Abstract
The effects of alginate/carboxyl methylcellulose composite coating incorporated with clove essential oil on quality of silver carp fillet chilled storage (4 + 1 °C) were examined over a period of 16 days. The control samples (c), alginate/carboxyl methylcellulose coating (C-A), alginate/carboxyl methylcellulose composite coating incorporated with clove essential oil (with different concentration 1 and 1.5 %) (C-A + CEO1 % and C-A + CEO 15 % respectively) were analyzed by bacteriological (total viable counts (TVC) and total psychrotrophic counts (TPC)), biochemical (Peroxide value (PV), free fatty acid (FFA), total volatile base nitrogen (TVB-N), and pH) and sensory characteristics. Also, the efficacy of these treatments was investigated in control of the population of Eschershia coli O157:H7 inoculated in silver carp fillet. According to the obtained results, C-A + CEO 1.5 % showed lowest (p < 0.05) and acceptable biochemical, bacteriological and sensory characteristics attributes up to 16 days storage at 4 °C compared to the others. Also, this treated sample was acceptable even at the end of the 16-day storage and it could reduce the population of E. coli O157:H7 below the acceptable level (<2) from day 4 until the end of the storage period. The results indicate Alginate/carboxyl methylcellulose composite coating with clove essential oil might be recommended as a preservative in the meat products.
Keywords: Silver crap, Alginate, Carboxyl methylcellulose, Clove essential oil, Shelf life, E.coli
Introduction
Consumption of fish and fish products is recommended because of good digestibility and high content of polyunsaturated fatty acids (PUFA). However, fish is also a highly perishable food; thus, it must be consumed immediately after fishing or must be stored under suitable conditions to reach consumers without losing its nutritional value (Andevari and Rezaei 2011). Moreover, seafood products could be a source of food borne infections or pathogens which emphasizes the need of a thorough control of its bacteriological characteristics (Albuquerque Costa 2013). It is well recognized that microbial concerns are widely associated with seafood (FDA 1998). Different types of seafood and the marine environment have been revealed as the sources of the isolation of foodborne pathogens, like Escherichia coli O157:H7 (Teophilo et al. 2002). The rising threat of the mentioned pathogen demands effective detecting and control methods to make sure that these pathogens are not present in the raw foods (Shin et al. 2004).
The development of an edible coating to inhibit the growth of pathogenic bacteria in food products is an active research area in the food science field (Seyfzadeh et al. 2013). Among the many materials that could form the edible films, alginate is the appealing film forming compound, as it is non-toxic, biodegradable, and biocompatible (Zactiti and Kieckbusch 2009). Alginates are linear polymers comprising residues of α-L-guluronic (G) and β-D-mannuronic (M) present in variable proportions and sequences in the cell wall and intercellular space of brown algae and could be synthesized from microorganisms (Alboofetileh et al. 2014). The edible films and coatings made from only one kind of natural film-forming polymer displays good properties in some aspects but are poor in the others. An alternative promising strategy to improve the properties of biodegradable coatings is through blending of biopolymers. Bio composite coatings are usually composed of two or three biopolymers and are prepared by varying methods (Ghanbarzadeh et al. 2010). Cellulose, the most abundant organic polymer in the world, and cellulose edible films are very efficient oxygen and hydrocarbon barriers, aroma compounds and it is insoluble in water. Carboxyl methylcellulose coatings are clear, relatively water resistant and relatively unaffected by oils, greases and most nonpolar organic solvents (Guilbert 1986). CMC and alginate edible coating was used for preserving of seafood by the other researchers (Hamzeh and Rezaei 2012; Ariaii et al. 2014; Raeisi et al. 2014).
In order to the control undesirable microorganisms on food surfaces, natural antimicrobial/antioxidant agents such as essential oils (EOs) could be incorporated into edible films and coatings. Clove (caryophillum aromaticcus) EO is of much attention due to its high content and wide spectrum of phenolic compounds, antimicrobial and antioxidant properties, and potential for use in meat and meat products. The use of EOs is reported in the literature to improve shelf life of meat (Jayasena and Jo 2013).
Silver carp (Hypophthalmicthys molitrix) is one of the most important economic freshwater fish species cultured in eastern countries due to its fast growth rate, easy cultivation, high feed efficiency ratio, and high nutritional value (Fan et al. 2009). Thus, this study was aimed to investigate the effect of alginate/carboxyl methylcellulose composite coating incorporated with clove essential oil on shelf life of silver carp fillet by determining chemical, microbiological, sensory analysis, and growth of E. coli O157:H7 inoculated onto it.
Materials and method
Materials
Commercial carboxyl methylcellulose (CMC) and sodium alginate were purchased from Sigma-Aldrich Chemical Co (St Louis, MO, U.S.). Polyethylene glycol and tween 80 were acquired from Merck (Frankfurt, Germany). Clove (caryophyllus aromaticus) was collected from the south of Iran (Khash, Iran) in March 2014. Essential oils were extracted by hydro-distillation from the dried samples by the Clevenger type apparatus, and the obtained oils stored in a dark container at 4 °C until used. All other chemicals were analytical grade.
GC-MS analysis of CEO
Clove essential oil (CEO) was analyzed using a Varian 3400 GC-MS (Agilent, USA) system equipped with a DB-5 fused silica column (30 m × 0.25 mm, film thickness 0.25 μm, J and W Scientific Corporation); the oven temperature was 50°-260 °C at a rate of 4 °C min−1. The transfer line temperature was 270 °C, carrier gas, helium at a linear velocity of 31.5 cm/s, split ratio 1:60, ionization energy 70 eV, scan time 1 s, mass range 40–300 amu (Ariaii et al. 2014).
Disc diffusion assay
Antibacterial properties CEO were studied using the agar diffusion method. Listeria monocytogenes (PTCC 1163), Escherichia coli (PTCC 3315) and Staphylococcus aureus (ATCC 9144), were purchased from the Iranian Research Organization for Science and Technology (Persian Type Culture Collection (PTCC), Tehran, Iran). Bacterial strains were cultured overnight in Brain Heart Infusion Broth (Scharlua, Spain) at 37 °C. 70 μl of different film-forming solutions were poured into Mueller Hinton (Scharlua, Spain) agar wells (7.9 mm diameter). Their plates had been seeded with 0.1 ml of inoculums by swab containing about106 to 107 CFU/ml of the indicated bacteria. In the same way, films were punched into discs of 13.4 mm diameter, and then placed on the plates. Next, the plates were incubated in incubator at 37 °C for 48 h. After incubation, the inhibitory zone was calculated, and then subtracted from the film disks' diameters. This difference was reported as the inhibitory zone of the CEO (Gómez-Estaca et al. 2010).
Coating preparation
The CMC coating was prepared by the method of Turhan and sahbaz (2004). 3 % CMC was dissolved into the ratio of 2:1 distilled water and ethanol and rotary shaking was undertaken concurrently for 30 min. Afterward, 33 % of Polyethylene glycol (PEG 400), as a plasticizer, was added to the sample solution.
Alginate solution was prepared by dissolving 30 g of sodium alginate powder in 2 L distilled water to obtain a 1.5 % w/v alginate solution using a magnetic stirring plate at 70 °C and 1200 rpm for 30 min, then cooled to room temperature (Heydari et al. 2015).
CMC and alginate solution with ratio of 50:50 mixed together to produce composite films. Then Tween 80, at a level of 0.2 % v/v of essential oil, was added as an emulsifier to aid essential oil dissolution in the mixture, After 30 min of stirring, food grade CEO at 1 and 1.5 % v/v concentration was added to the solution. The solution was homogenized with homogenizer Model D500 (Wiggenhauser Machinenbau, 10,965 Berlin, Germany) at room temperature for 2 min at 7000 rpm (Ojagh et al. 2010). The solution was kept overnight at 4 °C in order to remove all bubbles.
Preparation of E. coli O157:H7
In order to prepare E. coli O157:H7, the thawed culture (0.1 mL) was transferred to 10 mL of BHI broth and grown in a shaker incubator at 37 °C for 24 h. A second transfer of 0.1 mL of culture into 10 mL of BHI broth was grown in a shaker incubator at 37 °C for 24 h to the end of the exponential phase of growth. Bacteria cell count was determined by the optical density (OD) method at 600 nm, a population of 1 × 106 cells/ml (according to pretest, 0.08–0.1 OD was equal to 1 × 106 cfu/ml). After dilution, the fish fillet was inoculated by 1 × 103 CFU/g of E. coli.
Preparation of silver carp fillets, coating and storage
36 live silver carp with an average weight of 1000 ± 100 g were obtained from a local aquaculture farm. In 1 h, they were transported to the laboratory in sealed foamed polystyrene boxes containing flaked ice. Then, the fish were gutted, skinned, filleted, and washed with potable water in laboratory. 15 fillets (25 ± 2 g) for each treatment were randomly subjected to one of four treatments as presented in the following:
C: control, without treatment
C-A: coated with CMC and sodium alginate
C-A + CEO 1 %: coated with CMC and sodium alginate containing 1 % CEO
C-A + CEO 1.5 %: coated with CMC and sodium alginate containing 1.5 % CEO
The fish fillets were dipped for 30 s in 500 mL of the each coating solution. Then, the coated fillets stood for 2 min, followed by a second immersion in CaCl2 (Sigma-Aldrich Chemical Co., USA) for 30 s to achieve better crosslinking. Next, the samples were allowed to drain completely in refrigerator temperature for about 30 min (Ojagh et al. 2010).
For E. coli analysis, samples were inoculated with 1 × 103 CFU/g of E. coli. Then, the samples were coated according to the above mentioned treatments.
Finally, all of samples stored at refrigerator (4 ± 1 °C) for 16 days. Chemical, microbiological and sensorial analyses were performed at 4 day intervals to determine the overall quality of fish.
Biochemical analysis
The pH was determined by homogenizing 5 g of the fish sample in 45 ml of distilled water with Ultra-Turrax (IKA T25-Digital Ultra-Turrax, Staufen, Germany) at 3000 rpm for 30 s. Then, the pH was measured with a digital pH meter.
The peroxide value (PV) was determined in the total lipid extracts according to the method of Pearson (Egan et al. 1997). Results were expressed in mEq oxygen/kg lipid.
The total volatile basic nitrogen (TVB-N) of the fish samples was determined by the micro-diffusion method as described by Ojagh et al. (2010). Results were expressed in mg N/100 g of fish.
Free fatty acid (FFA) was estimated by the procedure explained by AOAC (2005) and its content was expressed as percentage of % oleic acid.
Bacteriological analysis
Bacteriological counts were determined by placing a 10 g sample in 90 ml of 0.85 % NaCl solution, and homogenising it with a stomacher. Total viable count (TVC) and total psychrotrophic count (TPC) determined by the pour plate method, using plate count agar (PCA, Merk, Darmstadt, Germany). The inoculated plates were incubated at 37 °C for 2 days for total viable counts, and at 10 °C for 7 days for psychrotrophilic counts (Ojagh et al. 2010). For counts of E.coli O157:H7, serial dilutions were made, and count of E. coli O157:H7 were determined by spreading 0.1 ml of serial dilutions on CHROMagartm STEC to screen E. coli O157:H7. The plates were incubated at 37 °C for 24 h, and the surviving cell population was counted (Kim et al. 2014). All counts were expressed as log colony-forming units (CFU/g) and performed in triplicate.
Sensory evaluation
The sensory quality of the silver carp fillets during 16 days of preservation was based on a five point scale. The six member trained panel were asked to judge the texture (5, firm; 1, very soft); color discoloration (5, no discoloration; 1, extreme discoloration); odor (5, extremely desirable; 1, extremely unacceptable/off-odors); and overall acceptability (5, extremely desirable; 1, extremely unacceptable) of the samples. The fish samples were defined as unacceptable when the sensory attributes declined below 4.0 (Ojagh et al. 2010).
Statistical analysis
One-way ANOVA was used and mean comparison was performed by Duncans' new multiple range test. Statistical analysis was prepared using the SPSS statistical software, (release 18.0) for Windows (SPSS Inc. Chicago, IL). All data are presented as mean ± SD.
Results and discussion
Chemical composition of CEO
Thirteen components were identified representing 97.03 % of CEO. The qualitative and quantitative of CEO compositions are in Table 1. The major constituents of CEO were Eugenol (59.29 %), Propylene Glycol (11.29 %), Benzothiophene (9.67 %), and Β-caryophyllene (5.01 %). The results are in agreement with the results of Fu et al. (2007) and Chaieb et al. (2007) that they all distinguish clove essential oil is rich with Eugenol.
Table 1.
Percentage of chemical compounds identified in clove essential oil (CEO)
| Peak | Compound | RI* | Gc area (%) |
|---|---|---|---|
| 1 | Eugenol | 22.768 | 59.29 % |
| 2 | Propylene Glycol | 3.642 | 11.29 % |
| 3 | Benzothiophene-3-carbonitrile, 4,5,6,7-tetrahydro-2-(3-ethoxy-4-hydroxybenzylidenamino) | 51.482 | 9.67 % |
| 4 | Β-caryophyllene | 22.837 | 5.01 % |
| 5 | Eugenol acetat | 22.789 | 0.73 % |
| 6 | 3-Hydroxy-4-methoxymandelic acid | 25.33 | 1.63 % |
| 7 | 3,4-Dimethoxyphenylacethydrazide | 29.739 | 1.24 % |
| 8 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 33.376 | 0.62 % |
| 9 | 4-Hydroxy-2-methoxycinnamaldehyde | 33.621 | 2.70 % |
| 10 | Coniferyl alcohol | 39.002 | 1.90 % |
| 11 | 3,4-Dimethoxyphenylacethydrazide | 41.215 | 0.57 % |
| 12 | Pregn-5-en-20-one, 3-(acetyloxy)-16,17-epoxy-6-methyl-, (3.beta.,16.alpha.) | 47.297 | 1.96 % |
| 13 | Propenone | 47.622 | 0.42 % |
*Retention indices
Antimicrobial activity by disc diffusion assay
The antimicrobial activity of the different concentration CEO (1 and 1.5 %) against three bacteria is presented in Table 2. Both concentration of CEO showed excellent antimicrobial activities against all microorganisms tested in the present study. The results are in agreement with the results of Chaieb et al. (2007), they reported that clove essential oil had excellent antimicrobial activities against L. monocytogenes and E. coli O157: H7, and increasing in the zone of inhibition was observed by increasing the concentration of essential oil.
Table 2.
Antibacterial activity (inhibitory zone) of clove essential oil (CEO) against Gram-positive and Gram-negative bacteria
| Bacteria | CEO (%) | Inhibitory zone*(mm2) |
|---|---|---|
| Staphylococcus aureus (Gram +) | 1 % | 15.5 ± 1.3b |
| 1.5 % | 23.43 ± 1.23a | |
| Listeria monocytogenes (Gram +) | 1 % | 18.21 ± 1.32a |
| 1.5 % | 27.32 ± 0.57b | |
| E coli 0157: H7 (Gram -) | 1 % | 12.3 ± 0.28a |
| 1.5 % | 19.5 ± 0.52b |
*For each microbial species, different letters in columns indicate a significative difference (p < 0.05)
The antimicrobial effect of CEO was dependent on the type of microorganisms. L. monocytogenes was the most sensitive bacteria at a level of 1.5 % of CEO; the inhibition zone was 27.32 mm, and E coli O157: H7 was the lowest sensitive bacteria. Gram positive bacteria strains were more susceptible than Gram negative bacteria strains. The existing inhibition zone diameter of tested microorganisms could be attributed to the fact that the mode of action of monoterpene competent especially, Limonene disintegrates the outer membrane of bacteria, releasing lipopolysaccharides and increases the permeability of the cytoplasm membrane to adenosine tri-phosphate (ATP) (Sánchez-González et al. 2011).
Assessment of biochemical spoilage
PH
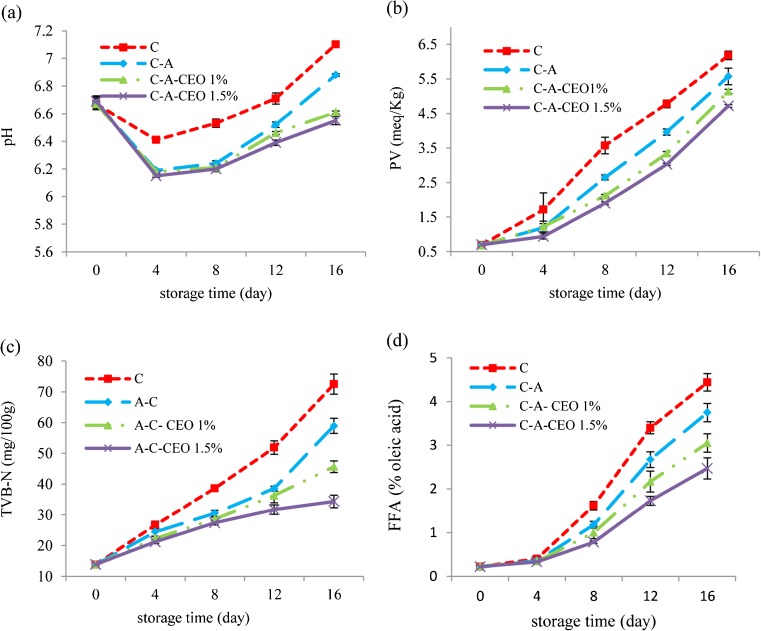
Sensory characteristics are negatively affected by a pH increase in fish during storage. The pH values of treatments during the refrigerated storage are shown in Fig. 1a. The initial pH of the samples was approximately 6.67, which decreased with storage up to the 4th day. A similar decrease has been reported by other researchers (Abdollahi et al. 2014, Ariaii et al. 2014). The reduction in initial pH of fishes presumably caused by the dissolution of CO2 in the fish sample, while the increase of pH was postulated to be due to an increase in volatile bases produced, e.g. ammonia and trimethylamine, by either endogenous or microbial enzymes (Manat et al. 2005). The value of pH increased until the end of the storage period. The pH values of coated samples significantly (p < 0.05) lower than the control samples during storage time. The lowest pH value at the end of storage period was observed in C-A + CEO 1.5 %. Lower pH in coated samples might be related to the potential of the coatings to decrease microbial growth and inhibit the activity of the endogenous proteases to different degrees (Fan et al. 2009). These potential had increased by CEO incorporation into the C-A matrix.
Fig. 1.
Changes in pH (a), peroxide value (PV) (b), total volatile basic nitrogen (TVB-N) (c) and free fatty acid (FFA) (d) of coated fillets during storage (C: control, C-A: coated with CMC and sodium alginate, C-A-CEO 1 %: coated with CMC and sodium alginate containing 1 % CEO, C-A-CEO 1.5 %: coated with CMC and sodium alginate containing 1.5 % CEO)
Peroxide value
Lipid oxidative rancidity has been long recognized as a main cause of seafood and food spoilage. The PV values of treatments during the refrigerated storage are shown in Fig. 1b. PV of fresh fish was approximately 0.69 MEq O2/Kg sample. The PV values of the control and coated samples increased significantly (p < 0.05) with storage time; by the end of the storage time (day 16), PV in coated samples was significantly lower than in the control. C-A have low oxygen permeability, which more produce an oxygen-resistant layer on the surface of fish fillet and decreased lipid oxidation (Abdollahi et al. 2014; Hamzeh and Rezaei 2012). These results are in agreement with those of Ariaii et al. (2014), who reported that methylcellulose coating was effective in retarding the production of primary lipid oxidation products in silver carp fillets stored by refrigeration. However, the lowest PV during the storage period was observed in the samples coated with C-A + CEO 1.5 % (P < 0.05). This could be caused by the combined effect of C-A (oxygen barrier) and antioxidant activity of CEO, which was mediated by polyphenols (Suppakul et al. 2003). Phenolic antioxidants do not function as oxygen absorbers; rather, they prevent the formation of fatty acid free radicals, which react with or absorb oxygen in the autoxidation process. This delays the onset of the autoxidative process in fat or oil (Turhan et al. 2009). The results were in agreement with Arfat et al. (2015) who reported that sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil had the lower PV, compared with the samples coated with wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film.
Total volatile basic nitrogen (TVB-N)
TVB-N is one of the most widely used indices of seafood quality. It is a general term which includes the measurement of trimethylamine (produced by spoilage bacteria), dimethylamine (produced by autolytic enzymes during storage), ammonia (produced by the deamination of amino-acids and nucleotide catabolites) and other volatile basic nitrogenous compounds associated with seafood spoilage (Gram and Huss 1996). TVB content of treatments during the refrigerated storage are shown in Fig. 1c. TVB content of all samples at day 0 was approximately 13.8 mg N/100 g, indicating the good quality of the fresh samples. The concentration of TVB-N in freshly caught fish is typically between 5 and 20 mg N/100 g, whereas levels of 30–35 mgN/100 g fish are generally regarded as the limit of acceptability for fish (Connell 1990). The TVB-N content increased in all treatments during storage. It exceeded the limit by day 7 for control and by day 10 for C-A coated samples. Such difference may be related to the antibacterial effect of cellulose and alginate coating as reported by other researchers (Hamzeh and Rezaei 2012; Ariaii et al. 2014; Raeisi et al. 2014; Heydari et al. 2015).
Ariaii et al. (2014) and Abdollahi et al. (2014) reported that exceeded limit for TVB-N of silver carp fillet was at day of 8 during the refrigerator storage.
At the end of the storage period, samples coated C-A + CEO 1.5 % reached a significantly (P < 0.05) lower TVB-N value of 34.35 in comparison with the others. Its value remained lower than acceptable limit for the C-A + CEO 1 and C-A + CEO 1.5 % samples until days 12 and 16 of storage, respectively. The lower value of TVB-N observed in samples coated C-A + CEO could be related to the antibacterial activity of the essential oil which would more rapidly reduce bacterial population and this could be due to the decreased capacity of bacteria for oxidative deamination of non-protein nitrogen compounds (Banks et al. 1980).
Free fatty acid (FFA)
Degree of lipid hydrolysis could be evaluated by measuring the amount of free fatty acids (FFA). FFA values of treatments during the refrigerated storage are shown in Fig. 1d. FFA of fresh fish was approximately 0.22 % oleic acid sample. A gradual increase in FFA content was observed in all treatments during the storage period. The overall increase displays hydrolytic oxidation in the fillets caused by internal or bacterial enzymes and the decrease might be related to the interaction of triacylglyceride products with proteins (Pereira de Abreu et al.2011). At the end of the storage period, samples coated A-C + CEO 1.5 % reached a significantly (P < 0.05) lower FFA value of 2.86 in comparison with the samples coated with A-C + CEO 1 %, A-C and the control, which attained higher levels of 3.05, 3.75 and 4.44, respectively. It might be attributed to antioxidant ability of clove which is related to its phenolic compounds, the major phenolic compounds of clove oil is eugenol, to which are attributed many of the antioxidant properties (Chaieb et al. 2007).
Microbiological changes during storage
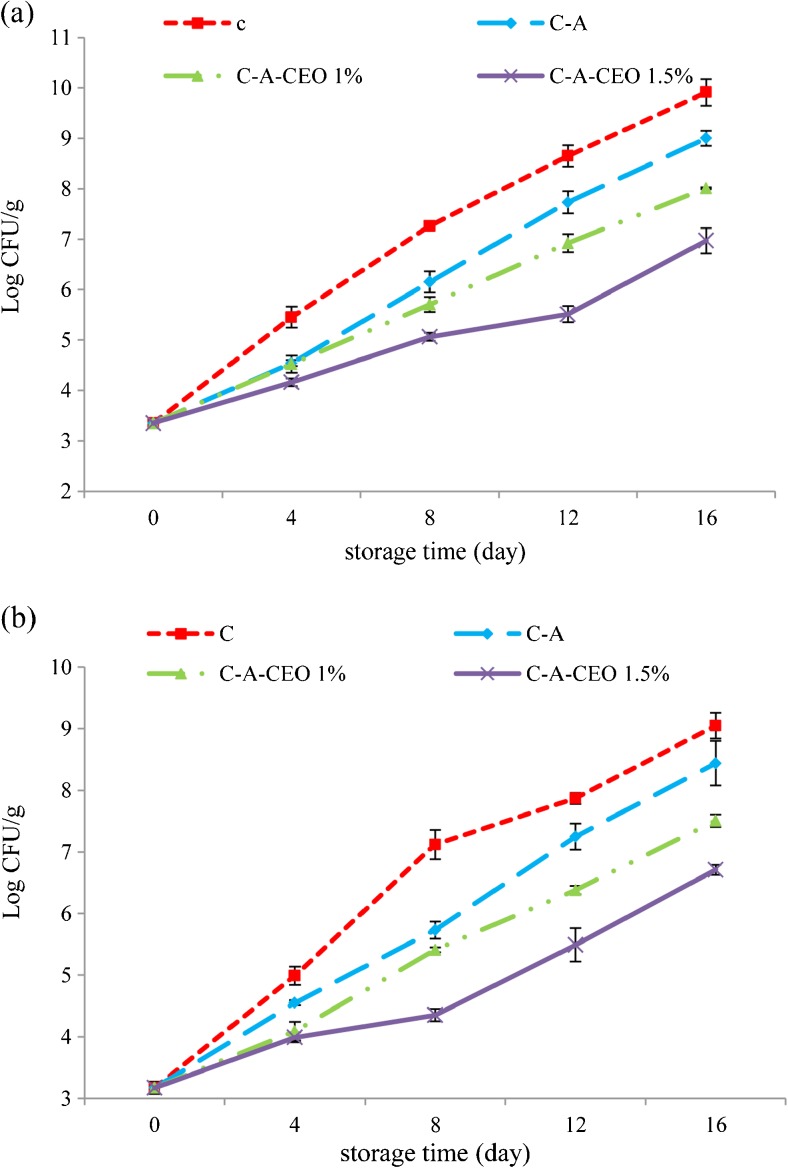
Changes in total viable count (TVC) and total psychrotrophic counts (TPC)
The composition of fish flesh makes it favorable for microbial growth, which manifests itself by changes in the sensory characteristics (Gram and Huss 1996). Variation in TVC and TPC of the samples treated with different treatments during the refrigerated storage are shown in Fig. 2a and b., TVC and TPC of silver carp samples were initially low (3.35 and 3.17 log10 CFU g-1 respectively) which is indicative of high fish quality and good manufacturing practices. In general, the initial microbial load of freshwater fishes can be different depending on water condition and temperature and it can be between 2.0 and 6.0 log10 CFU/g (ICMSF 1986). Both TVC (Fig. 2a) and TPC (Fig. 2b) showed a similar increasing trend in the all treatments; however, the value of the control increased faster than that of the others, Both TVC and TPC of control exceeded the maximum acceptable limit of 7 log10 CFU/g (ICMSF 1986) for freshwater and marine fish at the day 8th. As could be seen, all coated samples significantly inhibited the growth of mesophilic bacteria compared with the control during the storage period. It is revealed that composites coating could obviously prohibit the growth of mesophilic bacteria. The TVC of fillets coated with C-A, C-A + CEO 1 % exceeded the limit at the 12th and 16th day of storage, At the end of storage period, samples coated C-A + CEO 1.5 % reached a significantly (P < 0.05) lower TVC value of 6.71 in comparison with the others. It also had a similar trend about TPC of fillets stored at 4 ± 1 °C. Consequently, the composite coating containing CEO 1.5 % could improve the shelf life of the silver carp samples by about 8 days as compared to the control. Clove oil contains eugenol and other phenolic compounds that are known to be either bactericidal or bacteriostatic agents. These components could attack on the phospholipid cell membrane, causing increased permeability and leakage of cytoplasm, or interact with enzymes located on the cell wall. However, addition of CEO into the composite caused it to be released to the surface of coated fillets and could keep its inhibitory effect for a longer period. Similar results were observed by the others (Ariaii et al. 2014; Heydari et al. 2105).
Fig. 2.
Changes in (a) total viable count (TVC) and (b) total psychrotrophic count (TPC) of coated fillets during the storage. (C: control, C-A: coated with CMC and sodium alginate, C-A-CEO 1 %: coated with CMC and sodium alginate containing 1 % CEO, C-A-CEO 1.5 %: coated with CMC and sodium alginate containing 1.5 % CEO)
Inhibition the growth of E. coli O157:H7 in fish fillet
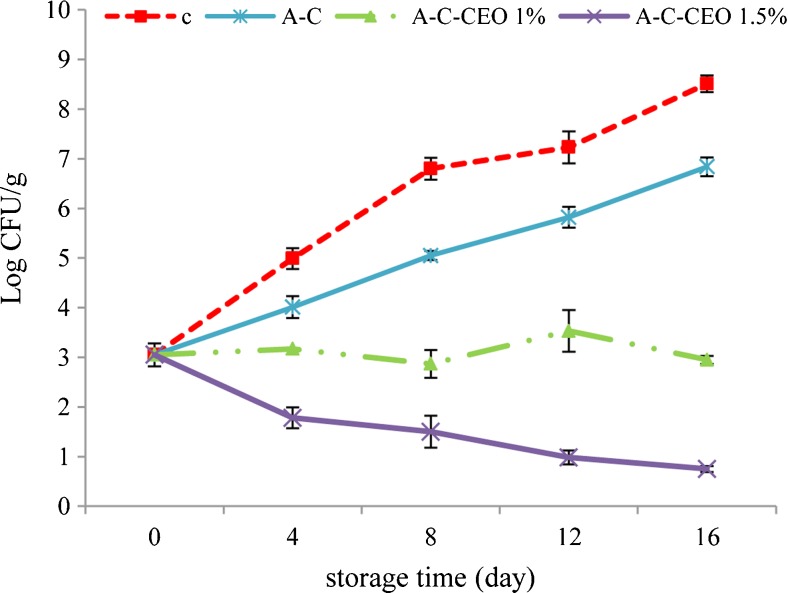
The effects of composite coated with clove essential oil on E. coli O157:H7 inoculated in silver carp fillet are presented in Fig. 3. According to the Food and Drug administration of IRAN related to the food microbiology, tolerated limit for fresh/frozen fish is 2 log CFU/g. As could be seen, the initial population of E. coli O157:H7 in the control group increased rapidly during the storage period. Although the sample coated with C-A showed a population of E. coli O157:H7 significantly lower than the control, it could not inhibit its growth completely during the storage period. C-A + CEO 1 % could significantly reduce the population of E. coli compared to the control and C-A treated samples from day 4 until the end of the storage. However, these treatments could not reduce its population below the acceptable level (<2 log CFU/g) until the end of the storage period. On the other hand, both treatment C-A + CEO 1 % and 1.5 % could reduce the population of E. coli O157:H7 significantly during the storage period, while C-A + CEO 1.5 % reduced its population below the acceptable level (<2 log CFU/g) from day 4 until the end of the storage. As mentioned before, some components of clove like eugenol, propylene Glycol and Benzothiophene have shown antibacterial properties (Chaieb et al. 2007). The results indicate that CEO at 1.5 % or higher concentrations has drastic bacterial effect against these pathogens in fish fillet at the refrigerated storage. These results were in agreement with those reported by Moreira et al. (2007) about the effect of clove essential oil on E. coli O157:H7 inoculated in minced cooked beef during the refrigerated storage. They reported that CEO showed an inhibitory activity against E. coli O157:H7; in addition, the antimicrobial action was dependent on the oil concentration.
Fig. 3.
Changes in Escherichia coli O157:H7 count (Log CFU/g) in coated fillets during storage. (C: control, C-A: coated with CMC and sodium alginate, C-A-CEO 1 %: coated with CMC and sodium alginate containing 1 % CEO, C-A-CEO 1.5 %: coated with CMC and sodium alginate containing 1.5 % CEO)
Sensory evaluation
The results of the sensory evaluation of samples are given in Table 3. Initially, all samples had a bright and acceptable appearance. Tang was not reported by any of the panelists. Therefore, the application of CEO in combination with A-C does not negatively affect sensory properties of fish products. The sensory scores of all treatment samples decreased with the storage time. The fish samples were considered to be acceptable for human consumption until the sensory score reached 4 (Ojagh et al. 2010). According to the results of the sensory analysis acceptability of control samples were given ‘unacceptable’ scores by the 4th day, 8th day for samples coated with A-C, 12th day for samples coated with A-C + CEO 1 %, and 16th day for samples coated with A-C + CEO 1.5 %. Changes in the sensory characteristics are correlated to microbial and chemical values; it is related to antioxidant and antibacterial properties of the CEO. Similar results were obtained by the other researches (Abdollahi et al. 2014; Ariaii et al. 2014).
Table 3.
Changes in the sensory attributes of coated fillets during the storage
| Sensory attributes | Treatment | Storage period (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| Texture | C | 5.00 ± 0.00 a | 4.23 ± 0.21 b | 3.14 ± 0.00 c | 2.50 ± 0.00 d | 1.95 ± 0.34 d |
| C-A | 5.00 ± 0.00 a | 4.56 ± 0.20 ab | 4.01 ± 0.15 b | 3.78 ± 0.17 c | 2.98 ± 0.27 c | |
| C-A-CEO 1 % | 5.00 ± 0.00 a | 4.75 ± 0.35 a | 4.23 ± 0.17 b | 4.02 ± 0.13 b | 3.52 ± 0.38 b | |
| C-A-CEO 1.5 % | 5.00 ± 0.00 a | 4.85 ± 0.25 a | 4.63 ± 0.15 c | 4.39 ± 0.10 a | 4.21 ± 0.28a | |
| Odor | C | 5.00 ± 0.00 a | 4.22 ± 0.15 a | 3.35 ± 0.25b | 2.25 ± 0.23 d | 1.85 ± 0.00 d |
| C-A | 4.85 ± 0.15b | 4.35 ± 0.20 a | 4.16 ± 0.28 a | 3.65 ± 0.27 c | 3.23 ± 0.07 c | |
| C-A-CEO 1 % | 4.75 ± 0.3 b | 4.48 ± 0.15 a | 4.33 ± 0.27 a | 4.06 ± 0.03 b | 3.82 ± 0.12 b | |
| C-A-CEO 1.5 % | 4.7 ± 0.19 b | 4.58 ± 0.23 a | 4.43 ± 0.28 a | 4.29 ± 0.10 a | 4.11 ± 0.22a | |
| Color | C | 5.00 ± 0.00 a | 4.1 ± 0.21 b | 3.14 ± 0.10 c | 2.00 ± 0.27 d | 1.75 ± 0.00 c |
| C-A | 5.00 ± 0.00 a | 4.38 ± 0.20 b | 4.11 ± 0.12 b | 3.33 ± 0.25 c | 3.35 ± 0.11 b | |
| C-A-CEO 1 % | 5.00 ± 0.00 a | 4.75 ± 0.25 a | 4.53 ± 0.27 a | 4.0 ± 0.15 b | 3.49 ± 0.23 b | |
| C-A-CEO 1.5 % | 5.00 ± 0.00 a | 4.89 ± 0.15 a | 4.63 ± 0.25 a | 4.35 ± 0.08 b | 4.0 ± 0.12 a | |
| Overall | C | 5.00 ± 0.00 a | 4.25 ± 0.12 b | 3.23 ± 0.21 d | 2.35 ± 0.23 d | 1.88 ± 0.35 d |
| C-A | 4.85 ± 0.05 b | 4.55 ± 0.2 a | 4.00 ± 0.11c | 3.46 ± 0.13 c | 3.02 ± 0.28 c | |
| C-A-CEO 1 % | 4.75 ± 0.10 b | 4.68 ± 0.09 a | 4.25 ± 0.07 b | 4.06 ± 0.03 b | 3.57 ± 0.32 b | |
| C-A-CEO 1.5 % | 4.75 ± 0.12 b | 4.65 ± 0.15 a | 4.43 ± 0.15 a | 4.25 ± 0.07 b | 4.1 ± 0.13 a | |
a, b, c With different small letters in the same row, represents significant difference (p < 0.05). (C: control, C-A: coated with CMC and sodium alginate, C-A-CEO 1 %: coated with CMC and sodium alginate containing 1 % CEO, C-A-CEO 1.5 %: coated with CMC and sodium alginate containing 1.5 % CEO)
Conclusions
Results of the present study demonstrate that the combination of clove essential oil with alginate/carboxyl methyl cellulose composite in 1.5 % v/v could improve the quality of coated silver carp fillets. A-C + CEO 1.5 % treatment could maintain silver fillet shelf life till 16 days without any significant loss of texture, odor, color or overall acceptability and it had lower bacterial count, TVB-N and lipid oxidation rate in comparison to the others. Moreover, both A-C + CEO at 1 and 1.5 % could decrease the population of E. coli O157:H7 significantly during the storage period, while A-C + CEO at 1.5 % reduced its population below the acceptable level (<2) from day 4 until the end of the storage. Therefore, these coatings could be promising alternatives to reduce conventional chemical additives and synthetic materials in food formulation, possibly contributing to improve food quality and prolong shelf life of fresh produce.
Compliance with ethical standards
Funding
This study was part of Nastaran Jalali’s of master thesis and it was supported by Islamic Azad University (Ayatollah Amoli Branch).
References
- Abdollahi M, Rezaei M, Farzi G. Influence of chitosan/clay functional bionanocomposite activated with rosemary essential oil on the shelf life of fresh silver carp. Int J Food Sci Technol. 2014;49:811–818. doi: 10.1111/ijfs.12369. [DOI] [Google Scholar]
- Alboofetileh M, Rezaei M, Hosseini H, Abdollahi M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common food borne pathogens. Food Control. 2014;36:1–7. doi: 10.1016/j.foodcont.2013.07.037. [DOI] [Google Scholar]
- Albuquerque Costa R. Escherichia coli in seafood: a brief overview. Adv Biosci Biotechnol. 2013;4:450–454. doi: 10.4236/abb.2013.43A060. [DOI] [Google Scholar]
- Andevari GT, Rezaei M (2011) Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. International Journal of Food Science and Technology 46:2305–2311
- AOAC (2005) Official method of analysis (17th ed). Washington DC: Association of Official Analytical Chemists
- Arfat Y.A, Benjakul S, Vongkamjan K, Sumpavapol P, Yarnpakdee S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J Food Sci Technol. 2015 doi: 10.1007/s13197-014-1706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariaii P, Tavakolipour H, Rezaei M, Elhamirad M, Bahram S (2014) Effect of methylcellulose coating enriched with pimpinella affinis oil on the quality of silver carp fillet during refrigerator storage condition. J Food Process Preserv ISSN 1745-4549. doi:10.1111/jfpp.12394
- Banks H, Ii R N, Finne G. Shelf-life studies on carbon dioxide packaged finfish from the gulf of Mexico. J Food Sci. 1980;45:157–162. doi: 10.1111/j.1365-2621.1980.tb02566.x. [DOI] [Google Scholar]
- Chaieb k, Hajlaoui H, Zmantar T, Kahla-Nakbi A B, Rouabbia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil Eugenia caryophyllata (syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- Connell JJ (1990) Control of fish quality (3rd ed.). Oxford, UK: Fishing News Books. Ehira, S. 1976. A biochemical study on the freshness of fish. Bull Tokai Reg Fish Res Lab, 88:130–132.
- Egan H, Kirk R. S, Sawyer, R (1997) Pearson’s chemical analysis of food. 9th ed. Edinburgh.Scotland. U.K: Churchill Livingstone 609–34.
- Fan W, Sun J, Chen Y, Qiu J, Zhang Y, Chi Y. Effects of chitosan coating on quality and shelf-life of silver carp during frozen storage. Food Chem. 2009;115:66–70. doi: 10.1016/j.foodchem.2008.11.060. [DOI] [Google Scholar]
- Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res. 2007;21:989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh B, Almasi H, Entezami A. A. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov Food Sci Emerg. 2010;11:697–702. doi: 10.1016/j.ifset.2010.06.001. [DOI] [Google Scholar]
- Gram L, Huss H H. Microbiological spoilage of fish and fish products. Int J Food Microbiol. 1996;33:121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- Guilbert S (1986) Technology and application of edible protective films. In Food Packaging and Preservation (M. Matholouthi, ed.) pp 371–392. Elsevier Applied Science Publishing Co. Inc. New York.
- Gómez-Estaca J, López de Lacey A, López-Caballero M E, Gómez-Guillén M C, Montero P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27(7):889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Hamzeh A, Rezaei M. The effects of sodium alginate on quality of rainbow trout (Oncorhynchus mykiss) fillets stored at 4 ± 2 °C. J Aquat Food Prod Technol. 2012;21:14–21. doi: 10.1080/10498850.2011.579384. [DOI] [Google Scholar]
- Heydari R, Bahram S, Javadian SR (2015) Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci Nutr 1-7. doi:10.1002/fsn3.202 [DOI] [PMC free article] [PubMed]
- ICMSF . Microorganisms in foods. 2. Sampling for microbiological analysis. 2nd. Toronto: University of Toronto Press; 1986. [Google Scholar]
- Jayasena DD, Jo C. Essential oils as potential antimicrobial agents in meat and meat products: a review. Trends Food Sci Technol. 2013;34:96–108. doi: 10.1016/j.tifs.2013.09.002. [DOI] [Google Scholar]
- Kim Y, Kim S, Chung H. Synergistic effect of propolis and heat treatment leading to increased injury to Escherichia coli O157:H7 in ground pork. J Food Saf. 2014;34:1–8. doi: 10.1111/jfs.12088. [DOI] [Google Scholar]
- Manat C, Sootawat B, Wonnop V, Caameron F. Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005;93:607–617. doi: 10.1016/j.foodchem.2004.10.035. [DOI] [Google Scholar]
- Moreira M, Ponce A, Delvalle C, Roura S. Effects of clove and tea tree oils on Escherichia coli O157:H7 in blanched spinach and minced cooked beef. J Food Process Preserv. 2007;3:379–391. doi: 10.1111/j.1745-4549.2007.00135.x. [DOI] [Google Scholar]
- Ojagh S M, Rezaei M, Razavi S H, Hoseini S M H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Pereira De Abreu D A, Paseirolosada P, Maroto J, Cruz J M. Natural antioxidant active packaging film and its effect on lipid damage in frozen blue shark (Prionace glauca) Innov Food sci Emerg. 2011;12(1):50–55. doi: 10.1016/j.ifset.2010.12.006. [DOI] [Google Scholar]
- Raeisi M, Tajik H, Aliakbalu J, Valipour S. Effect of carboxymethyl cellulose edible coating containing zataria multifloraessential oil and grape seed extract on chemical attributes of rainbow trout meat. Vet Res Forum. 2014;5:89–93. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M T. Use of essential oils in bioactive edible coatings. Food Rev Eng. 2011;3:1–16. doi: 10.1007/s12393-010-9031-3. [DOI] [Google Scholar]
- Seyfzadeh M, Motalebi AA, Kakoolaki S, Gholipour H (2013) Chemical microbiological and sensory evaluation of gutted kilka coated with whey protein based edible film incorporated with sodium alginate during frozen storage. Iranian Journal of Fisheries Sciences 12(1): 140–153
- Shin J H, Chang S, Kang D H. Application of antimicrobial ice for reduction of foodborne pathogens (Escherichia coli O157:H7, Salmonella Typhimurium, Listeria monocytogenes) on the surface of fish. J Appl Microbiol. 2004;97:916–922. doi: 10.1111/j.1365-2672.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Suppakul P, Miltz J, Sonneveld K, Bigger SW. Antimicrobial properties of basil and its possible application in food packaging. J Agric Food Chem. 2003;51:3197–3207. doi: 10.1021/jf021038t. [DOI] [PubMed] [Google Scholar]
- Teophilo G N, dos Fernandes-Vieira R H, dos Prazeres-Rodrigues D, Menezes F G. Escherichia coli isolated from seafood: toxicity and plasmid profiles. Int Microbiol. 2002;5:11–14. doi: 10.1007/s10123-002-0052-5. [DOI] [PubMed] [Google Scholar]
- Turhan K, Sahbaz F. Water vapor permeability, tensile properties and solubility of methylcellulose-based edible films. J Food Eng. 2004;61:459–466. doi: 10.1016/S0260-8774(03)00155-9. [DOI] [Google Scholar]
- Turhan S, Sagir I, Temiz H. Oxidative stability of brined anchovies (Engraulis encrasicholus) with plant extracts. Int J Food Sci Technol. 2009;44:386–393. doi: 10.1111/j.1365-2621.2008.01777.x. [DOI] [Google Scholar]
- Zactiti E, Kieckbusch T. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag Technol Sci. 2009;22(6):349–358. doi: 10.1002/pts.860. [DOI] [Google Scholar]