Abstract
Pomegranate extract, vanillin and geraniol were studied as natural antimicrobials on strawberry juice. Strawberry juice was treated with each agent at two concentrations: pomegranate extract at 180 and 360 μg/mL; vanillin at 2.5 and 5 mg/mL; and geraniol at 0.6 and 1.2 μL/mL. After being treated, juices were stored at 5 °C and microbiological, physicochemical and sensory studies were carried out. Also, a second batch of juice was inoculated with Escherichia coli O157:H7 (105 CFU/mL) before being treated, to safety study. Geraniol and vanillin, at both concentrations tested, were highly effective in reducing the native microflora on strawberry juice (more than 3 log cycles), extending the microbiological shelf-life of the product. Moreover, both antimicrobials improved the product safety by reducing inoculated E. coli O157:H7. Furthermore, vanillin showed a significant increase in polyphenol content compared to untreated juice. On the other hand, pomegranate extract applied at the highest concentration showed important reductions on mesophilic and psychrophilic bacteria, but no effect on yeast and molds and inoculated E. coli. Even though vanillin and geraniol incorporation on strawberry juice had a negative effect on its sensory quality, pomegranate extract had no impact on the sensory attributes evaluated. Combinations of the biopreservatives could be studied in order to decrease the concentration of the antimicrobials, reducing the effects on strawberry juice sensory characteristics.
Keywords: Pomegranate extract, Vanillin, Geraniol, Native microflora, Antioxidant capacity, Escherichia coli O157:H7
Introduction
Thermal pasteurization is one of the most popular preservation technologies in the food and beverage industry because of its effectiveness in eliminating microorganisms and pathogens, and inactivating enzymes. Nevertheless, thermal treatments are known to produce loss of nutritional and sensory characteristics, reducing the quality of the final product (Elez-Martínez and Martín-Belloso 2005). Therefore, consumers are demanding fresh-like products such as unpasteurized fruit juices, due to their high nutritional and sensory qualities, as well as their health benefits (Mittal and Griffiths 2005).
In the past, fresh fruits and their juices were considered safe products because of their low pH caused by naturally occurring organic acids, which may generally prevent the growth of pathogen bacteria (Parish 1997). However, many illness outbreaks have been reported due to the consumption of unpasteurized fruit juices. Food pathogens such as strains of Escherichia coli O157:H7 and the psychrotrophic bacterium Listeria monocytogenes, have shown a significant resistance to acid medium, and have survived in fruit juice for several days, even in refrigerated conditions (Moon et al. 2006).
Control of both food spoilage and pathogenic microorganisms is mainly achieved by chemical control. However, consumers have a negative perception of industrially synthesized food antimicrobials due to undesirable aspects including carcinogenicity, acute toxicity and teratogenicity (Faleiro 2011). This has generated an increasing interest in the use of more naturally occurring compounds. In this work, three novel natural antimicrobials were studied as biopreservatives in strawberry juice: vanillin (V), geraniol (G) and pomegranate extract (PE).
Vanillin, obtained from the bean or pod of the tropical Vanilla orchid, exhibits antioxidant and antimicrobial activity against bacteria, moulds and yeast and could be used also as food preservative (Vasantha Rupasinghe et al. 2006).
Geraniol is a common constituent of several essential oils and occurs in Monarda fistulosa (> 95 %), ninde oil (66.0 %), rose oil (44.4 %), palmarosa oil (53.5 %) and citronella oil (24.8 %), among others (Chen and Viljoen 2010). In addition, geraniol exhibits various biochemical and pharmacological properties. Researchers have shown geraniol to be an effective plant-based insect repellent (Barnard and Xue 2004) and its potential as an antimicrobial agent has been highlighted in several studies (e.g. Bard et al. 1988).
Finally, pomegranate fruit extracts possess several health benefits such as anti–inflammatory, cardioprotective, free radical scavenging, hepatoprotective, tyrosinase inhibition and anti–diabetic (Mphahlele et al. 2014). In addition to antioxidant properties, gallic acid, ellagic acid and punicalagin present in pomegranate extracts possess antimicrobial activities (Ismail et al. 2012).
Few studies have been made, where these natural agents were applied in vivo in fruit juices. Therefore, in this research, strawberry juice treated with the selected natural antimicrobials was stored at 5 °C for 21 days and the evolution of native microflora, physicochemical parameters, antioxidant capacity and sensory quality were evaluated. Also, inoculation with E. coli O157:H7 was carried out in order to evaluate the effectiveness of the antimicrobials in improving the safety of the strawberry juice.
Materials and methods
Juice preparation
Strawberries (Fragaria x ananassa) were grown and harvested in Sierra de los Padres, Mar. del Plata, Argentine. Stems of strawberries were removed and the juices were prepared with a commercial juice extractor. Once the treatments were applied, the strawberry juices were stored in sterile polypropylene flasks at 5 °C for 21 days to assess the evolution of native microbial populations, antioxidant capacity, total phenolic content, total soluble solids, total acidity and sensory evaluation.
These parameters were also measured on the strawberry juice before the application of treatments (untreated sample). Table 1 shows the results of this characterization.
Table 1.
Characterization of strawberry juice
| Parameters | Strawberry juice |
|---|---|
| Native microflora (log CFU/mL) | |
| Total mesophillic bacteria | 4.45 ± 0.25 |
| Yeast and molds | 5.40 ± 0.13 |
| Total psychrophilic bacteria | 5.23 ± 0.17 |
| Physicochemical determinations | |
| °Bx | 8.5 ± 0.1 |
| Total tritable acidity (% TA) | 0.781 ± 0.013 |
| Antioxidant capacity | |
| DPPH radical scavenging activity (% inhibition) | 61.4 ± 3.4 |
| Total phenols (mg GAE/100 mL of juice) | 119.5 ± 7.7 |
Data is shown as means ± standard errors
Inoculation with Escherichia coli O157:H7
Strain and inoculum preparation
Escherichia coli O157:H7, ATCC 43895 was used. A stock culture was maintained in tryptic soy broth (Britania, Buenos Aires, Argentina) at 4 °C. Before use, E. coli O157:H7 was cultured in brain heart infusion (BHI) broth (Britania) for 24 h at 37 °C. A 0.1 mL aliquot of the culture was transferred to 9.9 mL of BHI broth at two consecutive 24 h intervals followed by incubation at 37 °C before each experiment. A bacterial suspension was prepared by adding 10 mL of the E. coli culture to 90 mL of sterile peptonated water (0.1 % w/v).
Inoculation of the samples
Samples were inoculated with E. coli O157:H7 before the application of the treatments, simulating an inadequate manipulation of the strawberries at postharvest. To carry this out, 100 μL of the bacterial suspension previously prepared were added to 9.9 mL of fresh strawberry juice to reach a final pathogen concentration of ∼5 log colony-forming units CFU/mL.
Strawberry juice processing
Pomegranate extract used in this study was purchased from PureBulk, USA. In order to quantify the main phenolic compounds present in the pomegranate extract used in the present work, an HPLC analysis was performed in a previous unpublished study. Table 2 shows the results of this characterization. Vanillin and geraniol were purchased from Sigma Aldrich (St. Louis, MO, USA).
Table 2.
Main phenolic compounds concentrations of pomegranate extract
| Component | Concentration (mg/g of extract) |
|---|---|
| Ellagic acid | 345 ± 7 |
| Gallic acid | 184 ± 4 |
| Punicalagin A | 98 ± 2 |
| Punicalagin B | 47 ± 1 |
| Caffeic acid | 19.0 ± 0.4 |
Data is shown as means ± standard deviations
Each of the natural antimicrobial agents was applied to strawberry juice at two different concentrations: 2.5 mg/mL and 5 mg/mL of vanillin (V1 and V2, respectively); 0.6 μL/mL and 1.2 μL/mL of geraniol (G1 and G2, respectively); and 180 μg/mL and 360 μg/mL of pomegranate extract (PE1 and PE2 respectively). For vanillin and geraniol, concentrations were selected according to Tomadoni et al. (2015), whereas concentrations used for pomegranate extract were selected according to Alvarez et al. (2012). A juice with no treatment (untreated) was used as control sample.
A batch of non-inoculated strawberry juice was prepared and treated with the selected antimicrobial agents for microbiological, physicochemical and sensory analysis. A second batch of inoculated strawberry juice was prepared and treated with the antimicrobial agents for E. coli O157:H7 survival study.
Microbiological analysis
Counts of yeast and molds, mesophilic and psychrophilic bacteria in non-inoculated strawberry juice were evaluated at 0, 3, 7, 14 and 21 days of storage to study the impact of vanillin, geraniol and pomegranate extract on the native microflora. On the other hand, counts of E. coli on inoculated strawberry juice were also carried out at 0, 3, 7, 14 and 21 days of refrigerated storage to evaluate the effect of the different treatments over the E. coli population. Microbiological analysis on day 21 of storage was not performed on those samples that on day 14 showed counts higher than 107 CFU/mL (maximum limit of mesophilic aerobic total count at expiry allowed by Spanish regulations).
Native microflora was monitored as follows: 10 mL aliquot of juice from each treatment was sampled at each time of refrigerated storage. Serial dilutions (1:10) of each sample were made in sterile peptonated water (0.1 % w/v) and surface spread by duplicate. The enumeration of the microbial populations was performed according to Ponce et al. (2008) by using the following culture media and culture conditions: mesophilic aerobic bacteria on Plate Count Agar (PCA) incubated at 35 °C for 48 h; psychrophilic bacteria on PCA incubated at 7 °C for 7 d; yeast and molds on Yeast-Glucose-Chloramphenicol (YGC) medium incubated at 25 °C for 5 d.
Viable E. coli counts were monitored as follows: 0.1 mL aliquot of each sample were spread on the surface of eosin methylene blue (EMB) agar plates and the colonies were counted after incubation at 37 °C for 24–48 h. EMB is a selective medium that allows the characterization of typical E. coli colonies; those that were dark centered, flat and with a metallic sheen were taken into account. Randomly, selected E. coli colonies were confirmed using an E. coli chromogenic test kit (Chromobrit, Britania).
All culture mediums were purchased from Britania, Buenos Aires, Argentina. Microbial counts were performed by duplicate and expressed as log CFU/mL.
Total soluble solids
°Bx is a measure of soluble solids present in fruit pulp and juice expressed as percentage of sucrose at 20 °C. Soluble solids were measured using an Atago refractometer (Abbe 1T74T, Tokio, Japón). °Bx was measured by duplicate.
Titratable acidity
For determination of titratable acidity, a known volume of each sample was placed in a 250 mL beaker, and 50 mL of distilled water were added. Further, this solution was titrated against standardized 0.1 N NaOH to the phenolphthalein end point (pH 8.2 ± 0.1). The volume of NaOH was converted to grams of citric acid per 100 mL of juice (% TA) based on the method of Sadler and Murphy (2010), and the total acidity was calculated using equation (1):
| 1 |
where V is the titer volume of NaOH and Vs is the volume of the strawberry juice sample.
TA was measured by duplicate.
Extraction of antioxidants and phenolic compounds
Antioxidants and phenolic compounds were evaluated at 0, 3, 7 and 10 days of storage to study the impact of vanillin, geraniol and pomegranate extract on the nutritional quality of strawberry juice. 2 mL of strawberry juice from each treatment was homogenized with 10 mL solution of ethanol (80 % v/v) in a vortex. The homogenate was then centrifuged at 8000 rpm for 15 min at 4 °C. The supernatant was collected and filtered using Whatman filter paper #1. The ethanolic extract was stored at −20 °C to be used in the determinations of total phenolic content (TPC) and antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method.
Determination of DPPH radical scavenging activity
The radical scavenging activity was measured in terms of hydrogen-donating or free radical-scavenging using the DPPH methodology proposed by Brand-Williams et al. (1995).
An ethanolic DPPH solution (100 μM) was used for determinations. Ethanol (0.1 mL) was mixed with 3.9 mL of DPPH (100 μM) to determine the initial absorbance of the DPPH solution. Next, 0.1 mL of sample extract was added to 3.9 mL of 100 μM DPPH solution. The mixture was shaken immediately and allowed to stand at ambient temperature in the dark. The decrease in absorbance at 517 nm was measured after 60 min.
The radical scavenging activity was expressed as the inhibition percentage of the DPPH radical and was measured by duplicate.
Total phenolic content
TPC was determined spectrophotometrically using the Folin–Ciocalteu reagent (FCR) according to the methodology proposed by Singleton et al. (1999) with modifications. Extract samples properly diluted were added to 1000 μL of FCR (diluted 1:10). After 3 min of incubation at ambient temperature, 800 μL of 7.5 % Na2CO3 solution was added and the reaction mixture was incubated for 2 h at the same temperature. The absorbance was measured at 765 nm using a UV-Vis spectrophotometer (1601 PC UV-visible, Shimadzu Corporation, Kyoto, Japan) and TPC was calculated using gallic acid as standard.
Results of TPC were expressed as mg gallic acid equivalents (GAE)/100 mL of juice and were measured by duplicate.
Sensory analysis
A sensory analysis was carried out in a similar way as Saftner et al. (2005). Strawberry juice prepared the same day of the test (t = 0) and also those stored at 5 °C for 7 days were evaluated by ten trained panelists. Samples labeled with 3 digit code numbers were randomly provided.
Water was provided to panelists for eliminating the residual taste between samples. The attributes evaluated were: color, vanillin odor, citric odor, off-odor, bitterness, sweetness and acidity. Unstructured line scales (5 cm) anchored at the ends with terms related with minimum and maximum intensities were used to evaluate each attribute.
Definitions, anchor terms and reference values (fresh strawberry juice with no treatment) for each attribute are shown on Table 3.
Table 3.
Description of the selected sensory attributes, anchor ends and consensus values for the reference sample
| Attributes | Description | 0 | 5 | Reference |
|---|---|---|---|---|
| Appearance | ||||
| Color | Depth of the color | Characteristic red | Dark red | 0 |
| Odor | ||||
| Vanilla Odor | Intensity of vanilla odor | Low | High | 0 |
| Citric Odor | Intensity of lemon and citric odors | Low | High | 0 |
| Off-odor | Intensity of fermentation or other non-characteristic odors | Low | High | 0 |
| Flavor | ||||
| Sweetness | The intensity of sweet | Low | High | 1 |
| Acidity | The intensity of acid | Low | High | 1 |
| Bitterness | The intensity of bitter | Low | High | 0 |
Statistical analysis
A completely randomized design was used. Three independent runs were performed. Data obtained was analyzed using R v. 2.12.2 (R Development Core Team 2011). Results reported in this article are mean values accompanied by their standard errors (Kuehl 2001). Analysis of variance ANOVA was performed and Tukey-Kramer comparison test was used to estimate significant differences between treatments (p < 0.05).
Results and discussion
Effect of natural antimicrobials on native microflora in strawberry juice
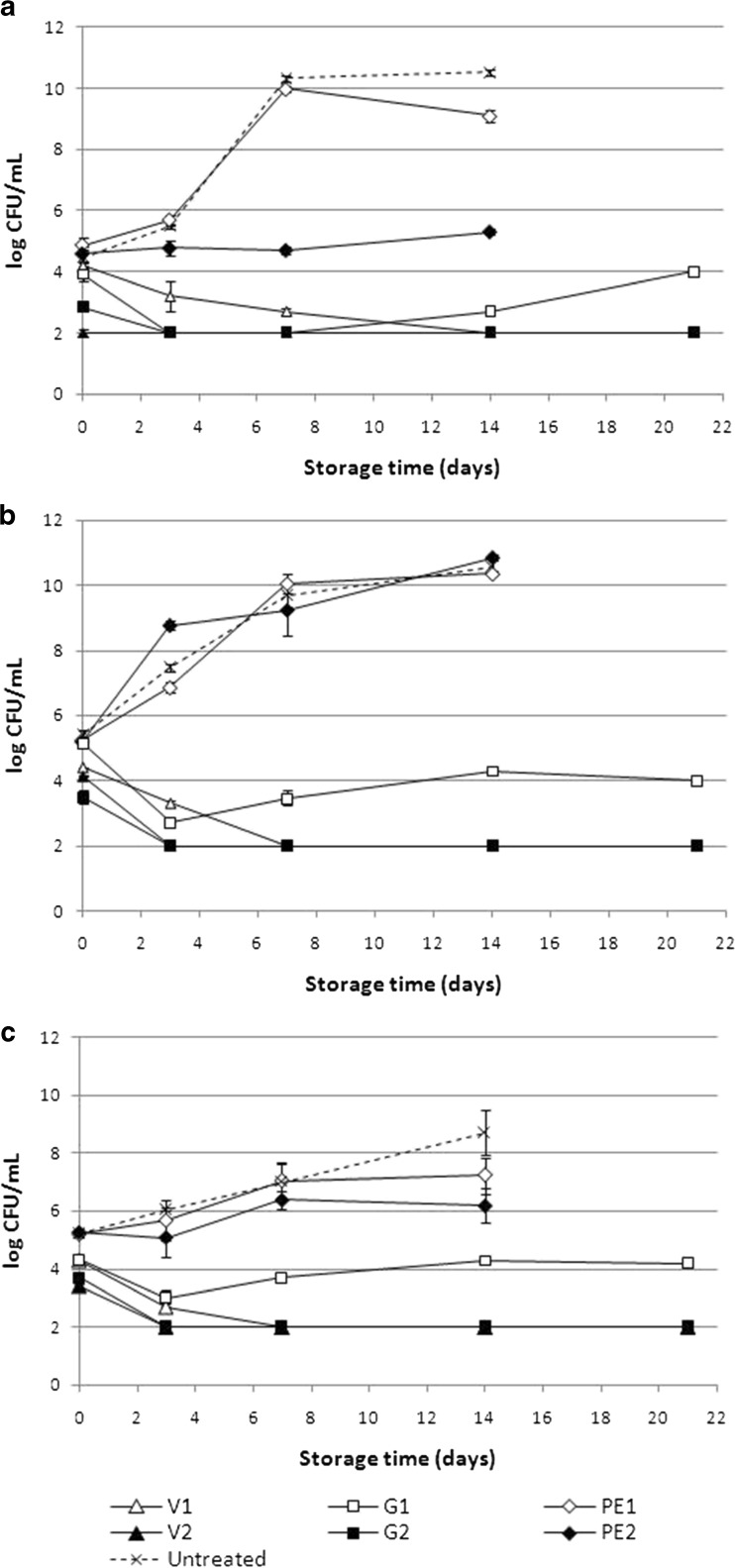
The results of selected natural antimicrobials effects on strawberry juice native microflora are shown on Fig. 1. Mesophilic microorganisms give an estimate of total viable populations and are indicative of the endogenous microorganisms and the contamination undergone by the material (Ponce et al. 2008).
Fig. 1.
Growth of mesophilic bacteria (a), yeast and molds (b), and psychrophilic bacteria (c) in non-inoculated strawberry juice with natural antimicrobials throughout refrigerated storage at 5 °C. Bars indicate standard errors. V1: 2.5 mg/mL of vanillin; V2: 5 mg/mL of vanillin; G1: 0.6 μL/mL of geraniol; G2: 1.2 μL/mL of geraniol; PE1: 180 μg/mL of pomegranate extract; PE2: 360 μg/mL of pomegranate extract
Initial population (day 0) of mesophilic microorganisms on untreated strawberry juice was 4.45 log CFU/mL (Fig. 1a). Significant reductions (p < 0.05) were observed for those samples treated with G2 and V2, with counts of 2.85 log CFU/mL, and below the detection limit (< 2 log), respectively. At day 3 of storage, mesophilic bacteria on untreated strawberry juice were 5.49 log, while samples treated with G1, G2 and V2 showed counts below the detection limit. Also, significant reductions were observed compared to untreated sample for V1 and PE2 treatments, where counts were 3.20 and 4.77 log, respectively.
At day 7 of refrigerated storage, untreated sample mesophilic counts were 10.32 log, with no significant difference from PE1. On the other hand, PE2 showed a significant reduction reaching 4.70 log, and V1 showed a greater effect, with counts of 2.70 log.
At day 14, mesophilic microorganisms on untreated juice, showed no significant difference from day 7, with counts of 10.53 log. PE1 showed a reduction of approximately 1 log, while PE2 showed a higher reduction, reaching counts of 5.30 log. On the other hand, V1 counts were below detection limit until the end of storage. G1 counts were 2.70 log and increased to 4.00 log by day 21. Mesophilic population on samples G2 and V2 remained below the detection limit until the end of refrigerated storage (day 21).
Figure 1b shows the evolution of yeast and molds populations through storage. Food spoilage by yeast and molds and the occurrence of their mycotoxins constitute a potential health hazard. Furthermore, yeasts produce different metabolites such as acids, alcohols and esters, which could affect odor and taste depending on the produced concentrations and threshold values (Ragaert et al. 2006). Therefore, yeast and molds growth has a direct impact not only on the product safety but also, on its sensory quality and, consequently on the consumers acceptability.
Initial population of yeast and molds on fresh strawberry juice was 5.40 log CFU/mL. PE1 and PE2 treatments were not significantly effective to reduce the initial population of yeast and molds in strawberry juice as well as for inhibit the growth through storage time since similar initial and final populations were found in untreated strawberry juice (Fig. 1b).
On the other hand, juice treated with G1 did not show an effect on day 0, but a significant reduction was observed through storage, maintaining yeast and molds counts on approximately 4 log by day 21, while untreated sample reached values of 10.56 log at day 14.
Juices treated with G2, V1 and V2 showed significant differences in the initial yeast and molds counts compared to control and also inhibit their growth through time, maintaining the populations below detection limits (< 2 log) until the end of storage.
Finally, psychrophilic bacteria growth is shown on Fig. 1c. Psychrophilic bacteria represent an important group of microorganisms, because at refrigerated temperatures recommended for storage, this microorganisms may become the predominant microbial population present in the product (Ponce et al. 2003).
Initial counts of psychrophilic bacteria on fresh strawberry juice were 5.23 log. PE1 and PE2 treatments were not able to reduce the initial load of psychrophilic microorganisms. Even though PE1 was also not effective to inhibit the growth of psychrophilic bacteria through storage, PE2 showed a significant reduction at day 14 with counts of 6.19 log, while psychrophilic bacteria on untreated juice was 8.70 log.
In this study, pomegranate extract showed effectiveness against mesophilic and psychrophilic bacteria when applied at the highest concentration (Fig. 1a and c). This antimicrobial effect can be attributed to ellagic acid, which is one of the most important bioactive compounds in pomegranate fruit extract (Table 2, Fig. 2c). Like other phenolic compounds, ellagic acid interacts with the phospholipid bilayer of the cell membrane, causing an increase of permeability and leakage of vital intracellular constituents or impairment of bacterial enzyme systems (Ponce et al. 2004). Also, phenolic compounds, especially punicalagins (Fig. 2c), have been proved to have antimicrobial activity (Burapadaja and Bunchoo 1995). On the other hand, pomegranate extract showed no effect on yeast and molds population. These results differ from those found by Tehranifar et al. (2011), where pomegranate extracts from seed, peel and leaf, proved to be effective in vitro to inhibit the growth of Penicillium italicum, Rhizopus stolonifer and Botrytis cinerea.
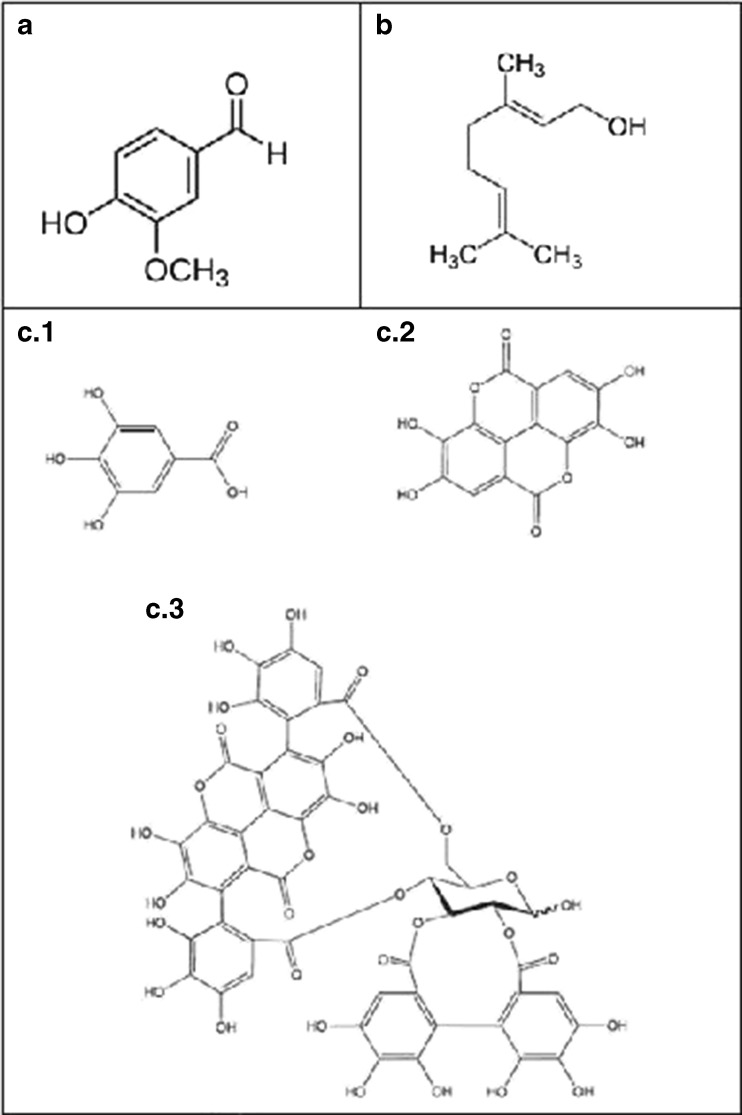
Fig. 2.
Chemical structures of (a) vanillin, (b) geraniol, and pomegranate extract principal components: (c.1) gallic acid, (c.2) ellagic acid and (c.3) punicalagins A and B
The incorporation of vanillin and geraniol was not only effective to reduce initial populations of mesophilic bacteria and yeast and molds population, and inhibit their growth through time, but also psychrophilic microorganisms naturally present in fresh strawberry juice.
In accordance with our findings, Vasantha Rupasinghe et al. (2006) demonstrated that the incorporation of vanillin (0.18 % w/v) into dipping treatments inhibited microbial growth on fresh cut apples during 19 days of storage at 4 °C compared to control. Also, vanillin applied at 10 and 20 mM has been shown to be effective against both yeasts and molds in fruit purees and laboratory growth media (Fitzgerald et al. 2003).
Vanillin (4-hydroxy-3-methoxybenzaldehyde, Fig. 2a), like many other low-molecular weight phenolic compounds, displays antioxidant and antimicrobial properties and hence has the potential for use as a food preservative (Naidu and Davidson 2000). A study conducted by Fitzgerald et al. (2004) suggests that the aldehyde moiety plays a key role in the antifungal activity of vanillin. Also, a number of studies using both prokaryotic and eukaryotic microorganisms have suggested that the inhibitory action of phenolic compounds is due to the presence of the hydroxyl group (Aziz et al. 1998; Dorman and Deans 2000; Ultee et al. 2002). The authors reasoned that the hydroxyl group either reacts with enzyme active sites through the formation of hydrogen bonds (Aziz et al. 1998) or acts as a transmembrane carrier for monovalent cations (Dorman and Deans 2000).
With regards to geraniol (3,7-dimethylocta-trans-2,6-dien-1-ol, Fig. 2b), scarce studies are found where its effectiveness as an antimicrobial agent is evaluated in vivo. Raybaudi-Massilia et al. (2008) has shown the antimicrobial capacity of geraniol against native microflora of fresh-cut melon, when incorporated at 0.5 % in edible alginate-based coating, extending the shelf-life of the product.
According to the Spanish regulation for hygienic processing, distribution and commerce of prepared meals, the maximum limit of allowed mesophilic total count at expiry allowed is 107 CFU/g ((BOE2001). Considering 107 CFU/mL as a maximum limit for all the populations, the treatments with vanillin and geraniol were able to extend strawberry juice microbiological shelf-life.
Shelf-life was mainly dependent on yeast and molds growth since they were the predominant flora in fresh strawberry juice in comparison with mesophilic and psychrophilic microorganisms. Fresh strawberry juice was highly perishable, with a microbiological shelf-life of approximately 3 days (Fig. 1).
Pomegranate extract at the lowest concentration tested was not able to extend the shelf-life of the product. However, at the highest concentration, pomegranate extract was able to reduce mesophilic and psychrophilic counts, reaching counts below the maximum limit of 107 CFU/mL by day 14 of refrigerated storage (Fig. 1). The ineffectiveness of PE2 to inhibit the yeast and molds population reduced the shelf-life of the product to 3 days.
An extension of the shelf-life of treated strawberry juice for more than 21 days was reached when vanillin and geraniol were used in all the concentrations tested (Fig. 1).Nevertheless, higher concentrations of geraniol were more effective than lower concentrations, whereas vanillin resulted equally effective at both concentrations.
Effect of natural antimicrobials on inoculated E. coli O157:H7 in strawberry juice
Table 4 shows the survival of E. coli O157:H7 in inoculated strawberry juice treated with natural antimicrobials. Immediately after applying the treatments (t = 0 days) significant reductions (p < 0.05) on E. coli O157:H7 counts were observed on samples treated with V2 (3.10 log), G2 (3.20 log) and G1 (3.00 log), compared to untreated juice (5.38 log). However, initial populations of E. coli O157:H7 in V1, PE1 and PE2 had no significant difference compared to control sample (Table 4).
Table 4.
Survival of E. coli O157:H7 in inoculated strawberry juice treated with natural antimicrobials
| Treatments | Survival fraction (log CFU/mL) | ||||
|---|---|---|---|---|---|
| Storage time (days) | |||||
| 0 | 3 | 7 | 14 | 21 | |
| Untreated | 5.38 ± 0.30Aa | 5.00 ± 0.10Aa | 4.48 ± 0.10Ba | 3.65 ± 0.30Ca | — |
| V1 | 4.89 ± 0.19Aa | 2.40 ± 0.05Bb | <2Cb | <2Cc | <2Ca |
| V2 | 3.10 ± 0.05Ab | <2Bc | <2Bb | <2Bc | <2Ba |
| G1 | 3.20 ± 0.10Ab | <2Bc | <2Bb | <2Bc | <2Ba |
| G2 | 3.00 ± 0.10Ab | <2Bc | <2Bb | <2Bc | <2Ba |
| PE1 | 5.28 ± 0.10Aa | 5.00 ± 0.13Aa | 4.49 ± 0.03Ba | 3.50 ± 0.20Ca | — |
| PE2 | 5.08 ± 0.10Aa | 5.00 ± 0.04Aa | 4.66 ± 0.24Ba | 2.80 ± 0.20Cb | — |
Data is shown as means ± standard errors. Values with different lower case letters in the same column indicate significant differences (p < 0.05) between treatments. Values with different capital letters in the same row indicate significant differences (p < 0.05) with respect to storage time. V1: 2.5 mg/mL of vanillin; V2: 5 mg/mL of vanillin; G1: 0.6 μL/mL of geraniol; G2: 1.2 μL/mL of geraniol; PE1: 180 μg/mL of pomegranate extract; PE2: 360 μg/mL of pomegranate extract
Table 4, shows that V2, G1 and G2 were the best to reduce E. coli population inoculated in strawberry juice during the first storage hours (more than 2 log reductions) in comparison with the rest of the treatments.
At day 3, V1 showed a reduction of 2.60 log, while V2, G1 and G2 counts were below detection limit (< 2 log) until the end of storage (day 21). From day 7, E. coli counts on sample V1 were also below detection limit. Therefore, high concentrations of natural antimicrobials resulted more effective to reduce E. coli than low concentrations.
In agreement with these results, Raybaudi-Massilia et al. (2006) found reductions on Salmonella Enteritidis, E. coli, and L. innocua inoculated in apple and pear juices, treated with a concentration of 2 μL/mL of geraniol. Also, similar reductions on L. monocytogenes and E. coli O157:H7 counts were found by Moon et al. (2006) adding 0.60 % w/v vanillin to apple juice, after 24 h of storage at 4 or 15 °C. In addition, these researchers indicated that the antimicrobial effect of vanillin was enhanced when lower pH and higher temperature were applied.
E. coli population on PE1 showed no significant differences from untreated strawberry juice throughout storage. However, at day 14, PE2 showed a significant reduction on E. coli counts (0.80 log compared to control). In accordance with these results, there are several works reporting that pomegranate and punicalagins significantly inhibited the growth of pathogenic Escherichia coli and Pseudomonas aeruginosa (Reddy et al. 2007). Significant reductions (p < 0.05) of E. coli population were also observed through the storage time for all treatment conditions including the control. Those reductions in E. coli observed in untreated strawberry juice along storage time could be due to effects such as storage temperature (5 °C) and competitive microflora (native flora), whereas the reductions observed through time in treated samples are due to combined effects of antimicrobials added and those other factors indicated (Raybaudi-Massilia et al. 2008).
The use of natural substances as preservatives was effective to reduce E. coli O157:H7 population in inoculated strawberry juice.
Effect of natural antimicrobials on physicochemical parameters of strawberry juice
The measurement of total acids (TA) in beverages and juices is required by all producers as a key quality control indicator. Another important quality parameter in fruit juices is total soluble solids (°Bx), which are primarily sugars: sucrose, fructose, and glucose.
The application of natural agents did not induce any significant changes in the titratable acidity and total soluble solids of strawberry juice compared to untreated samples, with mean values of 0.779 ± 0.019%TA and 8.2 ± 0.2 °Bx. These values were similar to those found by Tiwari et al. (2009) working with strawberry juice, where total soluble solids (°Bx) and titratable acidity as citric acid were 9.82 and 0.75 g/100 g citric acid, respectively.
Effect of natural antimicrobials on antioxidant properties of strawberry juice
Strawberries are a very rich source of antioxidant compounds including vitamins C, E, β-carotene, melatonin and phenolic compounds (Stürtz et al. 2011). Among the bioactives, phenolic compounds are one of the main groups of phytochemicals present in strawberries that strongly influence quality, contributing to sensory attributes and health properties (Buendia et al. 2010). Since antioxidant content is an important property of fruits and vegetables, it is of great interest to evaluate changes in the antioxidant status of strawberry juice during storage.
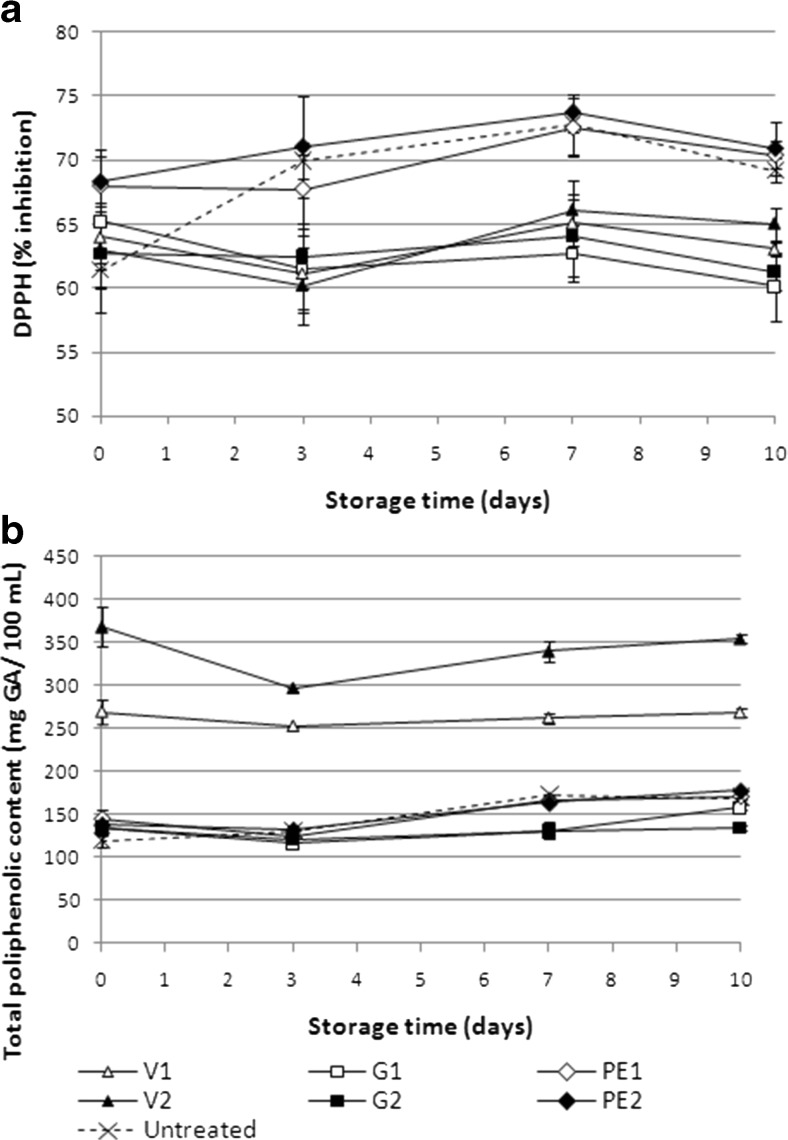
Results regarding the effect of natural antimicrobials on the antioxidant capacity and total phenolics of strawberry juice are shown on Fig. 3.
Fig. 3.
Effects of natural antimicrobials on antioxidant capacity of strawberry juice through refrigerated storage at 5 °C: (a) DPPH radical scavenging activity, (b) total phenolic content. Bars indicate standard errors. V1: 2.5 mg/mL of vanillin; V2: 5 mg/mL of vanillin; G1: 0.6 μL/mL of geraniol; G2: 1.2 μL/mL of geraniol; PE1: 180 μg/mL of pomegranate extract; PE2: 360 μg/mL of pomegranate extract
Antioxidant capacity, shown on Fig. 3a, is expressed as the inhibition percentage of the DPPH radical. The initial inhibition percentage in the fresh strawberry juice was 61.4 %. Vanillin and geraniol treatments had no initial effect on the antioxidant capacity in any concentration tested, with no significant differences compared to untreated juice. On the other hand, juice samples treated with pomegranate extract at both concentrations, showed a significant increment on the antioxidant capacity, with approximately 68 % of inhibition.
In accordance with these results, Bortolomeazzi et al. (2007) studied the antioxidant capacity of different phenols, and found that vanillin antioxidant capacity was significantly decreased at low pH. Villaño et al. (2007) evaluated the radical scavenging ability of different polyphenolic compounds towards DPPH free radical, concluding that the highest antioxidant capacity was found in those phenols with higher number of hydroxyl groups available. Gallic acid-like structures seem to be important for efficacy towards DPPH• radical (Villaño et al. 2007). This justifies the higher inhibition percentage of DPPH radical found in juices treated with pomegranate extract.
With regards to geraniol, in acid medium like strawberry juice, it reacts and transforms into α-terpineol (Baxter et al. 1978). In a recent study, Bicas et al. (2011), evaluated the antioxidant capacity of different bioflavors, and found that α-terpineol showed a very low radical scavenging capacity when compared to conventional antioxidants. The weak performance in the DPPH test is probably due to the low number of conjugated bonds capable of trapping free radicals in terpene molecules (Bicas et al. 2011).
The evolution of total phenolic compounds (TPC) through storage time is shown on Fig. 3b. Initial TPC on untreated strawberry juice was 119.5 mg of GAE/100 mL of juice, with no significant differences from juices treated with pomegranate extract and geraniol, along refrigerated storage. However, vanillin treatments showed a significant increment in TPC of strawberry juice at day 0, with significant differences between V1 and V2 (269.6 and 368.7 mg of GAE/100 mL of juice, respectively). This increment was expected given that vanillin is a phenolic compound. TPC in juices treated with vanillin was maintained throughout refrigerated storage (Fig. 3b).
Sensory evaluation of strawberry juice
A wide variety of natural compounds have proven to be powerful antimicrobials; however, their aromatic volatile constituents can be absorbed and greatly affect the sensory characteristics of the food product. As the aroma of fruit juices is a key marketing feature of these products, it is necessary to consider the sensory impact generated by the application of natural antimicrobials as biopreservatives in fruit juices (Ayala-Zavala et al. 2008).
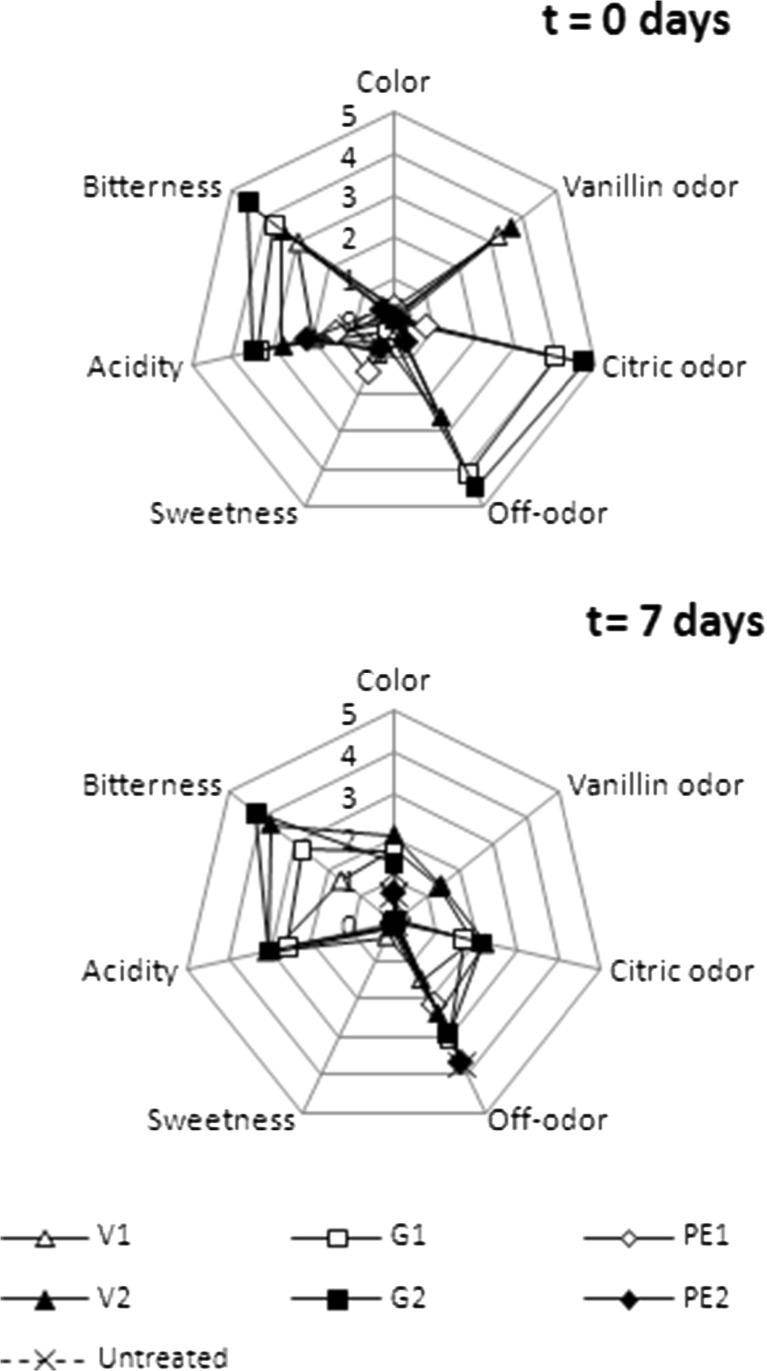
Therefore, a sensory evaluation was carried out and its results are shown on Fig. 4.
Fig. 4.
Influence of storage time on sensory characteristics of strawberry juice treated with natural antimicrobials. Bitterness, Sweetness and Acidity at 7 days in strawberry juices untreated and treated with PE1 and PE2 were not carried out due to their high microbial load. V1: 2.5 mg/mL of vanillin; V2: 5 mg/mL of vanillin; G1: 0.6 μL/mL of geraniol; G2: 1.2 μL/mL of geraniol; PE1: 180 μg/mL of pomegranate extract; PE2: 360 μg/mL of pomegranate extract
At day 0, no significant differences were found on strawberry juice color between treated and untreated samples, concluding that the incorporation of selected natural antimicrobials had no initial effect on the appearance of the product.
Strawberry juice treated with pomegranate extract at both concentrations tested, showed similar scores in every sensory attribute to fresh untreated juice, since no significant differences (p < 0.05) were found from each other.
Contrary to that result, odor attributes were significantly affected (p < 0.05) by incorporation of geraniol and vanillin, since significantly higher scores were observed in comparison with pomegranate extract or untreated samples. Panelists perceived vanillin odor in samples V1 and V2, with mean values of 3.2 and 3.6, respectively. However, this odor was not perceived as non-characteristic odor (off-odor), indicating the compatibility of vanillin with fruit juices. On the other hand, citric odor on samples G1 and G2 (4.0 and 4.7, respectively) was perceived as off-odor by the panelists, with values of 4.2 and 4.5, respectively.
At day 7, panelists found off-odor on untreated, PE1 and PE2 samples (3.7, 2.15 and 3.7, respectively). This may be a result of the fermentation, given the important microbial load on those samples by day 7 of storage. On the other hand, vanillin odor on V1 and V2, and citric odor on G1 and G2 decreased with respect to those values evaluated on day 0.
With regards to taste attributes, at day 0, no significant differences were found on sweetness between the treated samples and the control. On the contrary, acidity and bitterness were significantly different on those samples treated with vanillin and geraniol, were the incorporation of these antimicrobials negatively affected the flavor of the strawberry juice, with significantly higher scores compared to PE1, PE2, and untreated samples.
Storage time showed an important effect on the evaluated sensory characteristics (color, vanillin odor, citric odor, off-odor, sweetness, acidity and bitterness) since statistically significant differences (p < 0.05) were found between the values obtained at 0 and 7 days, indicating that changes throughout storage time appeared to be perceived by the panelists.
Conclusions
Vanillin and geraniol, at both concentrations evaluated, improved the shelf-life of strawberry juice from microbiological points of view in comparison with untreated juice. In addition, a reduction of inoculated E. coli O157:H7 population in strawberry juice using vanillin and geraniol was achieved, improving thus, the fruit juice safety. Total soluble solids and total acidity were not affected by the incorporation of the biopreservatives tested. Total polyphenol content was increased in those samples treated with vanillin, indicating higher nutritional value of the product.
On the other hand, pomegranate extract had no impact on the sensory characteristics evaluated. This result indicates that pomegranate extract may be a potential preservation alternative since maintains the sensory characteristics similar to fresh strawberry juice. Furthermore, pomegranate extract was effective to inhibit or decrease the microbial growth of mesophilic and psychrophilic bacteria through storage time when applied at the highest concentration evaluated, without affecting physicochemical parameters and improving the antioxidant capacity of the product. Since pomegranate extract had no effect on reducing yeast and molds population, or inoculated E. coli, higher concentrations could be evaluated or combination with vanillin and geraniol, in order to evaluate synergism between the biopreservatives, and extend the shelf-life of the product without affecting its sensory attributes.
Acknowledgments
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and Universidad Nacional de Mar. del Plata (UNMDP).
References
- Alvarez MV, Moreira MR, Ponce A. Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J Food Safety. 2012;32:379–387. doi: 10.1111/j.1745-4565.2012.00390.x. [DOI] [Google Scholar]
- Ayala-Zavala JF, Del Toro-Sanchez L, Alvarez-Parrilla E, Gonzalez-Aguilar GA. High relative humidity in-package of fresh-cut fruits and vegetables: advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J Food Sci. 2008;73:41–47. doi: 10.1111/j.1750-3841.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Aziz NH, Farag SE, Mousa LAA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93:43–54. [PubMed] [Google Scholar]
- Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W. Geraniol interferes with membrane functions in strains of candida and saccharomyces. Lipids. 1988;23:534–538. doi: 10.1007/BF02535593. [DOI] [PubMed] [Google Scholar]
- Barnard DR, Xue R. Laboratory evaluation of mosquito repellents against aedes albopictus, culex nigripalpus, and ochlerotatus triseriatus (diptera: culicidae) J Med Entomol. 2004;41:726–730. doi: 10.1603/0022-2585-41.4.726. [DOI] [PubMed] [Google Scholar]
- Baxter RL, Laurie WA, McHale D. Transformations of monoterpenoids in aqueous acids: the reactions of linalool, geraniol, nerol and their acetates in aqueous citric acid. Tetrahedron. 1978;34:2195–2199. doi: 10.1016/0040-4020(78)89026-7. [DOI] [Google Scholar]
- Bicas JL, Neri-Numa IA, Ruiz ALTG, De Carvalho JE, Pastore GM. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol. 2011;49:1610–1615. doi: 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Boletín Oficial del Estado (BOE)(2001) Normas de higiene para la elaboración, distribución y comercio de comidas preparadas. Orden del 29 de diciembre del 2000. Boe 11:1435–1441.
- Bortolomeazzi R, Sebastianutto N, Toniolo R, Pizzariello A. Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. 2007;100:1481–1489. doi: 10.1016/j.foodchem.2005.11.039. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Buendia B, Gil MI, Tudela JA, Gady AL, Medina JJ, Soria C, et al. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in strawberry cultivars. J Agr Food Chem. 2010;58:3916–3926. doi: 10.1021/jf9030597. [DOI] [PubMed] [Google Scholar]
- Burapadaja S, Bunchoo A. Antimicrobial activity of tannins from Terminalia citrine. Planta Med. 1995;61:365–366. doi: 10.1055/s-2006-958103. [DOI] [PubMed] [Google Scholar]
- Chen W, Viljoen AM. Geraniol — a review of a commercially important fragrance material. S Afr J Bot. 2010;76:643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Elez-Martínez P, Martín-Belloso O. Food safety aspects of pulsed electric fields. In: Sun DW, editor. Emerging technologies for food processing. Boston: Academic Press; 2005. pp. 183–218. [Google Scholar]
- Faleiro ML. The mode of antibacterial action of essential oils. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. 2011;2:1143–1156. [Google Scholar]
- Fitzgerald DJ, Stratford M, Narbad A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int J Food Microbiol. 2003;86:113–122. doi: 10.1016/S0168-1605(03)00059-X. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DJ, Stratford M, Gasson MJ, Ueckert J, Bos A, Narbad A. Mode of antimicrobial action of vanillin against Escherichia coli, lactobacillus plantarum and listeria innocua. J Appl Microbiol. 2004;97:104–113. doi: 10.1111/j.1365-2672.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. 2012;143:397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Kuehl R (2001) Diseño de experimentos, 2nd edn. Thompson Learning Intl
- Mittal GS, Griffiths MW. Pulsed electric field processing of liquid foods and beverages. In: Sun DW, editor. Emerging technologies for food processing. Boston: Academic Press; 2005. pp. 99–139. [Google Scholar]
- Moon K, Delaquisb P, Toivonenb P, Stanichm K. Effect of vanillin on the fate of Listeria monocytogenes and Escherichia coli O157:H7 in a model apple juice medium and in apple juice. Food Microbiol. 2006;23:169–174. doi: 10.1016/j.fm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mphahlele RR, Fawole OA, Stander MA, Opara UL. Preharvest and postharvest factors influencing bioactive compounds in pomegranate (Punica granatum L.) - a review. Sci Hort. 2014;178:114–123. doi: 10.1016/j.scienta.2014.08.010. [DOI] [Google Scholar]
- Naidu AS, Davidson PM. Phyto-phenols. In: Naidu AS, editor. Natural food antimicrobial systems. Boca Raton, London, New York, Washington DC: CRC Press LLC; 2000. pp. 265–294. [Google Scholar]
- Parish ME. Public health and non-pasteurized fruit juices. Crit Rev Microbiol. 1997;23:109–119. doi: 10.3109/10408419709115132. [DOI] [PubMed] [Google Scholar]
- Ponce A, Fritz R, Del Valle C, Roura S. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT- Food Sci Technol. 2003;36:679–684. doi: 10.1016/S0023-6438(03)00088-4. [DOI] [Google Scholar]
- Ponce A, Del Valle C, Roura S. Shelf life of leafy vegetables treated with natural essential oils. J Food Sci Technol. 2004;69:550–556. [Google Scholar]
- Ponce AG, Agüero MV, Roura SI, del Valle CE, Moreira MR. Dynamics of indigenous microbial populations of butter head lettuce grown in mulch and on bare soil. J Food Sci. 2008;73:257–263. doi: 10.1111/j.1750-3841.2008.00789.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- Ragaert P, Devlieghere F, Loos S, Dewulf L, Van Langenhove H, Debevere J. Metabolite production of yeasts on a strawberry-agar during storage at 7 °C in air and low oxygen atmosphere. Food Microbiol. 2006;23:154–161. doi: 10.1016/j.fm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O. Antimicrobial activity of essential oils on salmonella enteritidis, Escherichia coli, and listeria innocua in fruit juices. J Food Protect. 2006;69:1579–1586. doi: 10.4315/0362-028x-69.7.1579. [DOI] [PubMed] [Google Scholar]
- Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int J Food Microbiol. 2008;121:313–327. doi: 10.1016/j.ijfoodmicro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;73:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
- Sadler GD, Murphy PA. pH and titratable acidity. In: Nielsen SS, editor. Food analysis. New York: Springer; 2010. pp. 231–233. [Google Scholar]
- Saftner RA, Abbott JA, Bhagwat AA, Vinyard BT. Quality measurement of intact and fresh-cut slices of Fuji, granny smith, pink lady, and gold rush apples. J Food Sci. 2005;70:317–324. doi: 10.1111/j.1365-2621.2005.tb09985.x. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Stürtz M, Cerezo AB, Cantos-Villar E, Garcia-Parrilla MC. Determination of the melatonin content of different varieties of tomatoes (lycopersicon esculentum) and strawberries (fragaria ananassa) Food Chem. 2011;127:1329–1334. doi: 10.1016/j.foodchem.2011.01.093. [DOI] [PubMed] [Google Scholar]
- Tehranifar A, Selahvarzi Y, Kharrazi M, Bakhsh VJ. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind Crop Prod. 2011;34:1523–1527. doi: 10.1016/j.indcrop.2011.05.007. [DOI] [Google Scholar]
- Tiwari BK, O’Donnell CP, Patras A, Brunton N, Cullen PJ. Effect of ozone processing on anthocyanins and ascorbic acid degradation of strawberry juice. Food Chem. 2009;113:1119–1126. doi: 10.1016/j.foodchem.2008.08.085. [DOI] [Google Scholar]
- Tomadoni B, Cassani L, Moreira MR, Ponce A. Efficacy of vanillin and geraniol in reducing Escherichia coli O157:H7 on strawberry juice. LWT- Food Sci Technol. 2015;64:554–557. doi: 10.1016/j.lwt.2015.06.039. [DOI] [Google Scholar]
- Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Eniron Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha Rupasinghe HP, Boulter-Bitzer J, Ahn T, Odumeru JA. Vanillin inhibits pathogenic and spoilage microorganisms in vitro and aerobic microbial growth in fresh-cut apples. Food Res Int. 2006;39:575–580. doi: 10.1016/j.foodres.2005.11.005. [DOI] [Google Scholar]
- Villaño D, Fernández-Pachón MS, Moyá ML, Troncoso AM, García-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]