC-clamp-ATTACCGCGGCTGCTGG-3′) according to Hovda et al. (2007). The 20 μl of PCR products were separated by DGGE operating under the specified condition (Uraipan et al. 2014) using DGGE system (Cleaver Scientific, York, UK). The DNA fragments were stained with SYBR® Gold (Invitrogen, Grand Island, NY, USA) and viewed by using UV transillumination (Alpha innotech corporation, San Leandro, CA, USA). The major bands were visualized and cut under UltraBright LED Transilluminator (Gellex, Hsinchu City, Taiwan). Each individual DNA band was purified by NucleoSpin® Extraction П (Düren, Germany) before its nucleotide sequence was determined by BioDesign Co. Ltd. (Bangkok, Thailand). The nucleotide sequences were compared with those deposited in GenBank using the BLAST search program for bacterial identification.
Statistical analysis
All data were subjected to analysis of variance (ANOVA). The comparison of means was carried out by Duncan’s multiple range test. The statistical analysis was performed using the Statistical Package (SPSS 15.00 for windows).
Results and discussion
In situ challenge of bacterial community naturally present in tonggol
The mean value of the initial histamine (average) was 5.86 ± 3.64 ppm (ranged from 2.1 to 11 ppm) and initial total viable count ranged from 4.6 × 102–1.0 × 103 CFU/g. Fish were later submerged in water at temperature of 25–28 °C for 7 h to naturally elevate total viable count to 3.5 × 104–7.9 × 104 CFU/g with the mean value of initial histamine (average) of 4.61 ± 1.66 ppm (ranged from 2.3 to 10.45 ppm) according to Fig. 3a. This slight reduction of histamine could be due to its dissipation into soaking water. The microbial challenged tonggol were then subjected to temperature abuse during canned tuna processing. When the challenged fish were exposed to temperature of 27.6–28.6 °C for 1.5 h at butchering and eviscerating area; the average histamine level significantly increased to 8.46 ± 2.46 ppm (ranged from 3.81 to 14.84 ppm) as demonstrated in Fig. 3b. Such increase within a short period indicated that histamine production had been entering the active phase.
Fig. 3.
Histamine distribution of ten microbial challenged raw tonggol in various sections; (1) (5) upper anterior, (2) (6) lower anterior, (3) (7) upper mid section and (4) (8) lower mid section (a) and 1.5 h delay at butchering area, at which fish exposed to temperatures of 27.6 – 28.6 °C (b)
Histamine content and its distribution in tonggol muscles
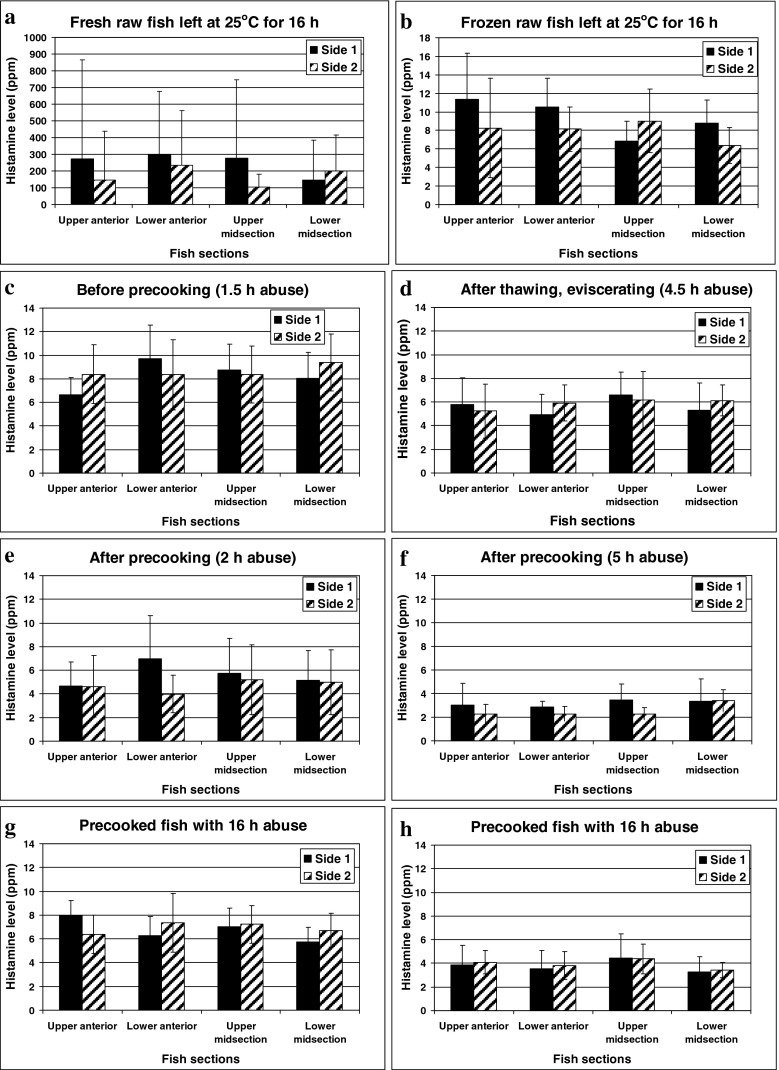
In this study, the highest histamine was detected in the anterior section of most fresh fish tested, particularly in the raw ones. This distribution pattern was supported by many authors (Baranowski et al. 1990; Lόpez-Sabater et al. 1996). The differences of maximum histamine level between anterior and mid sections became even greater in fish approaching advanced stage of decomposition (Fig. 4a). In addition, histamine level in the same section located at each side of fish was slightly different in the initial challenged fish (Fig. 4c to h). The greatest variation between each side of fish was clearly observed in fish approaching advanced stage of decomposition (Fig 4a). The wide range of standard deviation (±12.3 to ±1946 ppm) was shown in this study when histamine developed to high level. The highest histamine level and the widest standard deviation were localized in the anterior section. Because this portion is in proximity to the gill, at which prolific HFB were frequently found and isolated (Kim et al. 2003; Taylor and Speckhard 1983). As previously proven by Tao et al. (2009), the highest histamine level in the sterile muscle of Thunnus obesus was detected at the specific location, where the prolific HFB, such as M. morganii and Photobacterium phosphoreum were inoculated.
Fig. 4.
Changes of histamine levels in various sides of the microbial-elevated, fresh (left panel: c, e, and g) and previously frozen (right panel: d, f, and h) tonggol exposed to temperature abuse during processing for 16 h. Histamine changes of the positive controls, in which fresh (a) and previously frozen (b) fish were left at 25–28 °C for 16 h without pre-cooking
However, such pattern of distribution was not always true in raw and precooked fish previously frozen (Fig. 4d, f, h) with the exception of the control raw fish previously frozen (Fig. 4b). Surprisingly, the histamine variation among sections of previously frozen fish became narrower. This could be due to an occurrence of histamine diffusion over the storage period. Accordingly, Tao et al. (2009) clearly proved that the produced histamine can be diffused from an HFB inoculation site to remote area of fish muscle. This result suggests that five week frozen storage can possibly allow homogenous distribution of histamine throughout the whole fish muscle. Thus, the uneven distribution of this particular hazard can be reduced in fish portion and consequently lower histamine risk of the canned tuna product.
Effect of pre-cooking on histamine formation in the challenged fresh tonggol
After pre-cooking, fish were cooled and then rested under intermittent spray of tap water for 6 h. The cooled fish were then left at the cleaning area until the cumulative abuse period reached 16 h. The average histamine level was reduced from 8.46 to 5.17 ppm right after pre-cooking, at which the lowest temperature among three largest fish reached BBT of 51 °C (Fig. 4a, c, e, g). Although the pre-cooked fish exhibiting slight increase of histamine throughout the abuse period, the final histamine level remained significant lower than the initial level in the fish before pre-cooking (Fig. 4c to f). On the contrary, high histamine level was obviously developed in the control set of fresh raw fish left under the same temperature abuse. Such evidence ensured the presence of active HFB in the challenged fish subjected to this experiment. Similarly, Lόpez-Sabater et al. (1994) showed no increase of histamine in any Thunnus thynnus batches throughout the canning process. However, the tuna fish were not microbiologically challenged and the length of the abuse period was not mentioned.
The loss of histamine in the drained fluid during pre-cooking due to its water soluble property was also reported and explained by Lόpez-Sabater et al. (1994) and Shakila et al. (2005). The latter noted that moisture of pre-cooked tuna reduced from 73 to 57–59 % through the drain of water-soluble exudates from fish during heating. In addition, the greatest loss was observed in delay-processed, pre-cooked fish. Accordingly, Köse et al. (2003) reported the significant loss of histamine after cooking and pressing during processing of mackerel fish meal. Meanwhile, high histamine content was observed in the press liquor. Apart from histamine loss during pre-cooking, the dramatic reduction of HFB also caused such slow development of histamine. Although heating at temperature of 51–59 °C is theoretically insufficient to destroy all microorganisms in the fish, the outermost of fish actually exposed to high temperature (96–98 °C) of steam. The majority of fish microbes localized on skin were present at much greater number than in the fish muscle as demonstrated by Guillén-Velasco et al. (2004), Kim et al. (2003) and Taylor and Speckhard (1983). Therefore, the pre-cooking could reduce significant numbers of bacteria. This consequently reset microbial growth cycle leading to the delay of subsequent histamine development in the challenge fish up to 16 h abuse.
Effects of frozen storage and pre-cooking on histamine formation in the challenged tonggol
The histamine content remained quite stable throughout 5 weeks of frozen storage (data not shown) confirming that histamine cannot be destroyed by freezing and frozen storage corresponding to Economou et al. (2007). The histamine of raw tonggol previously frozen increased slowly from 5.86 ± 3.64 ppm to 8.70 ± 3.67 ppm (ranged from 3.71 to 21.7 ppm) throughout 16 h abuse (Fig 4b). In contrast, the average histamine of fresh raw fish elevated up to 209 ± 347 ppm (24 folds) under the same temperature abuse (Fig. 4a). Similar delay of histamine development in previously frozen mahi-mahi (40 week storage) was also observed by Baranowski et al. (1990). Kim et al. (1999) could not detect histamine formation in frozen albacore although fish were exposed to the temperatures of 25 °C for 4 days and 30 °C for 5 days. The total viable count reached maximum level of 1.0 × 108 CFU/g, at which the albacore developed spoilage appearance. Therefore, freezing and frozen storage can be applied as an effective strategy to extend the subsequent histamine formation during tuna canning process.
In addition, further reduction of histamine was observed once fish were thawed and pre-cooked thereafter. The histamine level of the challenged fish remained low throughout 24 h of temperature abuse (5.01 ± 1.32 ppm). At 16 h abuse, the average histamine of the precooked, thawed fish (3.85 ± 1.38 ppm) was significantly lower than the pre-cooked, fresh one (6.84 ± 1.69 ppm) and the initial frozen fish (5.86 ppm) as shown in Fig 4. This slow development of histamine in the pre-cooked, previously frozen fish was observed even at 24 h abuse (5.01 ± 1.32 ppm). The combination of these hurdles can therefore ensure product safety from histamine although the exposure was extended to 24 h. The conclusion supports the long history of canned tuna safety, which may be the consequence of pre-cooking. This practice is common among all tuna canners to facilitate skinning, cleaning and packing. Interestingly, frozen storage alone could prolong histamine formation of raw fish up to 16 h although fish were microbiologically challenged. Thus, the combined hurdles of pre-cooking and frozen storage could be accounted as the preventive strategy to control histamine hazard and to ensure safety of canned tuna production.
Effects of frozen storage and pre-cooking on bacteriological changes in tonggol muscles
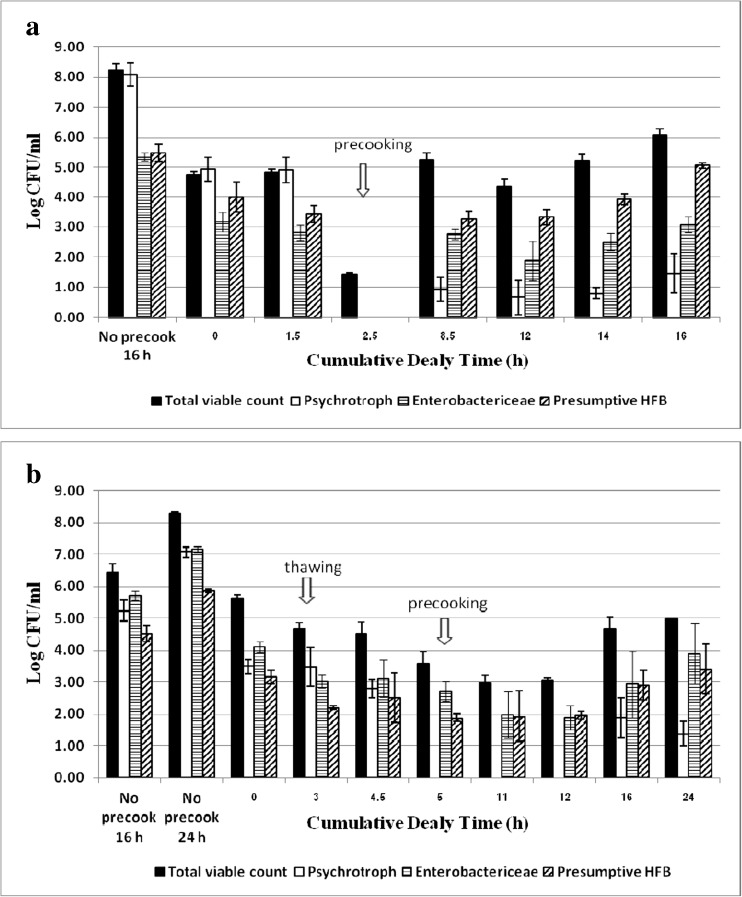
The pre-cooking significantly halted growth of all bacterial groups tested as shown in Fig. 5. The psychrotrophic bacteria, Enterobacteriaceae and presumptive HFB were greatly reduced to non-detectable levels in the fresh challenged tuna (Fig. 5a). However, the bacterial growth recovered and reached the level of 6.08 log CFU/g at 16 h abuse. Without pre-cooking, total viable counts reached 8.25 and 8.31 log CFU/g at 16 and 24 h abuse in raw muscles of fresh (Fig. 5a) and previously frozen fish (Fig. 5b), respectively. Moreover, the spoilage sign and histamine formation became correspondingly evident. The presumptive HFB were dominant bacteria in the pre-cooked fish, but Enterobactericeae were the major group observed in raw fish.
Fig. 5.
Changes of total viable count, psychrophile, Enterobacteriaceae and presumptive histamine-forming bacteria (HFB) counts in fresh (a) and frozen (b) tonggol exposed to temperature abuse in processing area before and after precooking. Histamine changes of the positive controls, in which fresh and frozen fish were left at 25–28 °C for 16 and 24 h, respectively without pre-cooking intervention. Bacteriological enumeration at each sampling period was analyzed from 3 fish with triplicate analysis of each parameter
Freezing and frozen storage had no impact on total viable and Enterobacteriaceae counts. However, HFB and psychrotrophic counts were significantly reduced. Thawing caused further reduction of HFB as well as Enterobacteriaceae. After pre-cooking, total bacterial count reduced only 0.93 log in the previously frozen fish, whereas significant 3.39 log reduction was observed in the fresh counterpart. This was because the thawed fish had lower initial temperature than the fresh ones. Nevertheless, the bacterial lag phase became longer after pre-cooking when fish was previously frozen. The majority of psychrotrophic bacteria were more susceptible to pre-cooking than Enterobacteriaceae and presumptive HFB. Slow bacterial recovery was observed in both fresh and thawed fish in relative to the delay of histamine development. After precooking, HFB became dominant flora in fresh fish, whereas Enterobacteriaceae dominated in the ones previously frozen.
No histamine formation was observed in the pre-cooked fresh fish although its presumptive HFB reached 5.05 log CFU/g. This was about the same level determined in the abused raw muscles of fresh (5.46 log CFU/g) and previously frozen fish (5.86 log CFU/g) with histamine concentration of 209.4 and 49.8 ppm, respectively. Thus, histamine level had no correlation with total bacteria and HFB numbers when fish was either pre-cooked or previously frozen. The result agrees with Kim et al. (1999) and Taylor and Speckhard (1983) in the regard of total bacteria but not in term of HFB. Taylor and Speckhard (1983) showed that histamine production of frozen tuna correlated to HFB concentration. This assumption may not be applicable for the processed fish. In this study, the high number of psychrotrophic bacteria seemed to correlate well with high histamine content in both raw muscles. In corresponding to no histamine formation, the majority of psychrotrophic bacteria was obviously reduced and hardly recovered after the fish was pre-cooked. The spoilage sign and histamine production were obviously noted at 24 h in the raw thawed fish corresponding to high loads of total bacteria and psychrotrophic bacteria up to 8.31 and 7.08 log CFU/g, respectively. The psychrotrophic bacteria appeared to play a major role in spoilage of raw fish correlating to Taylor and Speckhard (1983). Once fish decomposition approaches, the large amount of free histidine is released from fish muscle resulting to high histamine generation.
HFB isolation and diversity of tuna microflora in the challenged fish
Multiple strains of M. morganii, Proteus penneri, Proteus mirabilin, Citrobacter sp. were found and isolated from raw tonggol muscles (Table 1). Interestingly, great variation of histamine production was observed among the prolific strains of M. morganii and P. penneri. The histamine produced by these prolific strains was about 100 times higher than those produced by the slow formers. The latter may therefore have limited contribution to build up histamine in tuna muscle. This observation explained great variation of histamine level in various sections of fish muscles as noted above (3.2). M. morganii and P. penneri were isolated from the challenged raw fish, but not from initial raw and pre-cooked fish. Only a prolific HFB was isolated from the pre-cooked fish but its nucleotide sequence of 16S rDNA sequence did not match any strains deposited in the GenBank database. Although the prolific HFB still remained and actively recovered from the tonggol stored frozen up to 5 weeks (Table 1), their ability to form histamine was delayed.
Table 1.
Histamine production of HFB isolated from raw and pre-cooked fish muscles stored at −20 °C for 5 weeks
| Raw fish | Precooked fish | ||
|---|---|---|---|
| Bacterial strains | Histamine level (ppm) | Bacterial strains | Histamine level (ppm) |
| Proteus mirabilin A3-1 | 102.1 ± 37.9 | Unidentified bacteria E1-3 | 5602 ± 2049 |
| Proteus penneri B2-4.1 | 139.6 ± 65.8 | ||
| Morganella morganii A3-4 | 78.78 ± 20.3 | ||
| Proteus penneri B2-4.2 | 17,449 ± 1138 | ||
| Morganella morganii subsp. morganii B1-2.2 | 12,743 ± 1924 | ||
| Morganella morganii subsp. morganii B1-4 | 12,206 ± 607.8 | ||
| Proteus penneri D1-3 | 93.08 ± 20.3 | ||
| Proteus penneri D1-2 | 100.2 ± 15.1 | ||
| Morganella morganii subsp. morganii D2-2 | 10,030 ± 3958 | ||
| Morganella morganii subsp. morganii D2-4 | 4027 ± 3923 | ||
| Citrobacter sp. | 159.33 ± 48.1 | ||
| Unidentified bacteria D1-4 | 12,707 ± 1974 | ||
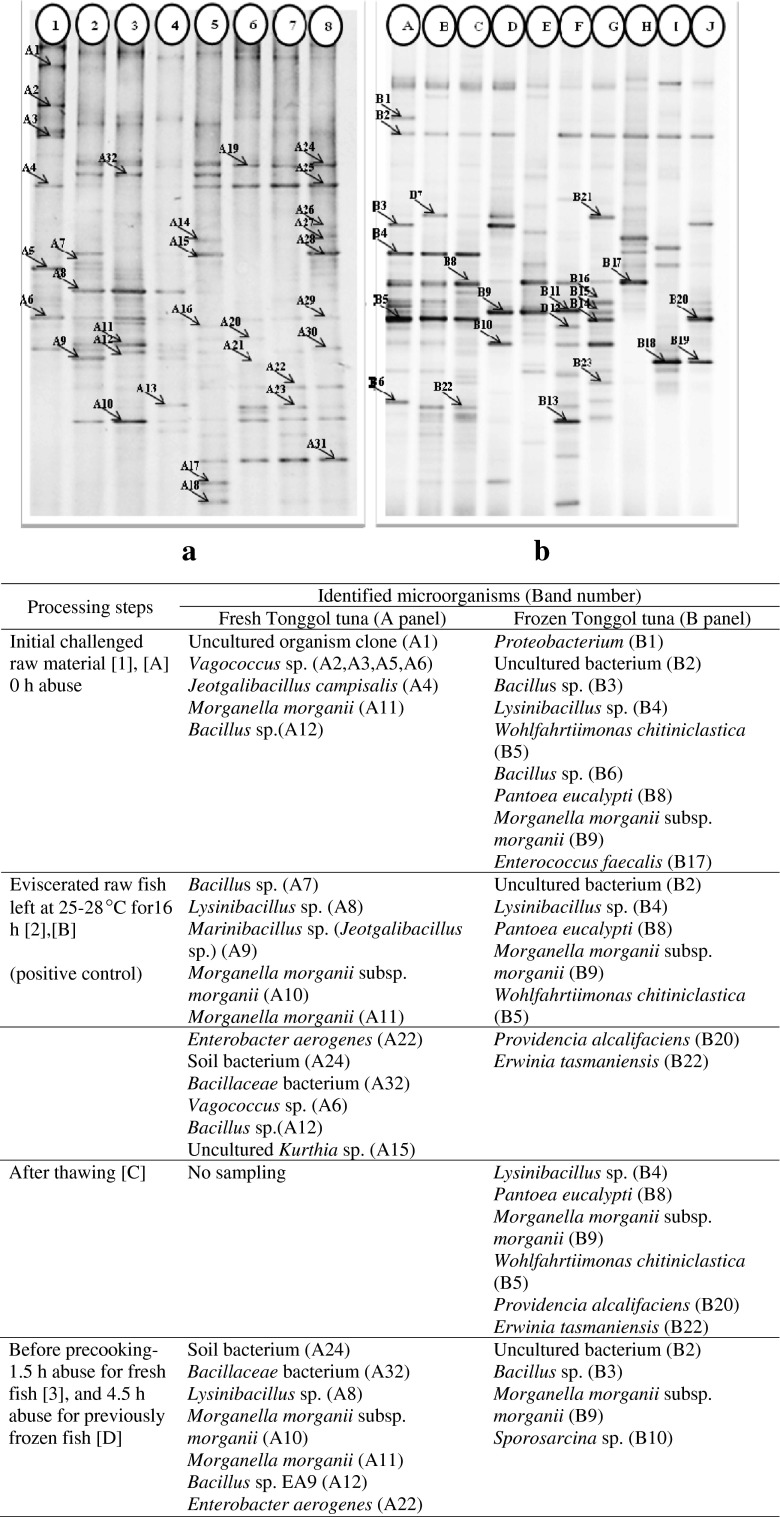
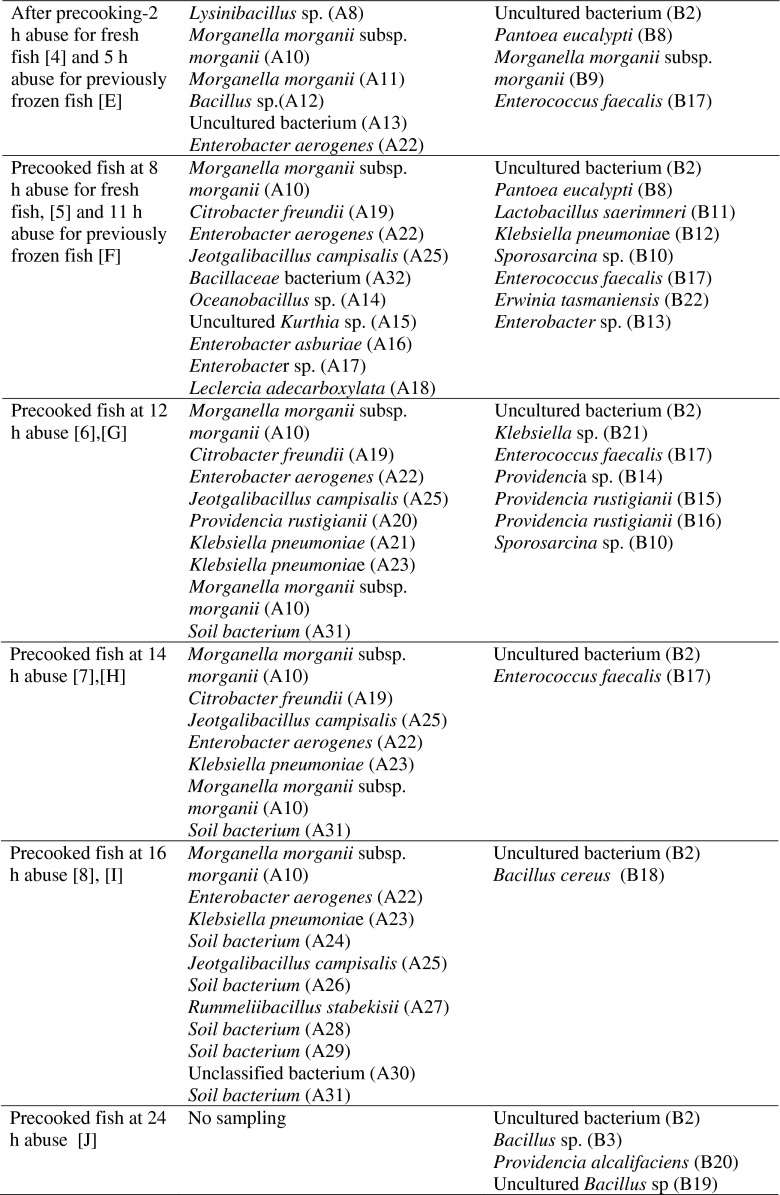
The PCR-DGGE technique targeting V3 region of bacterial 16S rDNA was applied to reveal bacteria population in the fish muscles (Fig. 6). This evidence strongly supported the presence of HFB in both fresh and frozen challenged fish which were either raw or pre-cooked. These included multiple strains of the well-known, prolific HFB such as Morganella morganii, Klebsiella pneumoniae, Citrobacter freundii, Enterococcus faecalis, Providencia rustigianii, Lactobacillus saerimneri etc. (Björnsdóttir-Butler et al. 2010; Bjornsdottir-Butler et al. 2009; Romano et al. 2013; Tembhurne et al. 2013; Wauters et al. 2004). Lysinibacillus spp., Pantoea spp. and Bacillus spp. were previously reported as potential histamine formers (Naila et al. 2011; Tsai et al. 2005). The PCR-DGGE profiles showed many potential HFB present in tonggol that could not be isolated and cultivated.
Fig. 6.
PCR-DGGE profiles and strain identification (below table) of bacterial diversity in fish muscles of fresh ([1] to [8]) and frozen ([A] to [J]) tonggol exposed to temperature abuse in processing area for 16 h and 24 h, respectively
The pre-cooking clearly altered bacterial diversity present in both fresh and frozen fish as shown by PCR-DGGE profile. For fresh tonggol, the dominant DGGE bands of raw fish muscle matched Vagococcus sp. (A2, A3, A5, A6); Lysinibacillus sp. (A8); Morganella morganii subsp. morganii (A10); Morganella morganii (A11); Bacillus sp. (A12). Right after pre-cooking, the band intensity significantly faded corresponding to low histamine development and bacterial count (Fig. 6a). Moreover, many bacteria, which could not be detected previously in the initial challenged fish, became dominant. These included Citrobacter freundii (A19); Providencia rustigianii (A20); Enterobacter aerogenes (A22); Klebsiella pneumoniae (A23); soil bacterium (A24, A26); Jeotgalibacillus campisalis (A25); Rummeliibacillus stabekisii (A27). Interestingly, Morganella morganii (A10, A11) and Klebsiella pneumoniae (A21) well-known as prolific HFB were significantly reduced right after pre-cooking. Strain A11 completely disappeared after cooling period at 16 h abuse, whereas strain A10 and A21 slowly resumed (lane 5–8, Fig. 6a). For previously frozen fish, Morganella morganii was not a dominant HFB after pre-cooking. In fact, the major HFB contributing to histamine development of pre-cooked, previously frozen fish were Enterobactericeae correlating to the culturable counts presented in Fig. 5. These included Enterobacter sp., Klebsiella pneumoniae, Pantoea eucalypti; Providencia sp. Many strains of Providencia appeared dominant in the pre-cooked fish muscle. Pantoea sp. was reported as a low histamine producer isolated from salted mackerel (Tsai et al. 2005). Contrarily, Providencia rustigianii were reported for its high histamine formation (Bjornsdottir-Butler et al. 2009).
Although the combination of pre-cooking and freezing effectively prolonged histamine formation, they could not destroy prolific HFB present in the challenged fish. Kim et al. (1999) concluded that HFB in previously frozen albacore may recover slowly at 25 °C. However, they lost the activity to decarboxylate histidine at 30 °C. This observation was in contradiction to Taylor and Speckhard (1983). These authors found no HFB isolated from any of samples taken from frozen skipjack tuna. Regarding this study, slow development of histamine in the pre-cooked, previously frozen tonggol could be due to slow recovery of HFB. Freezing somewhat injured bacterial cell wall, therefore enhanced cell rupture. In the meantime, fish tissue was similarly disrupted leading to the significant loss of water, many soluble nutrients and more importantly free histidine as well as histamine through fish exudates during thawing and steaming.
It is logically suspected that loss of water content, histamine, free histidine, many soluble nutrients and co-factors may contribute to such slow bacterial growth and their ability to develop histamine in fish muscle. Loss of many free amino acids in fish muscle induced by frozen storage were previously reported by Castrillón et al. (1996) and Jiang and Lee (1985). The significant loss of sulfur containing amino acids and protein solubility was observed when sardine was stored at −20 °C. The greatest drop of S-amino acids followed by histidine, tyrosine, leucine, lysine and phenylalanine was noted by Castrillón et al. (1996). Jiang and Lee (1985) concluded that the NH3 group of free amino acids (histidine, lysine, taurine, glycine, proline and glutamic acid) interacted with carbonyl groups on protein. Thus, denaturation of mackerel proteins was accelerated during frozen (−20 °C) storage.
Conclusion
The combination of frozen storage with pre-cooking effectively prolonged the subsequent histamine formation in the bacterial challenged tonggol up to 24 h. The temperature abuse occurred before pre-cooking was critical for histamine formation during canning process of fresh tonggol. However, frozen storage significantly reduced such risk. Frozen storage did not only promote even histamine distribution throughout fish muscle but also enhanced histamine loss during thawing and pre-cooking due to the rupture of fish tissue by ice crystal. Freezing and frozen storage are therefore recommended as preventive measures to ensure safety of canned tuna manufacture. The pre-cooking reduced histamine, bacterial recovery and histamine forming ability. Thus, it accentuated losses of water and soluble nutrients leading to alteration of fish bacterial profile. The combined hurdles of pre-cooking and frozen storage therefore delay subsequent histamine production of the challenged tonggol up to 24 h abuse throughout canning manufacture.
Acknowledgments
This research was funded by the PSU-General Income Budget 2012 (AGR550139S). Our great appreciation is expressed to Songkla Canning Public Company Limited for the canned tuna processing facility and their excellent technical assistance.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DL. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- AOAC . Histamine in Seafood: Fluorometric Method. Sec. 35.1.32. Method 977.13. nineteenth. Maryland: AOAC International; 2012. [Google Scholar]
- Barancin CE, Smoot JC, Findlay RH, Actis LA (1998) Plasmid-mediated histamine biosynthesis in the bacterial fish pathogen Vibrio anguillarum. Plasmid 39:235–244 [DOI] [PubMed]
- Baranowski JD, Frank HA, Brust PA, Chongsiriwatana M, Premaratne RJ. Decomposition and histamine content in mahimahi (Coryphaena hippurus) J Food Prot. 1990;53:217–222. doi: 10.4315/0362-028X-53.3.217. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir-Butler K, Bolton GE, McClellan-Green PD, Jaykus L-A, Green DP. Detection of gram-negative histamine-producing bacteria in fish: a comparative study. J Food Prot. 2009;72:1987–1991. doi: 10.4315/0362-028x-72.9.1987. [DOI] [PubMed] [Google Scholar]
- Björnsdóttir-Butler K, Bolton GE, Jaykus L-A, McClellan-Green PD, Green DP. Development of molecular-based methods for determination of high histamine producing bacteria in fish. Int J Food Microbiol. 2010;139:161–167. doi: 10.1016/j.ijfoodmicro.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Castrillón A, Alvarez-Pontes E, García-Arias M. Influence of frozen storage and defrosting on the chemical and nutritional quality of sardine (Clupea pilchardus) J Sci Food Agric. 1996;70:29–34. doi: 10.1002/(SICI)1097-0010(199601)70:1<29::AID-JSFA461>3.0.CO;2-2. [DOI] [Google Scholar]
- Commission Regulation (EC) No. 1441/2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Off J Eur Union L. 2007;322:12–29. [Google Scholar]
- Economou V, Brett MM, Papadopoulou C, Frillingos S, Nichols T. Changes in histamine and microbiological analyses in fresh and frozen tuna muscle during temperature abuse. Food Addit Contam. 2007;24:820–832. doi: 10.1080/02652030701278321. [DOI] [PubMed] [Google Scholar]
- Fletcher G, Summers G, Van Veghel P. Levels of histamine and histamine-producing bacteria in smoked fish from New Zealand markets. J Food Prot. 1998;61:1064–1070. doi: 10.4315/0362-028x-61.8.1064. [DOI] [PubMed] [Google Scholar]
- Guillén-Velasco S, Ponce-Alquicira E, Saravia FG, Guerrero-Legarreta I. Histamine production by two Enterobacteriaceae strains isolated from tuna (Thunnus thynnus) and jack mackerel (Trachurus murphyii) Int J Food Prop. 2004;7:91–103. doi: 10.1081/JFP-120022984. [DOI] [Google Scholar]
- Guizani N, Al-Busaidy MA, Al-Belushi IM, Mothershaw A, Rahman MS. The effect of storage temperature on histamine production and the freshness of yellowfin tuna (Thunnus albacares) Food Res Int. 2005;38:215–222. doi: 10.1016/j.foodres.2004.09.011. [DOI] [Google Scholar]
- Hovda MB, Sivertsvik M, Lunestad BT, Lorentzen G, Rosnes JT. Characterisation of the dominant bacterial population in modified atmosphere packaged farmed halibut (Hippoglossus hippoglossus) based on 16S rDNA-DGGE. Food Microbiol. 2007;24:362–371. doi: 10.1016/j.fm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Jiang ST, Lee TC. Changes in free amino acids and protein denaturation of fish muscle during frozen storage. J Agric Food Chem. 1985;33:839–844. doi: 10.1021/jf00065a018. [DOI] [Google Scholar]
- Kim SH, An H, Price RJ. Histamine formation and bacterial spoilage of albacore harvested off the U.S. northwest coast. J Food Sci. 1999;64:340–343. doi: 10.1111/j.1365-2621.1999.tb15896.x. [DOI] [Google Scholar]
- Kim SH, An H, Wei C-I, Visessanguan W, Benjakul S, Morrissey MT, Su Y-C, Pitta TP. Molecular detection of a histamine former, Morganella morganii, in albacore, mackerel, sardine, and a processing plant. J Food Sci. 2003;68:453–457. doi: 10.1111/j.1365-2621.2003.tb05693.x. [DOI] [Google Scholar]
- Korashy NT, Farag HEl-SM (2005) Histamine and histamine producing bacteria in some local and imported fish and their public health significance. Res J Agric Biol Sci 1:329–336
- Köse S, Quantick P, Hall G. Changes in the levels of histamine during processing and storage of fish meal. Anim Feed Sci Technol. 2003;107:161–172. doi: 10.1016/S0377-8401(03)00127-5. [DOI] [Google Scholar]
- Lehane L, Olley J (2000) Histamine fish poisoning revisited. Int J Food Microbiol 58:1–37 [DOI] [PubMed]
- Lin JS, Baranowski JD, Olcott HS. Rapid thin-layer chromatographic densitometric determination of histamine in tuna. J Chromatogr A. 1977;130:426–430. doi: 10.1016/S0021-9673(00)89838-6. [DOI] [PubMed] [Google Scholar]
- Lόpez-Sabater EI, Rodríguez-Jerez JJ, Roig-Sagués AX, Mora-Ventura MAT. Bacteriological quality of tuna fish (Thunnus thynnus) destined for canning: effect of tuna handling on presence of histidine decarboxylase bacteria and histamine level. J Food Prot. 1994;57:318–323. doi: 10.4315/0362-028X-57.4.318. [DOI] [PubMed] [Google Scholar]
- Lόpez-Sabater EI, Rodríguez-Jerez JJ, Roig-Sagués AX, Hernádez-Herrero M, Mora-Ventura MT. Sensory quality and histamine formation during controlled decomposition of tuna (Thunnus thynnus) J Food Prot. 1996;59:167–174. doi: 10.4315/0362-028X-59.2.167. [DOI] [PubMed] [Google Scholar]
- Naila A, Flint S, Fletcher GC, Bremer PJ, Meerdink G. Biogenic amines and potential histamine-forming bacteria in rihaakuru (a cooked fish paste) Food Chem. 2011;128:479–484. doi: 10.1016/j.foodchem.2011.03.057. [DOI] [PubMed] [Google Scholar]
- Oliveira RBA, Evangelista WP, Sena MJ, Gloria MBA. Tuna fishing, capture and post-capture practices in the northeast of Brazil and their effects on histamine and other bioactive amines. Food Control. 2012;25:64–68. doi: 10.1016/j.foodcont.2011.10.011. [DOI] [Google Scholar]
- Romano A, Trip H, Campbell-Sills H, Bouchez O, Sherman D, Lolkem JS, Lucas PM. Genome sequence of Lactobacillus saerimneri 30a (Formerly Lactobacillus sp. Strain 30a), a reference lactic acid bacterium strain producing biogenic amines. Genome Announc. 2013;1:1–2. doi: 10.1128/genomeA.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakila RJ, Jeyasekaran G, Vyla SAP, Kumar RS. Effect of delayed processing on changes in histamine and other quality characteristics of 3 commercially canned fishes. J Food Sci. 2005;70:M24–M29. doi: 10.1111/j.1365-2621.2005.tb09042.x. [DOI] [Google Scholar]
- Silva CCG, Da Ponte DGB, Dapkevicius MLNE. Storage temperature effect on histamine formation in big eye tuna and skipjack. J Food Sci. 1998;63:644–647. doi: 10.1111/j.1365-2621.1998.tb15803.x. [DOI] [Google Scholar]
- Tao Z, Sato M, Yamaguchi T, Nakano T. Formation and diffusion mechanism of histamine in the muscle of tuna fish. Food Control. 2009;20:923–926. doi: 10.1016/j.foodcont.2009.01.011. [DOI] [Google Scholar]
- Taylor SL, Speckhard MW. Isolation of histamine-producing bacteria from frozen tuna. Mar Fish Rev. 1983;45:35–39. [Google Scholar]
- Tembhurne M, Ghag A, Sanathkumar H, Nayak BB. Dominance of enterobacteria among histamine-producing bacteria isolated from Indian mackerel. Adv Microbiol. 2013;3:537–542. doi: 10.4236/aim.2013.37072. [DOI] [Google Scholar]
- Tsai Y-H, Lin C-Y, Chang S-C, Chen H-C, Kung H-F, Wei C-I, Hwang D-F. Occurrence of histamine and histamine-forming bacteria in salted mackerel in Taiwan. Food Microbiol. 2005;22:461–467. doi: 10.1016/j.fm.2004.11.003. [DOI] [Google Scholar]
- Uraipan S, Brigidi P, Hongpattarakere T. Antagonistic mechanisms of synbiotic between Lactobacillus plantarum CIF17AN2and green banana starch in the proximal colon model challenged with Salmonella Typhimurium. Anaerobe. 2014;28:44–53. doi: 10.1016/j.anaerobe.2014.05.002. [DOI] [PubMed] [Google Scholar]
- USFDA (2011) Scombrotoxin (histamine) formation. In: Fish and Fishery Products Hazards and Controls Guidance, fourth ed. U.S. Food and Drug Administration, Maryland, pp 113–152
- Wauters G, Avesani V, Charlier J, Janssens M, Delmée M. Histidine decarboxylase in Enterobacteriaceae revisited. J Clin Microbiol. 2004;42:5923–5924. doi: 10.1128/JCM.42.12.5923-5924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesudhason P, Al-Zidjali M, Al-Zidjali A, Al-Busaidi M, Al-Waili A, Al-Mazrooei N, Al-Habsi S. Histamine levels in commercially important fresh and processed fish of Oman with reference to international standards. Food Chem. 2013;140:777–783. doi: 10.1016/j.foodchem.2012.11.030. [DOI] [PubMed] [Google Scholar]