Abstract
Arrhythmia subcellular mechanisms are constantly being explored. Recent knowledge has shown that travelling Ca2+ waves in cardiac cells are critical for delayed afterdepolarisations and in some cases, early afterdepolarisations. In this review, we comment on the properties of cardiac Ca2+ waves and abnormal Ca2+ releases in terms of properties used to describe electrical waves; propagation, excitability and refractoriness.
Keywords: Delayed afterdepolarisation, early afterdepolarisation, Ca2+ waves, arrhythmias
Abnormalities in electrical rhythm were studied by Einthoven at the start of the 20th century. In the 1940s, studies by Bozler et al.1 described contractile signals that appeared to be ‘triggered’ heart beats. Today we use the term delayed afterdepolarisations (DADs) to refer to oscillations in voltage that follow a driven action potential.
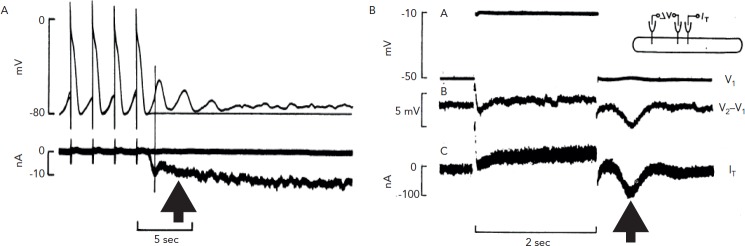
In the mid-1970s, progress was made when Lederer and Tsien developed a method to study the underlying electrical mechanism of DADs2 (see Figure 1). In a voltage clamped, multicellular canine Purkinje fibre, the transient depolarisation of the resting potential of the fibre was found to be due to a transient inward current (Iti) (see Figure 1A). Many initially challenged this idea but these authors went on to show that Iti was not an artifact and that the Iti they recorded in Purkinje fibres was Ca2+ dependent (see Figure 1B).2,3 This was a relatively new concept for cardiac electrophysiology; that is, the idea that Ca2+ inside the cell could feed back and affect the electrics of the cell’s membrane. In a recent review this was referred to as reverse mode excitation–contraction (EC) coupling.4
Figure 1: Evidence of Iti in Multicellular Canine Purkinje Fibres.
Panel A: The first manifestation of Ca2+ waves in cardiac cells, as observed by Lederer and Tsien 1976,2 was the appearance of Iti currents (arrows) in voltage-clamped multicellular Purkinje fibres. Note phase 4 activity in this Purkinje strand when clamp was off. Panel B: Multiple experiments including the one shown here illustrated the Cao dependence of Iti (Kass et al., 1978).3
Here we will discuss the Ca2+ wave and address the question: ‘Is it like the electrical wave with which we are all familiar?’
Functional Anatomy
A propagating electrical wave utilises the energy of the chemical gradients set up by the cardiac sarcolemma.5 Electrical waves rely on activation of a series of ion channels (eg. Na channel proteins) for forward propagation of the wave.
Propagation of a Ca2+ wave also depends on the energy stored in the myocyte. But in this case the energy comes from the presence of Ca2+ stored in the sarcoplasmic reticulum (SR). The SR is a specialised intracellular membrane structure that in a myocyte stores Ca2+ that has been pumped into it by a SR membrane pump, SERCA2. In the absence of Ca2+ influx through the plasma membrane or mischievous Ca2+ wandering the cytosol, the Ca2+ in SR stays in the SR. This is because the SR ligand-operated Ca2+ channel, the ryanodine receptor channel (RyR), which guards this SR Ca2+ store, has a low probability of opening.
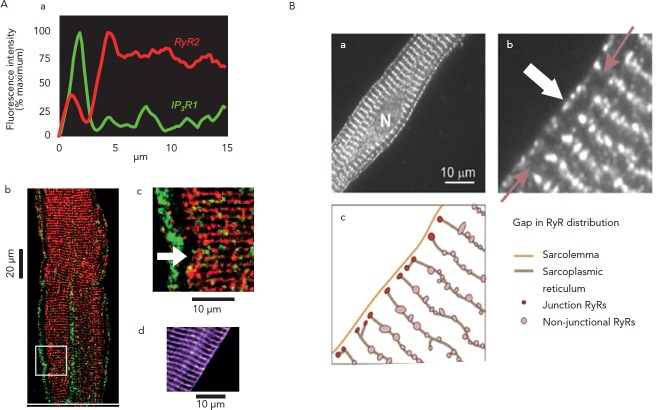
Interestingly, just as surface membrane ion channels (eg. Na channels) are positioned in a specific array6 to provide for smooth electrical wave propagation, RyR channel proteins in myocytes, Purkinje and atrial cells are clustered and aligned in a specific micro-anatomic pattern (see Figure 2).7,8,19 Presumably, and particularly in the tubulated structures of ventricular myocytes, this specific patterning is to allow for uniform Ca2+ release from SR during the action potential (forward mode EC coupling). The orderly pattern of RyRs on the SR sets up a series of potential release sites of Ca2+ in the cell.
Figure 2: Architecture of Ca2+ Release Channels in Purkinje (A) and Atrial (B) Cells.
The Ca2+ release channels (RyR) are organised into clusters (red dots in A.b and A.c); the clusters distribution follows a transverse striated pattern that matches the striation of the contractile filaments (not shown here). In Purkinje cells, junctional RyRs co-localise with IP3 receptor channels under the membrane. Note that both cell types show a gap in the RyR2 distribution. The gap is absent when isoform non-specific RyR antibody is used (A.d), indicating the presence of a different RyR isoform in the ‘gap’ of Purkinje cells. The same RyR2 organisation is found in atrial cells (B.a, B.b) wherein the same gap is interpreted here as a space filled by sarcoplamic reticulum with no channel and separating ‘junctional’ and ‘Non-junctional’ RyRs (B.c). In both cell types, this microanatomy shown schematically in B.c sets the stage for successful Ca2+ wave propagation. Adapted from Boyden et al.,7 Thul et al.8 and Stuyvers et al.19
Ca2+-induced Ca2+ Release
Fabiato’s work on the properties of the cardiac SR provided a potential explanation for spontaneous Ca2+ release in mechanically skinned cells in which the SR and RyR were intact and excessive Ca2+ loading of the SR caused spontaneous Ca2+ release.9,10,11 The mechanism for increased probability of opening of RyR when the SR is heavily loaded with Ca2+ is still uncertain, but suggests that the RyR channel is sensitive to both cytosolic and luminal [Ca2+] of the SR. Hence, the oscillatory character of a triggered arrhythmia in myocardium with a high cellular Ca2+ load may be due to further increase of Ca2+ entry into the cells during driven action potentials, which causes even more Ca2+ loading of the SR. So as soon as the release process has recovered after the electrically evoked Ca2+ release, the overloaded SR again releases a fraction of its Ca2+ into the cytosol. The requirement that the Ca2+ release mechanism must recover first (refractoriness) would explain the presence of a delay between aftercontractions and afterdepolarisations and the preceding beat.
Ca2+ waves occurring in cardiac cells depend on the regenerative production of a diffusible molecule that triggers Ca2+ release from adjacent SR stores. Cytosolic Ca2+ is one such ion and thus the process is called Ca2+ induced (intracellular) Ca2+ release (CICR) (see Figure 3B).12 This schematic shows Ca2+ wave propagation from one RyR cluster to another. Calsequestrin (CASQ), a Ca2+ binding protein, is found in the SR lumen and aids wave propagation inside cells (see Figure 3C). The released Ca2+ constitutes a leak from the SR and tends to reduce the overload. This phenomenon has been observed in different forms, all of which fall under the general definition of Ca2+ leak: increased probability of opening of RyR in lipid bilayer experiments,13 a biochemically detectable loss of Ca2+ from the SR;14 Ca2+ sparks in isolated cells and muscle;15,16 micro Ca2+ waves in isolated cells and muscle13,14 and Purkinje cells after infarction;7,19 and multicellular cellular Ca2+ waves.17–19 The threshold for Ca2+ leak is reduced in some arrhythmogenic mutations of the RyR,13 CASQ20 and in acquired dysfunction of the RyR such as in congestive heart failure and post MI.7,21–23
Figure 3: Simple Schematic Showing the Important Components of Ca2+ Wave Propagation.
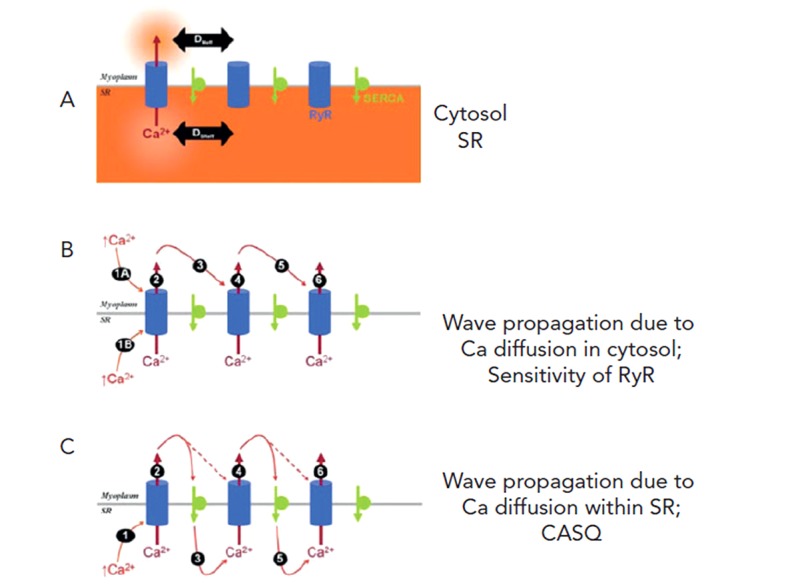
Panel A: Elements included are RyR Ca2+ release channel (blue), SERCA pump (green) both in the sarcoplasmic reticulum (SR) membrane that separates myoplasm and SR lumen (orange). Panel B: Ca2+ in myoplasm (step 1A) acts on first RyR cluster to open channel to release Ca2+ stored in SR to the myoplasm (step 2). This Ca2+ then diffuses away (step 3) and interacts with anatomically close RyR cluster (see Figures 2B and C) to cause further CICR (step 4). This process is regenerative and relies on sensitivity of RyR to Ca2+. Panel C: Ca2+ wave propagation as in Panel B but now wave propagation is also occurring in SR lumen. This process is thought to depend on calsequestrin (CASQ). Adapted from Swietach et al.12
Intracellular Ca2+ waves can be seen in normal canine atrial and Purkinje myocytes during forward mode EC coupling (see Figure 4).7 Here, a line of Ca2+ release is seen peripherally just after the plateau of the AP and this Ca2+ then via CICR, sets up a Ca2+ transient that moves to the core of the Purkinje cell (see Figure 4).
Figure 4: AP-evoked Global Ca2+ Transients in Purkinje Cells are Robust.
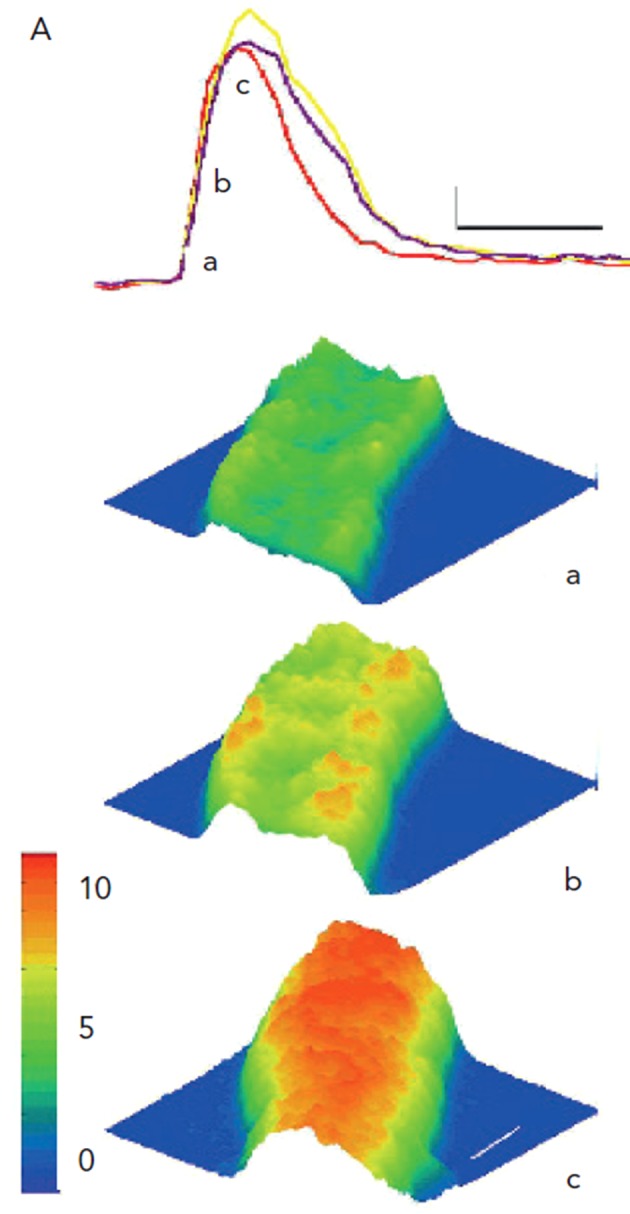
During the interval between stimuli, Purkinje cells are quiescent, except for occasional large Ca2+ waves (not shown here), which propagate along the cells of the aggregates. Panel A shows fluorescence intensity (Ca2+ changes) in Purkinje cells during electrically evoked depolarisation. Note the fluorescence during an electrically-evoked Ca2+ transient in three ROIs is indicated. Small letters correspond to 3D surface plots (below) of ratio images of a section of this aggregate during the transient. Ca2+ concentration is reflected by both the colour and height of the surface. The first response to a stimulus is an increase in Ca2+ (panel a), which is present mostly at the aggregate’s periphery (panel b). Peak Ca2+ change occurs later in core of aggregate (panel c). Thin calibration bars correspond to 1 F/F0 unit and 333 ms, respectively, while white lines on surface plot (panel c) correspond to 10 µm. From Boyden et al., 2003.7
Figure 5A shows that spontaneous Ca2+ waves occur often during diastolic intervals in Purkinje cells dispersed from the infarcted heart.7 In some cases the waves formed varied oscillatory changes in voltage as Ca2+ of wave is pumped out of cell via the sodium-calcium exchanger (Iti) (see Figure 5B). However, in the same cell the oscillatory voltage change is large enough to reach threshold, triggering a nondriven AP (see Figure 6). In these cells the small voltage signals and triggered activity are sensitive to an agent that blocks Ca2+ release of the RyR protein, ryanodine.
Figure 5: Ca2+ Waves Lead to Spontaneous Depolarisations.
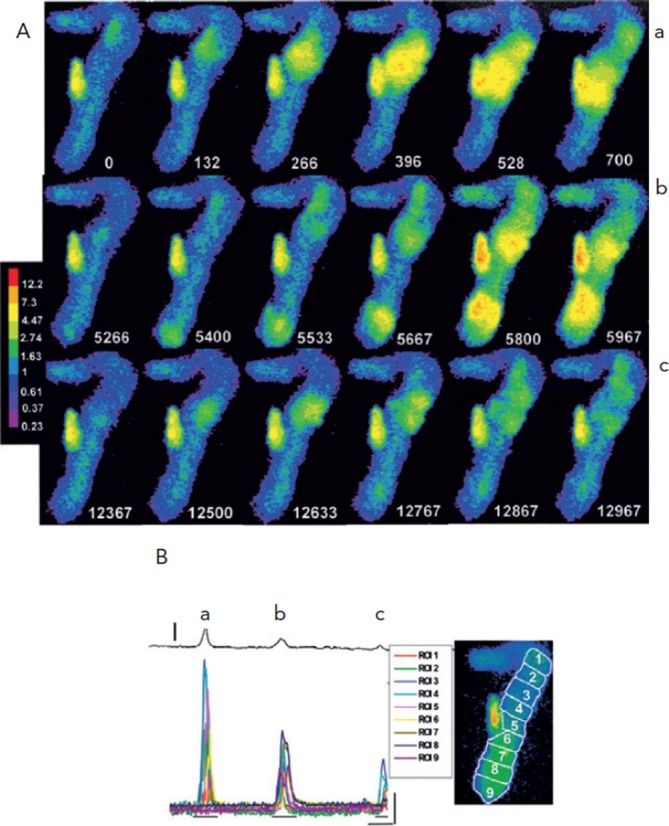
The magnitude of the depolarisation produced by the Ca2+ wave corresponds to the [Ca2+] and extent of the Ca2+ transients. Panel A: Selected F/F0 image frames from a Purkinje aggregate from the infarcted heart and concomitant membrane depolarisations (panel B). Relative time (t=0 first frame) are white numbers. In panel Aa sequence, t=0 to 700 ms corresponds to F/F0 during a large Ca2+ wave (see also a, panel B); In panel Ab, t=5266 to 5967 ms corresponds to F/F0 during two smaller waves which occur nearly simultaneously and propagate toward center of aggregate but stop before colliding (b, panel B); in panel Ac, t=12,367 to 12,967 ms corresponds to small micro Ca2+ transients which meander along upper section of aggregate (c, panel B). Colour bar indicates ratio values. Panel B: Transmembrane potential changes (thin black line above) and changes in F/F0 of ROIs in the spontaneously active Purkinje cell of panel A. Amplitude as well as spatial extent of the waves (see panel A) varied considerably, giving rise to a corresponding membrane depolarisation (denoted by a, b, c). Vertical line 15 mV. Image to right indicates positions of ROIs for this panel and panel 6B. Thin vertical and horizontal lines correspond to 1 F/F0 and 1,667 ms, respectively. Image frames of panel A derived from sections indicated by thin horizontal lines under Ca2+ tracings. From Boyden et al., 2003.7
Figure 6: Large Extensive Ca2+ Waves Lead to Sufficient Depolarisation to Elicit Nondriven APs.
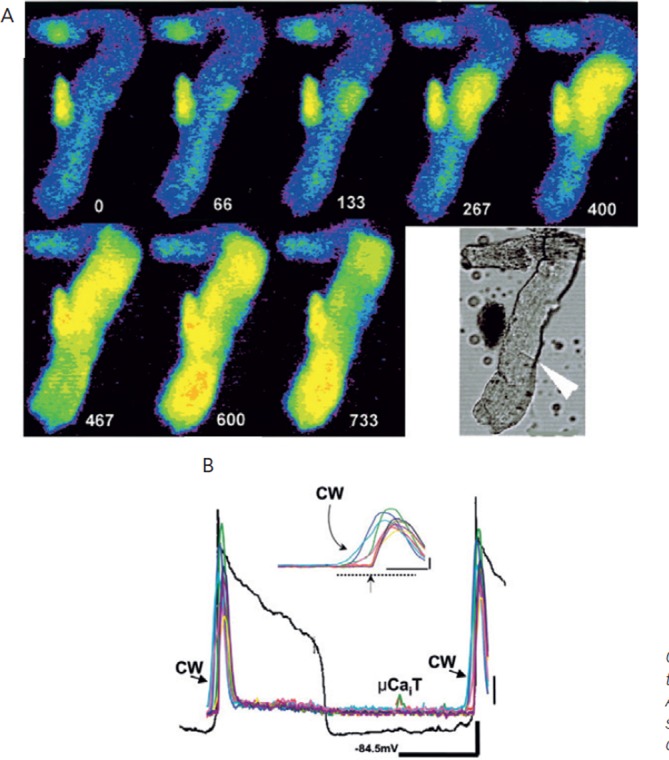
Panel A: Selected F/F0 image frames from the same infarct Purkinje shown in Figure 5 but during Ca2+ wave induced electrical activity. Time relative to t=0 of first frame is depicted by white numbers. Lower right image is bright field image of this aggregate early during experiment. The arrow denotes a probable cell border. Colour code as in Figure 5A. Panel B: Transmembrane potential changes (black line) and changes in F/F0 in several ROIs of spontaneously active Purkinje cell of panel A (MDP=-84.5 mV). Nondriven action potentials are triggered by the large cell wide Ca2+ waves (CW). Inset shows enlargement of the Ca2+ wave preceding synchronised Ca2+ release induced by the second action potential (arrow). Images presented in panel A are derived from those occurring during time of dotted line. Thick vertical and horizontal lines are one F/F0 unit and 3 s (1 F/F0, 417 ms for inset), respectively. Thin vertical black line is 12 mV. From Boyden et al., 2003.7
Propagation Between Cardiac Cells
Intercellular electrical transmission occurs via a set of ion channel proteins and specialised membrane structures called gap junctions.24 Each channel is formed by close apposition of two hemichannels each of which is in an opposing cell.25 Gap junctions can provide passage of many molecules (cAMP, Ca2+, IP3, ATP).26–28 In cardiac cells gap junctional conductance can be regulated acutely by pH, Ca2+, Camp and cGMP.29 Therefore Ca2+ ions can flow through gap junctions as well as inhibiting gap junctional conductance.
Since Ca2+ waves propagate along the cell, it is important to know whether they propagate between cells via gap junctions. Many have observed Ca2+ waves passing between two cardiac cells30 and have assumed a role for gap junctions. Ca2+ ions released upon RyR activation can travel as a wave across cells31 and propagate to adjoining cells via gap junctions.19 In cells transfected with both connexin 43 (Cx43) and RyR receptors the propagation of Ca2+ waves between cells was sensitive to octanol.32 Furthermore, in this experimental cell model, both Ca2+ wave propagation and gap junctional conductance between paired cardiac cells are related to the state of tyrosine phosphorylation of Cx43.33 How the Ca2+ wave crosses the gap junction is unknown but extracellular disulphide bonds of the Cx43 proteins between the adjoining cells appear critical for wave propagation.32
Arguably the occurrence of Ca2+ wave propagation from one cell to another is not a frequent event, but when one does happen, it appears to be due to a CICR mechanism (see Figure 7). In adult rat cells, Li et al.30 assert that Ca2+ wave propagation between cells mostly occurs at side-to-side junctions and the ultrastructure of the connections between the gap and SR release units is critical. Propagation failure occurs when distance between the disc membrane and neighbouring SR release unit is too large, such as occurs at end-to-end junctions.
Figure 7: Intercellular Ca2+ Wave Propagation.
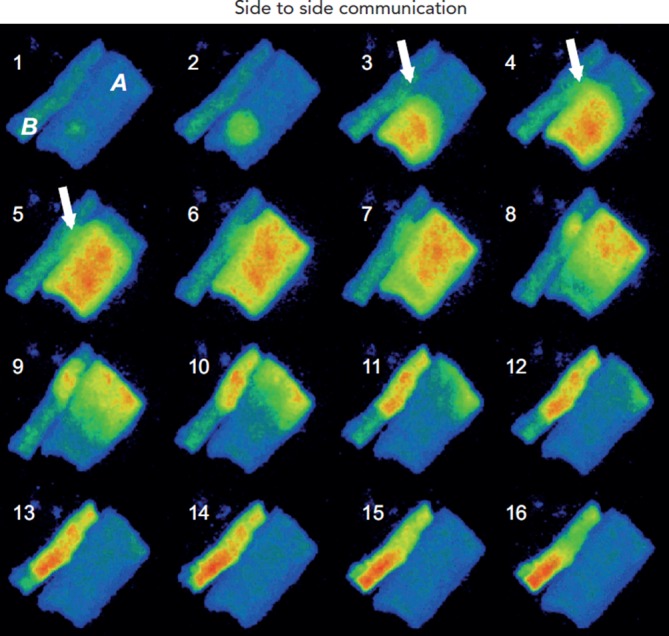
Ca2+ wave propagation in a pair of side-to-side canine Purkinje cells. Frames 1 and 2 show the initial Ca2+ release from the SR in cell A. The Ca2+ release then propagates by CICR in cell A. Arrows in frames 3–5 indicate the passage of the wave from cell A to cell B. By frame 8 sufficient Ca2+ has changed in cell B (yellow spot) to cause Ca2+ waves to propagate in both directions. The intracellular propagation velocity is ~60 µm/s-1.
At the tissue level, the subcellular Ca2+ dynamics combine with cell coupling and tissue architecture to generate multicellular Ca2+ wave dynamics. We understand focal electrical excitations due to triggered activity but are the dynamics of Ca waves similar? Spontaneous Ca2+ releases that triggered Ca2+ waves have been mapped in both normal and failing heart tissues34 (see Figure 8).35 Notably each spontaneous event occurred in a region of myocardium comprised of many cells (~3000 cells).34 In failing myocardium it is the rate of rise of the Ca2+ releases (waves) rather than their amplitude that is associated with triggered beats. In atria from CASQ-/- mice, the runs of APs during nondriven electrical activity were always preceded by rises in Ca2+, however total time of atrial activation increases with number of beats.36
Figure 8: Multicellular Ca2+ Wave Propagation.
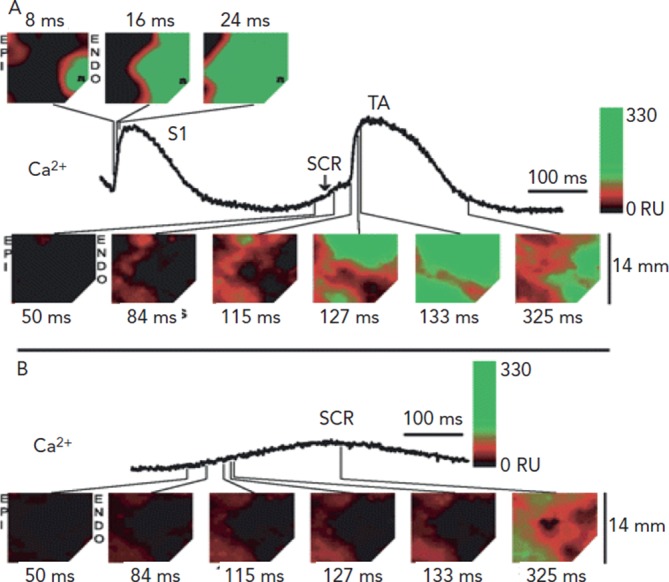
Shown are traces of two spontaneous Ca2+ release events (SCR) recorded from the same site in a single wedge preparation from a failing canine heart: one that resulted in a triggered beat (A) and one that did not (B). In A, above the last paced beat (S1) are three frames showing transmural (14 x 14 mm) calcium level (amplitude) at select time points, demonstrating the rapid, uniform pattern of calcium release during pacing (pacing symbol). Below the trace are several frames of calcium levels from select time points during the SCR and subsequent triggered beat (TA). All times shown are relative to the earliest site of calcium release, during pacing (top) or the SCR (bottom). The Epi and Endo are shown on the left and right sides of each contour, respectively. For the colour scale, black corresponds to diastolic calcium, red corresponds to subthreshold SCR, and the transition from red to green corresponds to the threshold for TA in A. Calcium release is much slower during the SCR compared with pacing. In B, the exact same format is shown, except that frames of calcium levels during pacing are not shown. The colour scale created for A was also used in B. In A and B, SCRs occur in a relatively large aggregate of myocardial cells and achieved a similar amplitude; however, the rate of SCR rise is much greater in A when TA occurred compared with B when TA did not. From Hoeker et al., 2009.35
Refractoriness
Electrical refractoriness is clearly related to time course of action potential repolarisation as well as the status of the sodium channel. In forward mode EC coupling, after the cellular action potential evoked Ca2+ transient due SR Ca2+ release, time is needed before a second Ca2+ release occurs of similar amplitude. Thus there is also a recovery process of Ca2+ release in cardiac cells that is independent of membrane voltage. In the electrically stimulated cell, the recovery of the SR Ca2+ release process or refractoriness is determined by recovery of the L-type calcium channel influx as well as the time course of the SR refilling. The latter can be examined by assessing the interval between spontaneous Ca2+ sparks occurring at the same release site (see Figure 9). Ca2+ spark termination is due to local depletion of Ca2+ within junctional SR. The time between one spark and another is related to the ryanodine sensitivity or threshold for Ca2+ release. How fast junctional SR refills after depletion is important for the recovery of both spark amplitude and CICR or Ca2+ wave formation.37 Shortened refractoriness of this process has been seen in both acquired (post MI)38 and genetic disease.39 For example, loss of calsequestrin (CASQ) in SR of cells in some CVPT patients produces fast SR refilling and greater likelihood of a trigger for re-release40 and Ca2+ waves.
Figure 9: Refractoriness of the Ca2+ Release Events.
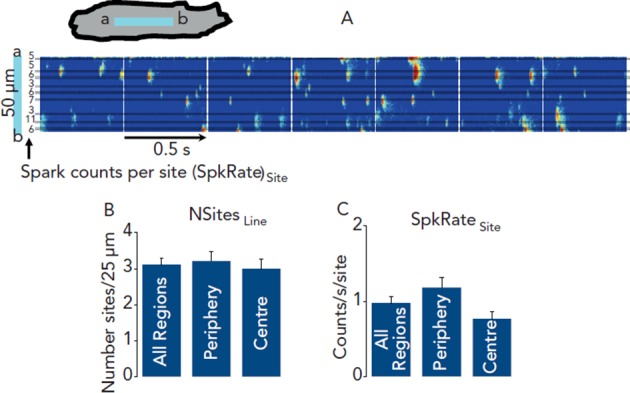
Panel A shows examples of line scan tracings (a–b) used to assess the refractoriness of Ca2+ releases in a normal Purkinje cell. Each fluorescent event represents a Ca2+ transient and corresponds to a Ca2+ release from a cluster of RyRs. Counting events along the scan indicates the regional density of active Ca2+ release sites (NSitesLine, Panel B) while horizontal event count reflects the frequency of Ca2+ release per site (SpkRateSite, Panel C).
Conclusion
While cellular electrical events and Ca2+ waves can occur independently of each other, it is when they interact and feed back on each other that complicated arrhythmogenic behaviour can occur (eg. alternans).4 Each physiological process has its own mechanisms of initiation, propagation and refractoriness and thus would be expected to have its own possible targets for effective therapeutic agents. For example, CamKII,41 sodium-calcium exchanger protein42 and EHD343 proteins have all emerged as possible targets for Ca2+-dependent arrhythmias.
References
- 1.Bozler E. The initiation of impulses in cardiac muscle. Amer J Physiol. 1943;138:273–82. [Google Scholar]
- 2.Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steriods in Purkinje fibers. J Physiol. 1976;263:73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass RS, Lederer WJ, Tsien RW, Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol (Lond) 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ter Keurs HEDJ, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 6.Dun W, Lowe JS, Wright PA Ankgrin-G participates in INa remodeling in myocytes from the broder zone of infarcted canine hearts. PloS ONE. 2013. p. e78087. [DOI] [PMC free article] [PubMed]
- 7.Boyden PA, Barbhaiya C, Lee T, Ter Keurs HEDJ. Nonuniform Ca2+ transients in arrhythmogenic purkinje cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–93. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thul R, Coombes S, Roderick HL, Bootman MD. Subcellular calcium dynamics in a whole-cell model of an atrial myocyte. Proc Natl Acad Sci. 2012;109:2150–5. doi: 10.1073/pnas.1115855109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiato A. Time and calcium dependence of activation and inactivation of calcium induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac Purkinje cell. J Gen Physiol. 1985;85:247–90. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabiato A. Simulated calcium current can both cause loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabiato A. Spontaneous versus triggered contractions of calcium tolerant cardiac cells from the adult rat ventricle. Basic Res Cardiol. 1985;80(Suppl 2):83–8. [PubMed] [Google Scholar]
- 12.Swietach P, Spitzer KW, Vaughan-Jones RD. Modeling calcium waves in cardiac myocytes: importance of calcium diffusion. Front Biosci. 2010;15:661–80. doi: 10.2741/3639. (Landmark Ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang D, Xiao B, Yang D et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci. 2004;101:13062–7. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–64. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wier WG, ter Keurs HE, Marban E et al. Ca2+ ‘sparks’ and waves in intact ventricular muscle resolved by confocal imaging. Circ Res. 1997;81:462–9. doi: 10.1161/01.res.81.4.462. [DOI] [PubMed] [Google Scholar]
- 16.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–4. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 17.Miura M, Boyden PA, terKeurs HEDJ. Ca2+ waves during triggered propagated contractions in intact trabeculae: determinants of the velocity of propagation. Circ Res. 1999;84:1459–68. doi: 10.1161/01.res.84.12.1459. [DOI] [PubMed] [Google Scholar]
- 18.Lamont C, Luther PW, Wier WG. Intercellular Ca2+ waves in rat heart muscle. J Physiol. 1998;512:669–76. doi: 10.1111/j.1469-7793.1998.669bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuyvers BD, Dun W, Matkovich SJ et al. Ca2+ sparks and Ca2+ waves in Purkinje cells: a triple layered system of activation. Circ Res. 2005;97:35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.di Barletta MR, Viatchenko-Karpinski S, Nori A et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circ. 2006;114:1012–9. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 21.Wehrens XHT, Lehnart SE, Reiken S et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. PNAS. 2005;102:9607–12. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yano M, Ono K, Ohkusa T et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circ. 2000;102:2131–6. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 23.Bers DM, Eisner DA, Valdivia HH. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ Res. 2003;93:487–90. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- 24.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 25.Yeager M, editor; Spooner PM, Joyner RW, Jalife J, editors. Structure of cardiac gap junction membrane channels. Discontinuous Conduction in the Heart. 1997. pp. 161–84. First ed. Armonk: Futura.
- 26.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol triphosphate. Science. 1992;258:292–5. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 27.Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositiol 1,4,5-trisphosphate and to calcium ions. Proc Natl Acad Sci. 1989;86:2708–12. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandberg K, Ji H, Iida Y, Catt KJ. Intercellular communication between follicular angiotensin receptors and Xenopus laevis oocytes: mediation by an inositol 1,4,5-trisphosphate- dependent mechanism. J Cell Biol. 1992;117:157–67. doi: 10.1083/jcb.117.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen MR, Boyden PA. Spooner PM, Joyner RW, Jalife J, editors. Is there a pharmacology of discontinuous conduction? Discontinuous Conduction in the Heart. 1997. pp. 471–82. First ed. Armonk: Futura.
- 30.Li Y, Eisner DA, O’Neill SC. Do calcium waves propagate between cells and synchronize alternating calcium release in rat ventricular myocytes? . J Physiol. 2012;590:6353–61. doi: 10.1113/jphysiol.2012.245241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Miura M, Ter Keurs HED J. Triggered propagated contractions in rat cardiac trabeculae; inhibition by octanol and heptanol. Circ Res. 1996;79:1077–85. doi: 10.1161/01.res.79.6.1077. [DOI] [PubMed] [Google Scholar]
- 32.Toyofukyu T, Yabuki M, Otsu K et al. Intercellular calcium signaling via gap junction in connexin43 transfected cells. J Biol Chem. 1998;273:1519–28. doi: 10.1074/jbc.273.3.1519. [DOI] [PubMed] [Google Scholar]
- 33.Toyofuku T, Yabuki M, Otsu K et al. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ Res. 1999;85:672–81. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 34.Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circ Res. 2005;96:535–42. doi: 10.1161/01.RES.0000159387.00749.3c. [DOI] [PubMed] [Google Scholar]
- 35.Hoeker GS, Katra RP, Wilson LD et al. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297:H1235–42. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou Q, Belevych AE, Liu B et al. Alternating membrane potential/calcium interplay underlies repetitive focal activity in a genetic model of calcium dependent atrial arrhythmias. J Physiol. 2015;593:1443–58. doi: 10.1113/jphysiol.2014.280784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramay HR, Liu OZ, Sobie EA. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc Res. 2011;91:598–605. doi: 10.1093/cvr/cvr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belevych AE, Terentyev D, Terentyeva R et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–77. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunello L, Slabaugh JL, Ho HT et al. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc Natl Acad Sci. 2013;110:10312–7. doi: 10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu N, Denegri M, Dun W et al. Abnormal propagation of calcium waves and ultrastructural remodeling in recessive catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;113:142–52. doi: 10.1161/CIRCRESAHA.113.301783. [DOI] [PubMed] [Google Scholar]
- 41.Pellicena P, Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Front Pharmacol. 2014;5:21. doi: 10.3389/fphar.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy N, Kormos A, Kohajda Z et al. Selective Na+/Ca2+ exchanger inhibition prevents Ca2+ overload-induced triggered arrhythmias. Br J Pharmacol. 2014;171:5665–81. doi: 10.1111/bph.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curran J, Makara MA, Little SC et al. EHD3-dependent endosome pathway regulates cardiac membrane excitability and physiology. Circ Res. 2014;115:68–78. doi: 10.1161/CIRCRESAHA.115.304149. [DOI] [PMC free article] [PubMed] [Google Scholar]