Abstract
Changes occurring in seven chestnut (Castanea sativa sp.) cultivars, caused by boiling and roasting, on starch content, cell and starch granules dimension were evaluated, and morphological changes were characterized by scanning electron microscopy. Three clear patterns of variation were detected after processing, namely: i) decrease of starch content with processing; ii) starch increase with the applied treatments; iii) increase of starch with boiling and decrease with roasting. Starch granules of raw chestnuts presented round, oval or elliptical form, external smooth surface and eccentric hilum, with rather ellipsoid-shaped growth rings. Processing resulted in modifications of the granules, with fusion of individual granules, and gelatinization taking place with the formation of elongated clusters. The present results indicate that boiling and roasting, besides changing the starch content of chestnut, causes important modifications in the starch granules, which can affect the sensory, rheological and chemical characteristics of chestnuts.
Keywords: Castanea sativa, Starch, Boiling, Roasting, Granule morphology
Introduction
Chestnut (Castanea sativa sp.) is an ancient cultivated tree crop in Portugal, with a fruit production estimated in 19,100 tons in 2012, ranking Portugal as the 8th world’s producer of this commodity (FAO 2012). The main production area is located in the Trás-os-Montes region, contributing to 85 % of the total Portuguese production and has a great social and economic importance to the local population, being part of the landscape patrimony (Borges et al. 2008). The favourable climatic, edaphic and ecological conditions associated to the promising chestnut market, result of recognition of the importance of this nut in human health, have been encouraging the establishment of new planting areas. The existence of many well-adapted native cultivars, with higher nutritional qualities and greater commercial value (Silva et al. 2011) is an important incentive to perform more research with these cultivars, since few studies had been conducted and published. Chestnuts present a starch content (an average of 22.3 g/100 g of raw edible portion) that places this fruit among the main sources of starch, even when compared with raw potatoes (about 16 g/100 g) or beans (around 20 g/100 g) (Pizzoferrato et al. 1999). Other important characteristic to be studied is the starch structure. Indeed, in Europe, the utilization of chestnuts to produce the marron glace, which depends of starch structure, becomes increasingly important. Furthermore, some studies about new food industry uses of starches with desired functional properties where starch from chestnuts is employed for production of gluten-free baked foods (Correia and Beirão-da-Costa 2012), points out that its characterization is very important for producers and consumers. Starch is made of two types of α-D-glucose linked polymers, amylase or amylopectin (Pizzoferrato et al. 1999), and the botanical origin of the starch determines the structure of starch. In chestnut, starch granules diameter can vary from 2 to 21 μm (Correia et al. 2012; Cruz et al. 2013), and present a round and oval shape (Correia et al. 2012; Cruz et al. 2013). According to Wen-Yang et al. (2008), in mature wheat grains, the starch is deposited in two types of granules, B-type granules (diameter < 9.9 μm) and A-type granules (diameter > 9.9 μm) and they have different rheological and swelling properties and baking characteristics leading to diverse uses in industrial food. For instance, an increase in the B-type granule content increases farinograph water absorption and improve breadmaking quality (Soh et al. 2006). In chestnut, the starch granule is generally classified into three types (A, B and C) based on the X-ray diffraction pattern given by the amylopectin crystalline structure, and recent studies have classified its granules either as B-type (Cruz et al. 2013) or as C-type (Correia et al. 2012).
Previous studies (Attanasio et al. 2004) have shown that during roasting or boiling process, starch suffers irreversible changes, losing its crystalline organization, that are dependent on the amount of water and heat available in the process. According to Thomas and Atwell (1999), the first set of changes during heating is termed as gelatinization and is characterized by the irreversible disruption of molecular order, and the initial increase in granule size, resulting in increased suspension viscosity. These changes in starch granules after heat treatment contribute to food properties such as rheological behaviour, texture, moisture retention, as well as to starch digestibility (Noda et al. 2008). Furthermore, the use of nonconventional baking (e.g. electrical or microwave ovens) processes have shown that the texture of the baked products varied with the baking conditions, as a result of different starch granule morphologies caused by altered heating regime (Yin and Walker 1995).
Hence, the present study intended to characterize the starch granule structure and the changes produced by two of the most common methods for chestnut cooking, boiling and roasting, on seven cultivars of sweet chestnut fruits, which were grown in northeast of Portugal, in order to evaluate its further use in baked or other processed products, like marron glace. For this, several parameters were evaluated, namely biometric parameters, starch content in fruits and the size and morphology of starch granule.
Material and methods
Samples
In this study, fruits from seven representative native cultivars, belonging to three protected designation of origin (PDO), previously characterized by Borges et al. (2008), were used: Boaventura, Côta, Lamela and Trigueira cultivars (PDO Terra Fria); Lada and Longal cultivars (PDO Padrela); Martaínha cultivar (PDO Soutos da Lapa). Three trees from each cultivar were selected, and three replications (1 kg each) of mature chestnuts were randomly collected from each tree, and kept in a refrigerator until further analysis.
Sample processing
Three different treatments were applied to fruits: i) raw fruits: 500 g of raw fruits were peeled; ii) boiling process: 500 g of raw chestnuts were cross-cut at the top, mixed with 2 L of water and 5 g of salt and boiled, for 20 min; iii) roasting process: 500 g of raw chestnuts were cut with a knife, spread over with 7 g of salt and placed, for 40 min, in an electric oven (Nabertherm Mod. – L9R) with air circulation, previously heated at 200 °C. In both boiling and roasting, the processing was performed in three replicates, and samples were peeled, cut into small pieces and milled in a Model SK 100 Cross Beater (Retsch GmbH, Haan, Germany) to a uniformly fine powder (0.5 mm sieve). Samples were stored in a freezer at-80 °C until analyses were performed.
Biometric parameters
For the determination of the fruit mass, 15 whole fruits, from each treatment, where individually weighed, and moisture content was determined by the official method of the Association of Official Analytical Chemists (AOAC 2000). Length, width and thickness were measure using a digital calliper. Sphericity and roundness indexes were calculated as follows:
and , where L is length, W is width and T is thickness. For both cases, the higher the values, a more round shape is presented by the fruit.
Starch content
For the determination of starch, the method of Salomonsson et al. (1984) was used. This method includes a first step of fat extraction, followed by starch hydrolysis. The percentage of starch was calculated as follows:
Light microscopy
Starch granules were stained using Lugol’s iodine solution. Starch granules were observed with an Olympus IX51 inverted light microscope (Olympus Biosystem, Munich, Germany) and photographed with a digital camera (ColorView III; Soft Imaging System GmbH, Münster, Germany).
Scanning electron micrographs
For scanning electron microscopy (SEM) observations, transverse sections of the raw, boiled and roasted chestnuts of all cultivars were conducted at 15 kV and 5 Torr, using a Philips/FEI SEM/ESEM Quanta 400 (FEI, Hillsboro, Oregon, United States). The samples were held with double-sided carbon adhesive tape on aluminium sample holder. Using the images obtained by electron microscopy, the area and perimeter of the chestnut cells and granules of starch were determined. For this purpose, the image analysis program Image-Pro Plus 6.2 was used, allowing the identification of the cells to measure, selecting study parameters, and export the data to a spreadsheet for further analysis.
Statistical analysis
Data from unit mass, dry mass, and starch content analysis, as well as length, width, thickness sphericity and roundness indexes, are presented as the mean of 15 analysed fruits displaying the respective standard deviation (SD) values. Values of cell and granule size were achieved by measuring 150 different individual cells or granules, and are presented as mean ± SD, with the minimum and maximum measure value also presented. Differences among means were determined by analysis of variance (ANOVA), using SPSS v.18 software and averages were compared using Duncan test (P < 0.05).
Results and discussion
Biometric parameters
Results regarding the biometric parameters evaluated in chestnut cultivars are presented in Table 1. There were significant differences detected between cultivars, for all analysed parameters, with the exception of dry mass in boiled samples. Furthermore, the values obtained in the present work are in accordance to the ones previously reported, regarding chestnut cultivars, including some presently described (Silva et al. 2007). These authors refer range values of length between 2.9 and 3.5 cm, of width between 2.5 and 3.5 cm and of thickness between 1.9 and 3.2 cm, similar to the ones reported in the present work. The length of the fruits where lower in Lada cultivar (2.9 cm), while higher in Côta cultivar (3.8 cm). By other hand, the lowest values of width were found for Trigueira cultivar (2.5 cm), being the highest value of this parameter detected in fruits of the Martaínha cultivar (3.4 cm). This cultivar also presented the thickest fruits (2.4 cm), although not statistically different from Boaventura cultivar, being the fruits of Longal cultivar the ones with lowest thickness (1.6 cm).
Table 1.
Biometric parameters (mean ± SD, n = 15) of the studied raw, boiled and roasted chestnut cultivars
| Length (cm) | Width (cm) | Thickness (cm) | Sphericity | Roundness | Fruit mass (g) | Dry mass (g/100 g edible part) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Raw | Raw | Boiled | Roasted | Raw | Boiled | Roasted | P (T) | ||||
| Boaventura | 3.34 ± 0.18 b | 3.28 ± 0.34 d | 2.20 ± 0.36 d | 86.31 ± 5.04 e | 0.82 ± 0.06 d | 18.67 ± 3.5 f | 18.81 ± 3.2 d | 15.32 ± 3.2 f | 49.39 ± 0.03 cd B | 53.77 ± 0.04 a C | 44.42 ± 0.06 e A | 0.0001 |
| Côta | 3.79 ± 0.10 c | 3.21 ± 0.29 d | 1.94 ± 0.21 c | 75.99 ± 3.70 b | 0.69 ± 0.05 b | 13.04 ± 1.5 cd | 13.07 ± 1.0 b | 10.06 ± 2.5 bcd | 49.27 ± 0.07 cd B | 55.87 ± 0.10 a C | 44.75 ± 0.01 f A | 0.0001 |
| Lada | 2.93 ± 0.17 a | 2.91 ± 0.24 c | 1.64 ± 0.22 a | 82.05 ± 5.34 d | 0.78 ± 0.07 c | 12.51 ± 2.6 bc | 12.65 ± 2.5 b | 10.61 ± 1.8 cde | 39.97 ± 0.07 a B | 48.98 ± 0.06 a C | 38.03 ± 0.04 a A | 0.0001 |
| Lamela | 3.26 ± 0.21 b | 2.86 ± 0.27 bc | 1.82 ± 0.29 bc | 78.67 ± 4.72 bc | 0.72 ± 0.06 b | 16.54 ± 4.0 ef | 16.91 ± 3.9 cd | 11.92 ± 2.4 de | 45.99 ± 0.10 bc B | 54.94 ± 0.10 a C | 42.81 ± 0.01 c A | 0.0001 |
| Longal | 3.38 ± 0.18 b | 2.72 ± 0.20 b | 1.61 ± 0.28 a | 72.52 ± 3.74 a | 0.64 ± 0.04 a | 10.51 ± 2.7 ab | 12.46 ± 1.8 b | 8.69 ± 1.3 abc | 43.98 ± 0.07 ab B | 55.67 ± 0.00 a C | 40.45 ± 0.02 b A | 0.0001 |
| Martaínha | 3.35 ± 0.19 b | 3.40 ± 0.28 e | 2.35 ± 0.27 d | 89.26 ± 4.93 f | 0.86 ± 0.06 e | 14.99 ± 2.4 de | 15.75 ± 2.3 c | 8.50 ± 2.3 ab | 49.97 ± 0.21 cd B | 56.72 ± 0.05 a C | 46.79 ± 0.04 g A | 0.0001 |
| Trigueira | 2.94 ± 0.12 a | 2.54 ± .15 a | 1.69 ± 0.22 ab | 79.01 ± 4.17 c | 0.72 ± 0.05 b | 9.01 ± 1.3 a | 9.48 ± 1.4 a | 7.76 ± 2.0 a | 51.27 ± 0.13 d A | 49.27 ± 0.13 a A | 43.44 ± 0.04 d A | 0.7012 |
| P(C) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0068 | 0.1841 | 0.0001 | |
Means within a column followed by the same letter and means within row followed by the same capital letter are not significantly different at P < 0.05, according to the Duncan multiple range test
In order to characterize the shape of the fruits, both sphericity and roundness indexes were calculated. Sphericity indexes ranged from 73 to 89, for Longal and Martaínha cultivars, respectively, while roundness values presented a minimum of 0.64 and a maximum of 0.86, for the same cultivars, respectively. Similar values of sphericity and roundness were previously reported for Portuguese (Silva et al. 2007) chestnut cultivars as well as for roundness, in Portuguese cultivars. Fruits from Longal cultivar showed the lower values of sphericity and roundness, presenting a more elongated form. By other hand, taking in consideration the high values of sphericity and roundness, the fruits from Martaínha cultivar are the ones which shape is closer to being round.
There were significant variations in fruit mass (F.M.) and dry mass (D.M.) among all cultivars and treatments. Regarding F.M., Boaventura cultivar presented the higher mass (19 g), with significant differences to all other cultivars, while Trigueira (9 g) cultivar presented the lightest fruits (Table 1), having the fruits of this cultivar half the mass of the heaviest cultivar (Boaventura). The processing of chestnut fruits resulted in two different behaviours for F.M.. By one hand, boiling the fruits increased their mass, in all cultivars, but particularly for Longal cultivar, with an increase of about 2 g (about 19 % more) and Martaínha cultivar, that increased its mass by 0.8 g (about 5 %). Contrarily, the roasting of the fruits resulted in the decrease of the mass, for all cultivars, being especially noticeable the reduction of this parameter on the fruits of Martaínha, which recorded a loss of around 6 g (about 44 %). Another noticeable fact is that in either raw or processed fruits, Boaventura cultivar presented always the highest F.M. and Trigueira cultivar the lowest, with significant differences to all other studied cultivars. These results are similar to the ones previously obtained by our research group (Silva et al. 2011). In this work, regarding eleven cultivars, the increase of mass for chestnut fruits, after boiling was also observed, as well as the decrease on this parameter, in roasted fruits. Similarly, the fruits from Boaventura and Trigueira cultivars presented the highest and lowest F.M., respectively.
In what concerns the dry mass (D.M.) analysis, values were higher in boiled fruits (average of 54 g/100 g edible part) followed by raw (average of 47 g/100 g edible part) and roasted (average of 43 g/100 g edible part) fruits. There were significant differences detected in the D.M. values, between raw, boiled and roasted fruits of all cultivars, with the exception of Trigueira. For all other six cultivars, a strong influence of the processing was found (P = 0.0001), and, in all situations, roasted samples presented the lowest values, followed by raw fruits, and in boiled fruits, the D.M. was detected in higher amount. These values, in raw fruits ranged from 40 g/100 g edible part, in Lada cultivar, to 50 g/100 g edible part, in Martaínha cultivar. These results agree with previous studies from our research team (Borges et al. 2008; Gonçalves et al. 2010; Silva et al. 2011). Indeed, data from Spanish samples show that moisture content of chestnut fruits is usually over 50 %, with an average value of 54 %, in some cases (Pereira-Lorenzo et al. 2006). After processing, the same pattern of results was observed: lowest D.M. in fruits from Lada cultivar after roasting (38 g/100 g edible part) or boiling (48 g/100 g edible part) and higher D.M. for fruits of Martainha cultivar (46 g/100 g edible part, after roasting and 57 g/100 g edible part, after boiling). The differences on the D.M., hence, the water content, of fruits are related to many factors including genetic, geographical location and also soil genesis of the production local (Pereira-Lorenzo et al. 2006). In the first case, Pereira-Lorenzo et al. (2006) showed that chemical composition of fruits varied significantly, due to genetic variations between cultivars, furthermore stating that correlations with environmental factors can be low. By other hand, the same study refers a significant effect of the geographical location, reflecting different amounts of summer rainfall. No correlation was found between the unit mass of chestnuts and the respective D.M. Indeed, the fruits with lower and higher D.M. were from Lada and Martaínha cultivars, which presented fruits with an intermediate mass.
Starch content
Starch is the main bioavailable carbohydrate in human diet, and chestnuts are among the main sources of starch in the diet, although as they are a product with low consumption, their contribution to dietary starch intake is low (Pizzoferrato et al. 1999). Results regarding the starch content of chestnut fruits from the studied cultivar are presented in Table 2. Significant differences (P = 0.0001) were detected between cultivars, as starch amounts in raw chestnuts ranged from 57 g/100 g on chestnuts of Trigueira cultivar to 71 g/100 g on Martainha cultivar (Table 2), with an average value of 63 g/100 g. Interestingly, raw fruits of Trigueira cultivar presented simultaneously the lower unit mass and starch amount, being this behaviour not found for the fruits with higher starch content (Martaínha cultivar). Furthermore, no other correlation was found between fruits mass or dry mass and starch content. The detected amounts of starch are similar to the ones available in other studies. The review provided by Vasconcelos et al. (2010) refer that starch can represent from 39 to 67 g/100 g of dry matter. Similar starch content variations were detected by Künsch et al. (2001) in Swiss, French and Italian cultivars (starch contents ranging from 42 to 58 g/100 g of dry weight), and by Mert (2010) for Turkish samples (between 34 to 45 g/100 g dry matter). The variations on the amount of starch can be related to genetic factors, known to influence chestnut composition, as well as geographic location (Borges et al. 2007; Gonçalves et al. 2012; Künsch et al. 2001; Pereira-Lorenzo et al. 2006).
Table 2.
Starch (g/100 g of dry mass) (mean ± SD, n = 15) in kernels of the studied raw, boiled and roasted chestnut cultivars
| Cultivar | Raw | Boiled | Roasted | P (T) |
|---|---|---|---|---|
| Boaventura | 66.50 ± 1.80 cd A | 62.12 ± 1.64 a A | 62.92 ± 5.72 b A | 0.2717 |
| Côta | 65.21 ± 1.32 bc A | 66.65 ± 0.80 bc A | 65.24 ± 2.45 bc A | 0.5437 |
| Lada | 59.26 ± 3.46 a A | 64.73 ± 1.03 ab B | 56.68 ± 1.10 a A | 0.0084 |
| Lamela | 70.63 ± 3.56 d A | 70.89 ± 2.98 d A | 71.51 ± 1.87 d A | 0.9273 |
| Longal | 57.79 ± 1.98 a A | 62.11 ± 1.38 a A | 64.17 ± 5.76 b A | 0.2248 |
| Martaínha | 71.11 ± 8.82 d A | 71.01 ± 7.57 d A | 70.07 ± 5.58 cd A | 0.9854 |
| Trigueira | 57.41 ± 1.67 a A | 70.33 ± 2.21 cd C | 64.60 ± 2.16 bc B | 0.0003 |
| P(C) | 0.0001 | 0.0001 | 0.0001 |
Means within a column followed by the same letter and means within row followed by the same capital letter are not significantly different at P < 0.05, according to the Duncan multiple range test
The processing of chestnut fruits didn’t result in a visible pattern of behaviour. Average values indicate (67 g/100 g of dry mass for boiled fruits and 65 g/100 g of dry mass for roasted fruits) that both boiling and roasting would result in an increase of starch, when compared to raw fruits. However, after processing, different variations occurred: i) both boiling and roasting resulted in a decrease of starch in the fruits (Boaventura and Martaínha cultivars); ii) the applied treatments resulted in an increase of starch amount (Côta, Lamela, Longal and Trigueira cultivars); iii) one of the treatments (boiling) increased the starch content, while roasting resulted in its decrease (Lada cultivar). It also caused that fruits of Trigueira cultivar, that presented the lowest amount of starch (57 g/100 g of dry mass) when raw, had one of the highest quantity when boiled (70 g/100 g of dry mass), and an intermediate amount when roasted (65 g/100 g of dry mass). However, only the values of starch content of Lada and Trigueira cultivars were significantly different, when treatments were compared. In fact, for Lada cultivar (P = 0.0084), both raw and roasted fruits presented statistically similar values, that were lower than the values recorded for boiled fruits. For Trigueira cultivar (P = 0.0003), raw fruits presented the lower content of starch, followed by roasted fruits, while the boiled samples present the higher content of starch, being all these values significantly different from each other. Different influence of boiling and roasting on chestnut chemical and physical properties have already been described (Barros et al. 2011; Gonçalves et al. 2010; Gonçalves et al. 2012; Silva et al. 2011), and showed that a similar response of fruits from different cultivars to the same processing cannot be expected. In fact, previous works dealing with the effect of roasting time (Künsch et al. 2001) showed, that, for similar times of processing, different cultivars present higher (Marrone de Cuneo) or lower (Luina, Pinca, Torción negro and Marigoule) starch content than when raw. By other hand, both Correia et al. (2009) and Attanasio et al. (2004) point out that, when drying chestnuts, as temperature increases, the starch content decreases, at least until reaching 60 °C. Above this temperature, starch content increases, as detected by Correia et al. (2009). The variations of starch content in the present work, are related to both the type of processing that was used, as well as to the studied cultivars, as only for two of them (Lada and Trigueira), the differences between treatments were of statistical significance.
Morphological properties of raw starch by light microscopy
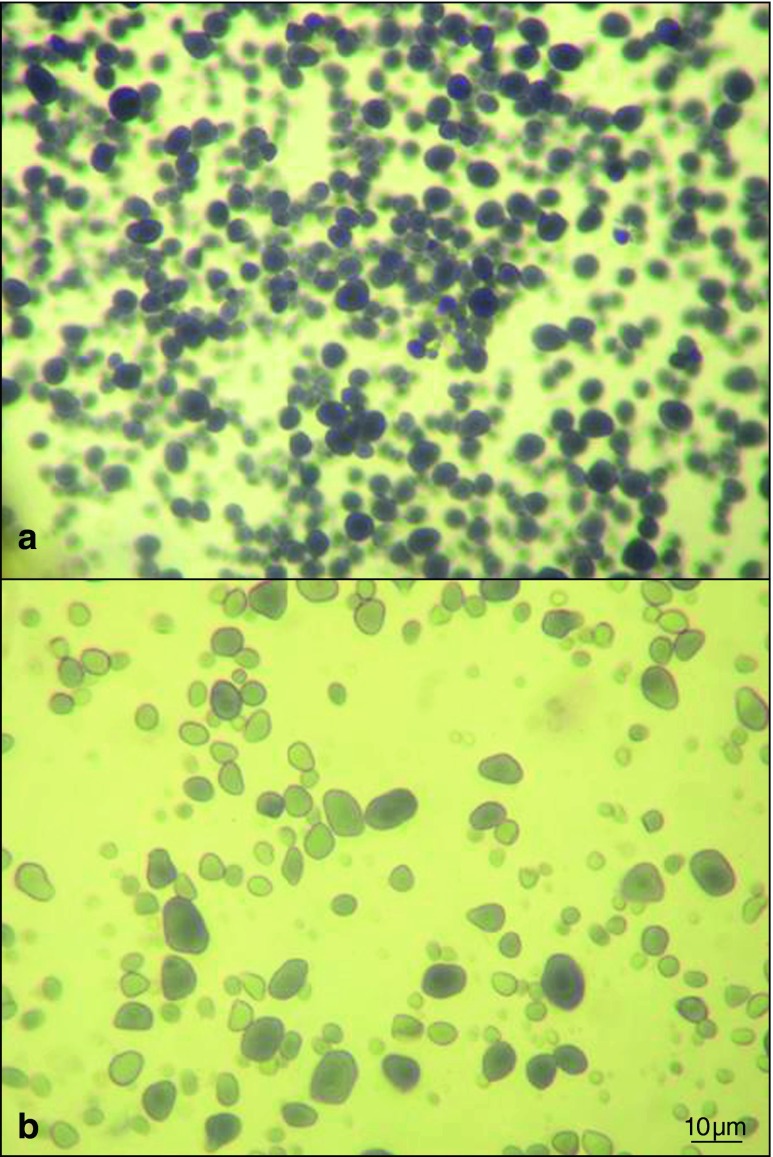
The use of light microscopy is an important way to gather information about the morphology and characteristics of starch granules. In Fig. 1, representative micrograph of starch granules are presented. All cultivars presented the same granular morphology, being of an irregular form, round, oval or elliptical. The surface of the granules appears to be smooth, without visible fractures or wrinkles. Same granular morphology of chestnut starch has been previously detected using light microscopy (Correia et al. 2009). The light micrograph allowed the visualization of the hilum. In the analysed chestnut cultivars, the hilum, core of the granule and starting point of growth (Cai and Wei 2013), presented an eccentric location, towards the end of the granule rather than a central positioning. This eccentric location indicates that the enzymes responsible for synthesis are pushed towards the distal end of the granules, while being absent or with reduced activity at the proximal part of the hilum (French 1984). Internal growth rings of increasing diameter extend from the hilum towards the surface of granules were also visible. The growth rings were somewhat ellipsoid-shaped and present around the eccentric hilum. These rings, usually ranging from 120 to 400 nm in thickness, are considered to reflect the diurnal fluctuations in the deposition of starch in storage tissues (Donald et al. 2001).
Fig. 1.
Light micrographs of starch granules of Martaínha cultivar stained using Lugol’s iodine solution
Morphological properties of raw starch by scanning electron microscopy (SEM)
Scanning electron microscopy was used to study the morphological characteristics of starch. Figure 2.1 to 2. 7a and B show the SEM photographs of chestnut parenchyma cell (600x magnification) and starch granules (2500 × magnification), respectively. Similar cell and starch granules shape was observed in all cultivars. Cellular area distribution of all analyzed cultivars is presented in Fig. 2.1 to 2.7c. This distribution is comparable in all cultivars, with the large majority of cells (from 68 in Côta and Martainha cultivars, to 84 % in Lada cultivar) presenting an area over between 1000 and 2000 μm2. Considerable small parenchyma cells (<600 μm2) where only found in Lamela and Martaínha cultivars, but in low percentages (1.3 % and 0.7 %, respectively). Furthermore, large cells (>3000 μm2) were detected in Boaventura, Côta and Martaínha cultivars, also in low percentages (1.3 %, 1.3 % and 0.7 %, respectively).
Fig. 2.

Scanning electron micrographs of raw chestnut starch using: a 600x magnification (bar of 100 μm) or b 2500× magnification (bar of 20 μm), 1 - Boaventura; 2 - Côta; 3 - Lada; 4 - Lamela; 5 - Longal; 6 - Martaínha; 7 – Trigueira and distribution (%) of b parenchyma cells, and d starch granules, for the studied cultivars: Scanning electron micrographs of raw chestnut starch using: a 600x magnification (bar of 100 μm) or b 2500× magnification (bar of 20 μm), and Size 1 - Boaventura; 2 - Côta; 3 - Lada; 4 - Lamela; 5 - Longal; 6 - Martaínha; 7 – Trigueira
The results of the measurement of the cells of chestnut parenchyma are presented in Table 3. There is a significant effect of the cultivar on the evaluated parameters, and fruits of Boaventura and Côta cultivars presented the higher area (1596 μm2 and 1727 μm2, respectively), length of major (55 μm and 58 μm, respectively) and minor axis (38 μm) and perimeter (154 μm and 161 μm, respectively). By other hand, the fruits with smaller cell areas were from Lamela cultivar (1239 μm2), although these results didn’t differ significantly for the ones recorded for fruits of Lada, Longal, Martaínha and Trigueira cultivars. Fruits from Lamela cultivar also presented the smallest length of the major axis (47 μm), statistically different from data for Boaventura, Côta and Longal cultivars, as well smaller perimeter length (133 μm), being these results significantly lower than the ones obtained for Boaventura, Côta, Lada and Longal cultivars. However, the smallest length for the minor axis was recorded in fruit cells of Longal cultivar (33 μm), although results are only significantly different from those recorded for the two cultivars with the largest cell size, Boaventura and Côta.
Table 3.
Dimensions of parenchyma cells and starch granules (mean ± SD, n = 150, and range: minimum-maximum) of the raw studied chestnut cultivars
| Cultivar | Cells | Granules | ||||||
|---|---|---|---|---|---|---|---|---|
| Area (μm2) | Axis (major) (μm) | Axis (minor) (μm) | Perimeter (μm) | Area (μm2) | Axis (major) (μm) | Axis (minor) (μm) | Perimeter(μm) | |
| Boaventura | 1596.31 ± 444.29 b | 54.62 ± 9.94 c | 37.52 ± 6.14 c | 153.75 ± 23.08 c | 46.35 ± 28.01 c | 8.62 ± 2.61 b | 6.40 ± 1.86 b | 23.93 ± 7.00 c |
| (666.83–3295.57) | (33.07–86.32) | (22.86–55.69) | (95.82–220–54) | (11.43–156.84) | (4.43–18.08) | (3.23–12.77) | (12.11–47.31) | |
| Côta | 1726.99 ± 529.68 b | 58.26 ± 13.67 d | 38.14 ± 6.55 c | 160.85 ± 29.85 c | 38.19 ± 17.2 a | 7.92 ± 1.84 a | 5.92 ± 1.45 a | 21.98 ± 4.96 ab |
| (678.51–4069.77) | (36.49–110.71) | (22.81–58.41) | (100.77–271.28) | (12.55–105.75) | (4.68–15.23) | (3.19–10.87) | (12.74–38.82) | |
| Lada | 1337.14 ± 331.82 a | 48.89 ± 7.85 a | 35.15 ± 5.24 b | 139.59 ± 18.70 b | 45.17 ± 24.46 c | 8.59 ± 2.26 b | 6.35 ± 1.68 b | 23.78 ± 6.07 c |
| (724.11–2383.48) | (34.79–80.41) | (23.90–51.12) | (101.76–191.96) | (13.79–158.59) | (4.63–15.92) | (3.52–12.69) | (13.49–45.34) | |
| Lamela | 1239.32 ± 390.63 a | 46.60 ± 9.15 a | 33.80 ± 5.58 ab | 132.98 ± 22.48 a | 43.73 ± 21.34 bc | 8.28 ± 2.08 ab | 6.39 ± 1.65 b | 23.34 ± 5.82 bc |
| (506.86–2465.71) | (26.42–78.36) | (23.71–49.19) | (81.51–200–99) | (6.73–112.8) | (3.14–13.99) | (2.73–10.32) | (8.93–38.81) | |
| Longal | 1320.68 ± 362.19 a | 51.18 ± 10.80 b | 33.21 ± 4.79 a | 140.64 ± 22.03 b | 39.33 ± 20.43 ab | 8.06 ± 2.31 a | 5.90 ± 1.45 a | 22.23 ± 5.88 ab |
| (638.47–2560.69) | (33.63–88.59) | (23.54–49.62) | (95.09–208.52) | (10.44–147.37) | (4.40–19.41) | (3.02–9.75) | (11.63–47.55) | |
| Martaínha | 1286.84 ± 395.62 a | 48.28 ± 9.21 a | 34.00 ± 5.64 ab | 136.73 ± 21.97 ab | 38.66 ± 16.65 a | 8.00 ± 1.87 a | 5.94 ± 1.36 a | 22.17 ± 4.91 ab |
| (558.75–2421.54) | (29.60–82.28) | (23.11–47.33) | (85.99–200.57) | (11.74–89.14) | (4.14–13.79) | (3.42–9.93) | (12.10–34.11) | |
| Trigueira | 1272.28 ± 369.08 a | 47.92 ± 8.54 a | 33.91 ± 5.35 ab | 135.13 ± 19.91 ab | 36.69 ± 14.76 a | 7.80 ± 1.60 a | 5.81 ± 1.27 a | 21.61 ± 4.34 a |
| (602.02–3190.71) | (30.03–85.95) | (22.50–47.39) | (91.53–217–62) | (13.48–80.15) | (4.98–12.49) | (3.28–9.22) | (13.49–33.04) | |
| P(C) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.001 | 0.0001 |
Means within a column followed by the same letter are not significantly different at P < 0.05, according to the Duncan multiple range test
Starch granules presented an irregular form, round, oval or elliptical, with an external smooth surface (Fig. 2.1 to 2.7b), as referred before, confirming previous reports on granule morphology (Attanasio et al. 2004; Correia et al. 2009; Correia et al. 2012; Gonçalves et al. 2012; Mert 2010). Starch granular area distribution of chestnut starch for all analyzed cultivars is presented in Fig. 2.1 to 2.7d. In all cultivars, the area distribution is similar, with the major percentage of granules presenting an area between 20 to 40 μm2, with fruits of Trigueira cultivar presenting higher (56 %) percentage of granules with area between 20 and 40 μm2, while fruits from Boaventura cultivar showed the lowest percentage (39 %) of granules with this area range. Interestingly, the sum of percentages for granules with < 20 and with area ranging from 20 to 40 μm2, results, in all cultivars, in over 50 % of granules. This indicates that in all cultivars, starch granules are of small to medium size. Similar results were observed by Correia et al. (2009; 2012) for Longal and Martainha cultivars.
The granular starch measurements are presented in Table 3. It is known that the granule size is one of the most important characteristic of starch, which can influence functional properties (Correia et al. 2012), digestibility and glycemic index of starch itself (Noda et al. 2008). Furthermore, the dimension of starch granules can also affect rheological properties, swelling properties, baking and processing characteristics, even colour properties, as well as the adaptation of starch to non-food related used (D’Appolonia and Gilles 1971). As for detected for cellular size, there is also a significant effect of the cultivar in this parameter. Indeed, as for cell dimensions, the fruits of Boaventura cultivar presented granules with higher area (46 μm2), length of major (9 μm) and minor axis (6 μm), as well as longer perimeter (24 μm). However, the smaller granular size was not detected in the fruits from the cultivar with the smallest cells (Lamela cultivar). In the granular size measurements, the fruits presenting granules with smaller area, length of major and minor axis and perimeter were from cv. Trigueira. The area of granules ranged from a minimum of 7 μm2 (Lamela cultivar) to a maximum of 159 μm2 (Lada cultivar). The range of values for length of major and minor axis, as well as perimeter, was minimum in Lamela cv. (3 μm, 3 μm and 9 μm, respectively), and reach the maximum values of major axis length (19 μm) and perimeter (48 μm) in Longal cultivar, and minor axis (13 μm), in Boaventura cultivar. The size of the granules of the studied cultivars in the present work is comparable to the ones previously reported. Earlier studies show average diameters of 11 μm (ranging between 4 to 21 μm) (Cruz et al. 2013), mean values of length (7 μm) and width (6 μm) (range from 1.4 to 23.3 μm) (Correia et al. 2009), and granule length with minimum and maximum values of 3 to 18 μm (Mert 2010) or 3 to 15 μm (Attanasio et al. 2004). These variations of the size of starch granules are explained by the factors known to influence this parameter. Starch properties, including granule size, are influenced by both cultivar and by environmental factors (Noda et al. 2008). Although little is known about changes in granular size caused by these factors on chestnut starch, previous research using other sources of starch show the effect of varieties (Tester and Karkalas 2001), development stage, and, of course, of environmental factors, such as high or low air and soil temperatures, salinity, nitrogen deficiency, CO2 concentration, source and intensity of light or water availability, as thoroughly reviewed by Beckles and Thitisaksakul (2014).
Usually, X-ray diffraction pattern analysis is performed to evaluate granule type, being classified into three types (A, B and C). An alternative classification of starch granules, B-type granules (diameter < 9.9 μm) and A-type granules (diameter > 9.9 μm), with C-type being a mixture of granules of both A and B-type have been used in another starch origins (Wen-Yang et al. 2008). Using this classification, the results of the present work indicates that chestnut granules are of C-type, as major axis length ranges from 3 μm (B-type) to 19 μm (A-type). Previous works, using X-ray diffraction, also classified chestnut starch granules as C-type (Correia et al. 2012). As formerly referred, the type of granules and their proportion influence the farinograph water absorption and improve breadmaking quality (Soh et al. 2006), as well as final product characteristics (e.g. Soulaka and Morrison 1985).
Effect of processing on starch morphology
The change in granular morphology of starch from chestnuts is showed in Fig. 3, where representative SEM micrographs are presented, using Martaínha cultivar samples. The processing of chestnut starch is known to physically changes granules, affecting morphological characteristics of granules, and sensory attributes of chestnuts (Attanasio et al. 2004). When heated, it suffers irreversible changes, occurring in the first place the breaking of molecular order and the increase of granular size (gelatinization), and, afterwards, granular swelling, exudation components from granule and eventually total disruption of granules (pasting) (Thomas and Atwell 1999). Furthermore, these changes are dependent on the available amount of heat and water (Patel and Seetharaman 2006). When starch is heated in enough water, the starch granule swells and its volume increases (Tester and Karkalas 1996). In the samples used in the present work, it is visible that the starch granules changed their configuration and surface morphology, after boiling and roasting (Fig. 3). In both cases, granules lost their round to oval shape, and showed a large elongated form, while the surface lost is apparent smoothness, and presented numerous wrinkles. Fusion of individual granules and gelatinization also occurred. Similar granular behaviour after heating was recorded by Mert (2010) and Gonçalves et al. (2012), with the changes in shape and surface smoothness being very similar to the ones detected in the present work. However, and unlike our results, the cultivars using by Mert (2010), didn’t show the same response to heating, as one of them (Alimolla), presented oval starch granules, rather than elongated ones. The use of the same treatments (boiling and roasting) has previously showed that it affect rheological properties of chestnut fruit shell and kernel, as well as colour parameters (Silva et al. 2011). This type of processing also showed to cause variation of the composition of the chestnut fruits, including vitamin C content (Barros et al. 2011), primary and secondary metabolites, as protein, ash, fat, fibre, organic acids and phenolics (Gonçalves et al. 2010), and free amino acid and mineral content (Gonçalves et al. 2012), as well of antioxidant activity of chestnut fruits (Barros et al. 2011). The application of heat or microwave irradiation to starches of other vegetable origins have been reported to cause the surface of granules to be roughened (Xie et al. 2013). Furthermore, the changes in the morphology of starch granules can be influenced by the heating rate (Patel and Seetharaman 2006). These authors showed that a lower rate of heating increased the folding of granules, while the increase of granular size occurred at a higher temperature when heated at a faster rate (25 ° C/min compared to 5 ° C/min). In our case, Figure 3 shows, although small, some differences in the granules, between the boiled and roasted samples. Even though no measurement of granular size was performed, as blending of granules occurred, as well as gelatinization, the granules observed in boiled samples (Fig. 3, 1 and 2b) appear to be slightly smaller than the ones observed in roasted samples (Fig. 3, 1 and 2c). Additionally, the number of wrinkles present on the surface of the granules is apparently higher in roasted than boiled samples. It could be expected that granules of boiled chestnuts increased their volume in a higher manner than roasted granules, as the swelling of starch granules in dependant on the water available in the system, which is much higher in the boiling processing. However, one of the other major factors affecting granular swelling is the temperature. Cruz et al. (2013) showed that an increase of 40 °C (from 50 to 90 °C) almost fourfold the swelling power of chestnut granules, which can partially explain that the size of the granules has increased in the same way than when samples were boiled. The fact that the temperature differences between boiling (100 °C) and roasting (200 °C) didn’t produce more considerable changes in granular morphology in linked to the effect of the water availability. Only when the temperature of starch is higher than the gelatinisation temperature can water molecules penetrate the granules, making them swell. Hence, at low water content (in this case, the roasting procedure), the melting temperature increases, and the addition of water lowers this value (Correia and Beirão-da-Costa 2012), therefore, making it easy to gelatinization occurs (Sandhu et al. 2007).
Fig. 3.
Scanning electron micrographs of a raw, b boiled and c roasted Martaínha cultivar starch using: (1) - 600× magnification (bar of 100 μm) or (2) - 2500× magnification, for raw and boiled, and 2000× magnification for roasted chestnuts (bar of 20 μm)
Conclusions
The results achieved in the present work provide further insight on cultivar effect on several parameters of chestnut fruits, as biometric measurements, starch content, as well as cell and starch granular size. In all parameters, there was a marked cultivar effect, which may reflect either one or both genetic and environmental factors. Furthermore, as far as we know, this is the first report on the morphological characteristics of starch of seven of the main cultivars of chestnut, and, additionally, on the effects of the most important cooking methods (boiling and cooking) on starch morphology. Chestnuts are predominately consumed cooked, either boiled or roasted, and the present results indicate that these processes alter the morphology of starch granules, and, more likely, their digestibility, therefore, the nutritional and health characteristics of chestnuts. This data is of extreme importance, either to increase consumer’s awareness to health benefits of this fruit, as well as to chestnut industry, as it can provide helpful information for the processing of chestnuts.
Acknowledgments
This work was supported by Project INNOFOOD - INNovation in the FOOD sector through the valorization of food and agro-food by-products - NORTE-07-0124-FEDER-0000029, financed by the North Portugal Regional Operational Programme (ON.2 – O Novo Norte) under the National Strategic Reference Framework (QREN), through the European Regional Development Fund (FEDER), as well as by National Funds (PIDDAC) through the Portuguese Foundation for Science and Technology (FCT/MEC).
References
- AOAC (2000) Association of Official Analytical Chemists. In Official methods of analysis chemists. Washington, DC
- Attanasio G, Cinquanta L, Albanese D, Di Matteo M. Effects of drying temperatures on physico-chemical properties of dried and rehydrated chestnuts (Castanea sativa) Food Chem. 2004;88:583–590. doi: 10.1016/j.foodchem.2004.01.071. [DOI] [Google Scholar]
- Barros A, Nunes F, Gonçalves B, Bennett R, Silva A. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.) Food Chem. 2011;128:165–172. doi: 10.1016/j.foodchem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Beckles D, Thitisaksakul M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch-Starke. 2014;66:58–71. doi: 10.1002/star.201300212. [DOI] [Google Scholar]
- Borges O, Carvalho J, Correia P, Silva A. Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. J Food Compos Anal. 2007;20:80–89. doi: 10.1016/j.jfca.2006.07.008. [DOI] [Google Scholar]
- Borges O, Gonçalves B, Soeiro J, Correia P, Silva A. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008;106:976–984. doi: 10.1016/j.foodchem.2007.07.011. [DOI] [Google Scholar]
- Cai C, Wei C. In situ observation of crystallinity disruption patterns during starch gelatinization. Carbohydr. Polym. 2013;92:469–78. doi: 10.1016/j.carbpol.2012.09.073. [DOI] [PubMed] [Google Scholar]
- Correia P, Beirão-da-Costa M. Effect of drying temperatures on starch-related functional and thermal properties of chestnut flours. Food Bioprod Process. 2012;90:284–294. doi: 10.1016/j.fbp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Correia P, Leitão A, Beirão-da-Costa M. The effect of drying temperatures on morphological and chemical properties of dried chestnuts flours. J Food Eng. 2009;90:325–332. doi: 10.1016/j.jfoodeng.2008.06.040. [DOI] [Google Scholar]
- Correia P, Cruz-Lopes L, Beirão-da-Costa L. Morphology and structure of chestnut starch isolated by alkali and enzymatic methods. Food Hydrocoll. 2012;28:313–319. doi: 10.1016/j.foodhyd.2011.12.013. [DOI] [Google Scholar]
- Cruz B, Abraão A, Lemos A, Nunes F. Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill) Carbohyd Polym. 2013;94:594–602. doi: 10.1016/j.carbpol.2012.12.060. [DOI] [PubMed] [Google Scholar]
- D’Appolonia B, Gilles K. The effect of various starches in baking. Cereal Chem. 1971;48:625–636. [Google Scholar]
- Donald A, Kato K, Perry P, Waigh T. Scattering studies of the internal structure of starch granules. Starch-Starke. 2001;53:504–512. doi: 10.1002/1521-379X(200110)53:10<504::AID-STAR504>3.0.CO;2-5. [DOI] [Google Scholar]
- FAO (2012) Available at http://faostat3.fao.org/home. April, 2014.
- French D. Organisation of starch granules. In: Whistler R, BeMiller J, Paschall J, editors. Starch: chemistry and technology. 2. New York: Academic; 1984. pp. 183–247. [Google Scholar]
- Gonçalves B, Borges O, Costa H, Bennett R, Santos M, Silva A. Metabolite composition of chestnut (Castanea sativa mill.) upon cooking: proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010;122:154–160. doi: 10.1016/j.foodchem.2010.02.032. [DOI] [Google Scholar]
- Gonçalves B, Borges O, Rosa E, Coutinho J, Silva A. Effect of cooking on free amino acid and mineral profiles of sweet chestnut (Castanea sativa Mill.) Fruits. 2012;67:201–214. doi: 10.1051/fruits/2012013. [DOI] [Google Scholar]
- Künsch U, Schärer H, Patrian B, Höhn E, Conedera M, Sassella A, Jermini M, Jelmini G. Effects of roasting on chemical composition and quality of different chestnut (Castanea sativa Mill) varieties. J Sci Food Agric. 2001;81:1106–1112. doi: 10.1002/jsfa.916. [DOI] [Google Scholar]
- Mert C. Proximate content and starch granule structure in raw and boiled chestnuts with different aptitude to candying. Acta Horticult. 2010;866:667–674. doi: 10.17660/ActaHortic.2010.866.89. [DOI] [Google Scholar]
- Noda T, Takigawa S, Matsuura-Endo C, Suzuki T, Hashimoto N, Kottearachchi N, Yamauchi H, Zaidul I. Factors affecting the digestibility of raw and gelatinized potato starches. Food Chem. 2008;110:465–470. doi: 10.1016/j.foodchem.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Patel B, Seetharaman K. Effect of heating rate on starch granule morphology and size. Carbohydr. Polym. 2006;65:381–385. doi: 10.1016/j.carbpol.2006.01.028. [DOI] [Google Scholar]
- Pereira-Lorenzo S, Ramos-Cabrer A, Díaz-Hernández M, Ciordia-Ara M, Ríos-Mesa D. Chemical composition of chestnut cultivars from Spain. Sci Hortic. 2006;107:306–314. doi: 10.1016/j.scienta.2005.08.008. [DOI] [Google Scholar]
- Pizzoferrato L, Rotilio G, Paci M. Modification of structure and digestibility of chestnut starch upon cooking: A solid state 13C CP MAS NMR and enzymatic degradation study. J. Agric. Food Chem. 1999;47:4060–4063. doi: 10.1021/jf9813182. [DOI] [PubMed] [Google Scholar]
- Salomonsson A, Theander O, Westerlund E. Chemical characterization of some Swedish cereal whole meal and bran fractions. Swed J Agric Res. 1984;14:111–117. [Google Scholar]
- Sandhu K, Singh N, Malhi N. Some properties of corn grains and their flours. I: physicochemical, functional and chapatti-making properties of flours. Food Chem. 2007;101:938–946. doi: 10.1016/j.foodchem.2006.02.040. [DOI] [Google Scholar]
- Silva A, Ribeiro R, Gonçalves B, Santos F, Borges O, Carvalho J, Costa H, Santos M, Fontes T, Mota C, Magalhães B, Marinho E, Moutinho N, Frias A, Ribeiro C, Guedes C, Costa H, Mendes J, Carvalho R, Pires R. Castanha – Um fruto Saudável. Vila Real: Minfo Gráfica; 2007. [Google Scholar]
- Silva A, Santos-Ribeiro R, Borges O, Magalhães B, Silva M, Gonçalves B. Effects of roasting and boiling on the physical and mechanical properties of 11 Portuguese chestnut cultivars (Castanea sativa Mill.) CyTA - J Food. 2011;9:214–219. doi: 10.1080/19476337.2010.518249. [DOI] [Google Scholar]
- Soh H, Sissons M, Turner M. Effect of starch granule size distribution and elevated amylase content on durum dough rheology and spaghetti cooking quality. Cereal Chem. 2006;83:513–519. doi: 10.1094/CC-83-0513. [DOI] [Google Scholar]
- Soulaka A, Morrison W. The bread baking quality of 6 wheat starches differing in composition and physical-properties. J Sci Food Agric. 1985;36:719–727. doi: 10.1002/jsfa.2740360812. [DOI] [Google Scholar]
- Tester R, Karkalas J. Swelling and gelatinization of oat starches. Cereal Chem. 1996;73:271–273. [Google Scholar]
- Tester R, Karkalas J. The effects of environmental conditions on the structural features and physico-chemical properties of starches. Starch-Starke. 2001;53:513–519. doi: 10.1002/1521-379X(200110)53:10<513::AID-STAR513>3.0.CO;2-5. [DOI] [Google Scholar]
- Thomas D, Atwell W. Starches. St. Paul: Eagan Press Handbook Series; 1999. [Google Scholar]
- Vasconcelos M, Bennett R, Rosa E, Ferreira-Cardoso J. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: fresh and processed products. J Sci Food Agric. 2010;90:1578–1589. doi: 10.1002/jsfa.4016. [DOI] [PubMed] [Google Scholar]
- Wen-Yang L, Su-Hui Y, Yan-Ping Y, Yong L, Tai-Bo L, Feng G, Zhong-Min D, Zhen-Lin W. Comparison of starch granule size distribution between hard and soft wheat cultivars in Eastern China. Agric Sci China. 2008;7:907–914. doi: 10.1016/S1671-2927(08)60129-7. [DOI] [Google Scholar]
- Xie Y, Yan M, Yuan S, Sun S, Huo Q. Effect of microwave treatment on the physicochemical properties of potato starch granules. Chem Cent J. 2013;7:113–119. doi: 10.1186/1752-153X-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Walker C. A quality comparison of breads baked by conventional versus nonconventional ovens – a review. J Sci Food Agric. 1995;67:283–291. doi: 10.1002/jsfa.2740670302. [DOI] [Google Scholar]