Abstract
Therapy for atrial fibrillation (AF) remains suboptimal, in large part because its mechanisms are unclear. While pulmonary vein ectopy may trigger AF, it remains uncertain how AF, once triggered, is actually sustained. Recent discoveries show that human AF is maintained by a small number of rotors or focal sources. AF sources are widely distributed in patient-specific locations, often remote from pulmonary veins and in the right atrium and stable for prolonged periods of time. In a multicentre experience, brief targeted ablation at sources (focal impulse and rotor modulation [FIRM]) terminated AF predominantly to sinus rhythm prior to pulmonary vein isolation and eliminated AF on rigorous followup. This review summarises the evidence for stable rotors and focal sources of human AF and their clinical role as ablation targets to eliminate paroxysmal, persistent and long-standing persistent AF.
Keywords: Atrial fibrillation, human, ablation, therapy, rotor, focal source, spiral wave, multiwavelet re-entry
Atrial fibrillation (AF) is the most prevalent arrhythmia in the world and a leading cause of hospitalisation and death.1 Current therapy for AF remains suboptimal, in large part because its mechanisms are uncertain. However, recent advances in our understanding of human AF, from meticulous mapping in patients and insights from animal models, are providing new therapeutic options for patients.
Seminal observations by Haïssaguerre in 19982 revealed that localised ectopic beats from the pulmonary veins (PVs) may trigger AF. These revelations launched the field of potentially curative AF ablation, with PV isolation as its cornerstone.3 Nevertheless, the mechanisms that sustain paroxysmal or persistent AF, once triggered, remained undefined.4,5 The multiwavelet hypothesis proposed that meandering electrical waves cause AF,6,7 but did not explain consistent observed activation patterns in AF,8,9 why AF may terminate after localised ablation,3,10 or why extensive ablation that should constrain wavelets often has little acute impact.3,11 Alternatively, the localised source hypothesis12 is supported by elegant experiments in which localised spiral waves (rotors)5,13 or focal sources9 disorganise into AF. Until recently, however, there had been little14,15 or no6 evidence to support localised sources in human AF. The ensuing imprecision in targeting AF sustaining mechanisms and relying on trigger modification has limited the promise of catheter ablation in many populations.3
Recent studies from multiple institutions show that human AF is sustained by a small number of rotors or focal sources16,17 for each individual. AF sources are remarkably stable over time, enabling targeted source ablation (focal impulse and rotor modulation [FIRM]) to acutely terminate AF within minutes (‘hyperacute’ termination) and subsequently eliminate AF on long-term followup.16
This review summarises the evidence for stable electrical rotors and focal sources for human AF and their role as targets for ablation and long-term maintenance of sinus rhythm in patients with all presentations of AF.16
Prior Mapping Studies of Human Atrial Fibrillation
Much of the debate on whether human AF reflects multiwavelet re-entry, localised sources or mixed mechanisms results from mapping that has not always met classical criteria for diagnosing any arrhythmia: to broadly map chambers of interest, then use interventions to prove that proposed mechanisms are causal and not bystanders. Failure to apply these criteria even to simple supraventricular18,19 and ventricular20 arrhythmias, for instance, is well recognised to lead to incorrect diagnosis and potentially undesirable therapy.
Clinical studies for over a decade suggest that human AF has preferred sustaining regions, evidenced by repeated activation patterns21 and rate gradients within and between atria8,22,23 and electrocardiogram (ECG) spectra suggesting conserved global spatiotemporal organisation for at least days.24 Moreover, ablation may successfully terminate AF at focal triggers and drivers2 or other localised regions.25,26 Although such sites are difficult to identify a priori, and widely distributed in both atria,27 they may be ablated by a systematic stepwise approach.10,28 Conversely, the results from high resolution (experimental) mapping in patients has been mixed. In elegant studies spanning2 decades,29 Allessie and colleagues described disordered activity in clinical AF subjects interpreted as multiwavelet re-entry without consistency. Their approach, however, mapped <20 % of the atria29 and could not exclude missed consistency or repeatability in unmapped tissue. Detailed mapping by Cox, Schuessler and colleagues30,31 found consistent circuits in AF that they interrupted by careful lesions in the Maze procedure,32 underscoring current extensive approaches to percutaneous ablation.3 More recent recordings of large epicardial atrial regions confirms localised but patient-specific regions of rapid AF activity consistent with sources.9,33 Global mapping of the atria has also been performed using elegant inverse solution mathematics applied to data acquired from a remote electrode vest on the body surface (EcVueTM, Cardioinsight, Cleveland, Ohio),14 or a noncontact intracardiac array in the atria (Ensite 3000TM, St Jude Medical, Minnesota).15 Both inverse solution methods agree show similar patterns in human AF, that regions consistent with sources can occasionally be revealed from ‘disordered’ AF,14,15 but both require prospective targeted ablation to validate whether they are causal or bystander patterns.
Mapping Rotors and Focal Sources in Human Atrial Fibrillation
We have recently described mapping of human AF using a wide field-of-view contact approach in the form of panoramic electrophysiological mapping (PEM)34 (RhythmViewTM, Topera Medical, San Diego, California) at clinical electrophysiology study and ablation. Multipolar basket catheters with 64-poles (4–6 mm nominal separation) are inserted into both atria, thus providing 128-pole bi-atrial contact recordings (see Figure 1) at 1 ms temporal resolution for periods of hours.
Figure 1: Method and Nomenclature for Panoramic Electrophysiological Mapping.
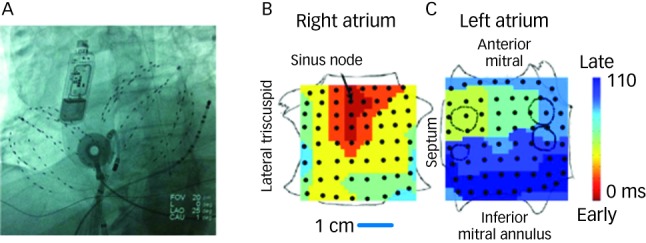
A: Fluoroscopy of multipolar basket catheters in the right atrium and in the left atrium across a trans-septal puncture, in the 25o LAO projection. An ablation catheter is shown in its right atrial sheath just about to be deployed and an oesophageal temperature probe is also seen. A subcutaneous electrocardiogram (ECG) monitor (Reveal XT, Medtronic, MN), used in the CONFIRM trial to ensure longterm freedom from AF, is also shown. B, C: Sinus rhythm map on biatrial schematic. Activation at basket electrodes, shown as dots, is displayed as a colour-coded map from the sinus node to the lateral inferior LA. The RA is opened between its poles with tricuspid annulus opened laterally and medially; the LA is opened along its equator, with mitral annulus opened superiorly and inferiorly. The pulmonary vein ostia are indicated by dashed lines.
Contact electrograms of AF are analysed using algorithms that analyse activation using the rate-dynamics of human left and right atrial (RA) action potential duration35–38 to limit the shortest cycle length at any rate and rate-dependent conduction velocity37,39 to explain propagation paths. Animated movies of voltage (isopotentials) are created34 and used to identify causal mechanisms for AF in each individual patient that are proven by direct targeted ablation.
Practically, panoramic electrophysiological mapping of AF rotors and focal sources is performed during electrophysiology study under heparin anticoagulation (target ACT >350 seconds). A commercially available basket (for instance, Constellation, Boston Scientific, MA) is advanced into the right atrium. Multiple epochs of AF are then recorded digitally over 10–20 minutes from the multipolar contact catheter and analysed using RhythmViewTM (Topera, Inc, Lexington, MA). Maps of AF propagation are generated within 8–10 minutes and used to directly target rotors or focal sources for ablation (FIRM) using a conventional ablation catheter. Once any or all RA sources have been ablated, if AF is still present (or if AF is re-inducible if AF terminated by FIRM), a trans-septal cannulation is performed and the process is repeated in the left atrium. All reported studies performed FIRM ablation prior to PV isolation.16,17
Results from Panoramic Electrophysiological Mapping
Illustrative maps from panoramic electrophysiological mapping (PEM) are shown in Figure 1 in sinus rhythm, for orientation. The atria are projected onto grids, with the right atrium opened vertically through the tricuspid valve and its lateral and medial halves opened. The left atrium is opened horizontally through the mitral valve and its superior and inferior halves folded upwards and downwards. Sinus activation is represented by colour-coded isochrones emanating from the sino-atrial node (red), crossing Bachmann’s bundle to the left atrium (blue).
Definitions of Localised Atrial Fibrillation Sources in Humans
In AF, isopotential (voltage) movies were analysed to identify rotors, as rotational activity around a centre, or focal impulses, identified as centrifugal activation from a point of origin, laminar activation patterns or disorganised activity that could not be classified into the above three categories. Rotors and focal sources are diagnosed if consistent for thousands of cycles detected in time lapse fashion in multiple epochs spanning 10–20 minutes. This enables practical mapping, while excluding transient or migrating rotational and/or focal activation seen in noninvasive/noncontact studies14,15 that are challenging ablation targets and may simply represent passive activation. We recently analysed AF rotors and focal sources continuously using PEM, and found that sources were stable in the same region with precession <1 cm, causing constantly varying surrounding non-repeatable AF activity, for at least 115 ± 57 minutes prior to ablation.40 Further studies are required to rationalise differences between rotors detected by PEM used to guide clinical ablation 16 from other methods.14,15
High Prevalence of Localised Sources in Human Atrial Fibrillation
We observed localised stable sources in nearly all patients (98 % or 98/101) with paroxysmal, persistent and long-standing persistent AF.16 Subjects exhibited 2.1 ± 1.0 sources concurrently, more in those with persistent than paroxysmal AF (2.2 ± 1.0 versus 1.7 ± 0.9; p=0.03). AF sources were stable over thousands of cycles analysed in ‘time lapse’ fashion16 and in a subset of subjects who failed conventional ablation then re-presented for FIRM-guided ablation, over months40. Sources lay in both atria with, surprisingly, 24 % in the right atrium. The number of rotors was greater than the number of focal beats.34 There were no complications during mapping.16
Figure 2 illustrates a left atrial (LA) rotor during persistent AF in a 63-year-old man, showing head-to-tail (red-to-blue) activation in its organised domain with peripheral disorganisation and/or collision. Tissue in the organised domain of the rotor activates sequentially (arrowed), but over slightly varying electrodes from cycle to cycle, reflecting the scientifically predicted41 precession of human AF sources within limited areas.16
Figure 2: Left Atrial Spiral Wave (Rotor) during Human Atrial Fibrillation on Panoramic Electrophysiological Mapping.
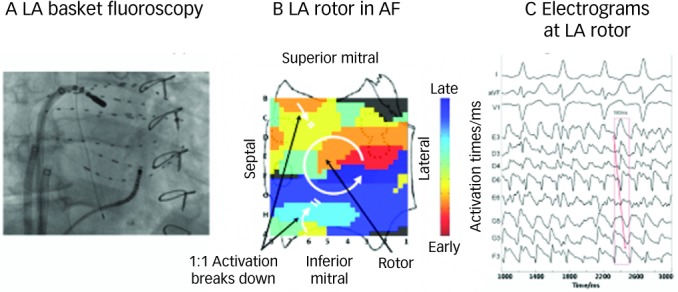
A: Fluoroscopy showing ablation catheter at centre of rotation of rotor relative to left atrial basket catheter. Coronary sinus catheter is also shown. B: Counterclockwise rotor in mid-posterior left atrium, with collision (white double lines) beyond organised domain of spiral arms. This is a single cycle snapshot of thousands of successive rotors with slight spatial precession. C: Electrograms from sites within the organised domain of the left atrial (LA) rotor site indicate sequential activation of counterclockwise rotation with variability representing AF. Pulmonary vein ostia indicated by dashed lines. ABL = ablation electrogram; CS = coronary sinus. Atrial orientations as in Figure 1B, C. Source: Adapted from Narayan et al., 2012.45
Recent studies have compared human AF rotors and focal sources to previously studied clinical targets. Detailed electrogram analyses has revealed a poor relationship42 between sustained AF rotors or focal sources and complex fractionated electrograms or low electrogram amplitude.25 There is great interest on whether AF sources are spatially or functionally related to atrial innervation or ganglionated plexi43,44 and is currently under investigation.
Targeted Ablation Only at Rotors and Focal Sources Acutely Terminates Atrial Fibrillation
Applying PEM during electrophysiology study has enabled prospective ablation at rotors and focal impulses (FIRM) prior to any other intervention (e.g. pulmonary vein isolation), to prove that rotors and focal beats are sources for human AF.
FIRM-guided ablation16,17,45 may use various energy sources, including irrigated and non-irrigated tip radiofrequency catheters and cryoablation. In each case, the catheter is manoeuvred to basket electrode(s) subtending each PEM-identified source precession area using fluoroscopy (see Figure 1A). The acute endpoint is ‘hyperacute’ AF termination (occurring within minutes of ablation at a predicted location, predominantly to sinus rhythm and accompanied by non-re-inducibility of AF using burst pacing and isoproterenol), or ≥10 minutes’ ablation, whichever comes first. If AF does not terminate or is re-inducible after termination, we assess for AF cycle length prolongation >10 %, that indicates elimination of a secondary AF source in our studies and in prior computer simulations (that used a more lenient 3–4 % cutpoint).46,47 We then used a composite acute endpoint of ‘hyperacute’ AF termination (with non-re-inducibility) or ≥10 % slowing.
These data were summarised in the Conventional ablation with or without focal impulse and rotor modulation (CONFIRM) trial16 in patients aged 63 ± 9 years, of whom 81 % had persistent AF, and a more recent first-case multicentre experience,17 in patients aged 58 ± 12 years, of whom 92 % had persistent AF. A videotaped case of FIRM-guided ablation is also available as an online video report45.
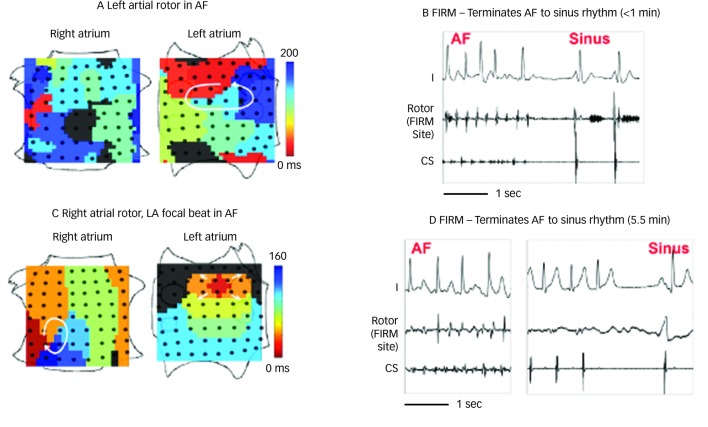
FIRM ablation achieved the acute endpoint in 86 % (31/36) of patients in the FIRM-guided limb of the CONFIRM trial16 and 100 % (12/12) of mapped patients in the first-case multicentre series.17 A 101 patient multicentre FIRM-guided experience shows similar results (manuscript in preparation). Figure 3 shows AF termination by FIRM ablation at ‘A’ a posterior LA rotor, ‘B’ a RA rotor and ‘C’ a LA focal beat. AF termination alone was observed in 5616 and 67 %17 of patients in each study, respectively, prior to PV isolation with a median of <5 minutes’ FIRM ablation at the primary source (hence the term ‘hyperacute’) and non-re-inducible AF thereafter. Notably, AF termination by FIRM was predominantly to sinus rhythm (23/28 terminations in both pooled studies, 82 %16,17), unlike conventional (non-guided) ablation in which AF terminates typically to an atrial tachycardia (<13 % to sinus rhythm47), and re-inducibility is not typically tested.
Figure 3: Acute Termination of Atrial Fibrillation to Sinus Rhythm By Focal Impulse and Rotor Modulation Ablation.
Each image is a single cycle snapshot of thousands of successive mapped rotors with slight spatial precession. A: Left atrial (LA) rotor with counterclockwise activation (red to blue) and disorganised right atrium in atrial fibrillation (AF). B: Focal impulse and rotor modulation (FIRM) ablation at LA rotor terminated AF to sinus rhythm in <1 minute. C: Right atrial (RA) rotor (clockwise) and simultaneous LA focal source during persistent AF. D: FIRM ablation at RA rotor terminated AF to sinus rhythm in 5.5 minutes. Both patients are AF-free on implanted cardiac monitors at >1 year. CS = coronary sinus electrogram. Atrial orientations as in Figure 1B, C. Source: Adapted from Narayan et al., 2012, with permission.16
We hypothesised that such AF termination (with subsequent non-re-inducibility) by brief targeted FIRM ablation only differs from AF termination by extensive empirical ablation, and may portend long-term maintenance of sinus rhythm.
Long-term Outcome After FIRM Ablation at Atrial Fibrillation Sources – The CONFIRM Trial
The CONFIRM trial was a prospective case cohort study.16 CONFIRM enrolled 107 consecutive AF patients with standard indications for ablation, excluding only those who did not consent. Patients had long-standing persistent, persistent AF or paroxysmal AF by standard criteria.3 Of this population, 71 underwent conventional ablation and 36 underwent FIRM-guided ablation followed by conventional ablation (2:1 allocation). Three-quarters of patients had persistent AF (conventional: 66 %, FIRM-guided 81 %), and subjects had a wide range of ages (20–81 years), left ventricular ejection fractions (20–75 %) and co-morbidities.
FIRM-guided ablation was delivered prior to PV isolation as described above, for the endpoint of ‘hyperacute’ AF termination (with non-re-inducibility) or organisation (slowing ≥10 %). When AF terminated, we vigorously attempted AF re-initiation (burst pacing and isoproterenol). If AF was re-induced, FIRM ablation was repeated for ≤3 sources (≤30 minutes) to the endpoint of AF non-inducibility. Of note, only one PEM map was performed because early versions of RhythmView in the CONFIRM trial were too slow to enable remaps. Conventional ablation was then performed by wide-area circumferential PV isolation.3 In FIRM-blinded (control) subjects, conventional ablation was performed immediately after acquiring basket data. In FIRM-guided subjects, conventional ablation was performed after FIRM-guided ablation, even if AF had terminated and was non-inducible. Follow-up in clinic for up to two years was rigorous, using implanted subcutaneous ECG monitors whenever possible. Antiarrhythmic medications were discontinued at three months. Repeat ablation was not permitted.
The single-procedure freedom from AF in CONFIRM was higher for FIRM-guided than conventional FIRM-blinded cases (82.4 versus 44.9 % p<0.001) after 273 days (median; IQR 132-681). Notably, no FIRM-guided case recurred after ≈7 months. FIRM-guided ablation maintained its benefit over FIRM-blinded therapy for first-time ablation cases, patients with prior unsuccessful ablation and all other pre-specified subgroups. Figure 4 illustrates a Kaplan Meier curve showing benefit for FIRM-guided versus FIRM-blinded cases for patients off anti-arrhythmic medications (p<0.001).
Figure 4: Cumulative freedom from Atrial Fibrillation.
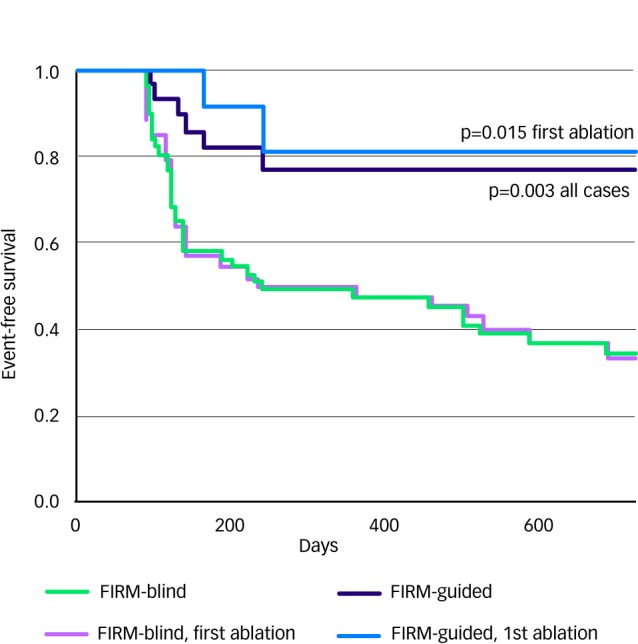
Cumulative freedom from atrial fibrillation off anti-arrhythmic medications, for all cases (solid lines) and those undergoing first ablation (dashed lines). Intention-to-treat analysis, and p values reflect the complete follow-up period. Source: Adapted from Narayan et al., 2012, with permission.16
Notably, the benefit of FIRM-guided ablation was achieved despite the fact that this group had greater co-morbidities and more rigorous follow-up (subcutaneous ECG monitors in 88.2 versus 26.1 %; p<0.001) than FIRM-blinded patients.
Summary of the CONFIRM Trial
The CONFIRM trial showed for the first time that brief ablation (FIRM) at patient-specific AF-maintaining sources can acutely terminate AF and substantially increase long-term AF elimination after a single procedure compared to conventional AF ablation alone. The CONFIRM trial is notable in that it reports the highest single procedure success rate of any AF ablation trial to date, verified using highly sensitive subcutaneous ECG monitors. CONFIRM is also among the largest AF trials to date11,48,49 and one of the few to compare an ablation strategy to conventional ablation50,51 rather than to a failed anti-arrhythmic medication.11,48,49
Results from the CONFIRM trial may explain the results and limitations of conventional AF ablation. First, while most sources lay in the left atrium, RA sources in one-quarter of patients may explain the 70 % multi-procedure ceiling of success3 for predominantly LA AF ablation. Second, diverse source locations are consistent with reports that widespread ablation may be required in both atria.10 Third, from several analyses of our data, a higher number and wider distribution of sources explains the apparent complexity of persistent AF compared to paroxysmal AF and may explain the lower success of PV-oriented anatomical ablation in persistent AF patients.
Limitations of CONFIRM include the fact that it was non-randomised, although subjects were enrolled consecutively and treated prospectively for pre-specified endpoints. In fact, FIRM-guided subjects were more likely to have persistent AF, had more co-morbidities and underwent more intense monitoring than FIRM-blinded subjects, that bias against and underestimate the benefit of FIRM-guided ablation. Ongoing studies will test this hypothesis. Other limitations include suboptimal basket resolution and the fact that PEM was performed only once in CONFIRM because early versions of the mapping system prevented re-maps. These limitations may help to guide future improvements to the FIRM-ablation technique.
Conclusions
Data from the CONFIRM trial builds upon human mapping data over the past decade to demonstrate that AF is sustained by a small number of surprisingly stable localised rotors and focal sources in patients with all forms of AF (paroxysmal AF, persistent AF and long-standing persistent AF).
The CONFIRM trial further showed that FIRM ablation to eliminate AF sources in patient-specific bi-atrial locations was able to abruptly terminate AF and render it non-inducible in a majority of patients with persistent and paroxysmal AF. Patients receiving FIRM-guided ablation with conventional ablation showed substantially improved long-term AF elimination over conventional ablation alone.
In conclusion, panoramic electrophysiological mapping can identify rotors and focal sources that sustain AF in individual patients, opening the possibility for patient-tailored FIRM ablation that may improve procedural success, reduce procedural time and potentially improve safety. Studies in the near future will further define the mechanistic underpinnings of localised rotors and focal sources and clinical outcomes following FIRM ablation alone.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. doi: 10.1007/s10840-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S et al. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 5.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–9. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allessie MA, de Groot NM, Houben RP. et al. The electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;122:1674–82. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 7.Moe GK, Rheinboldt W, Abildskov J. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–20. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 8.Lazar S, Dixit S, Marchlinski FE et al. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–6. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 9.Sahadevan J, Ryu K, Peltz L et al. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004;110:3293–9. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 10.Haissaguerre M, Sanders P, Hocini M et al. Catheter ablation of long-lasting persistent atrial fibrillation: Critical structures for termination. J Cardiovasc Electrophysiol. 2005a;16:1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 11.Oral H, Pappone C, Chugh A et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 12.Lewis T et al. Observations upon flutter and fibrillation as it occurs in patients. Heart. 1921a;8(193) [Google Scholar]
- 13.Skanes AC, Mandapati R, Berenfeld O et al. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–48. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 14.Cuculich PS, Wang Y, Lindsay BD et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Huang W, Yao Y Electropathological substrate detection of persistent atrial fibrillation – a novel method to analyze unipolar electrograms of noncontact mapping. Conf Proc IEEE Eng Med Biol Soc. 2011. pp. 1471–4. [DOI] [PubMed]
- 16.Narayan SM, Krummen DE, Shivkumar K et al. Treatment of atrial fibrillation by the ablation of localized sources: the conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation: Confirm trial. J Am Coll Cardiol, 2012;59 doi: 10.1016/j.jacc.2012.05.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shivkumar K, Ellenbogen K, Kenneth A Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First multicenter experience of focal impulse and rotor modulation (firm) ablation. J Cardiovasc Electrophys. 2012. in review. [DOI] [PMC free article] [PubMed]
- 18.Jackman WM, Wang XZ, Friday KJ et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–11. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 19.Jackman WM, Beckman KJ, McClelland JH et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992;327:313–8. doi: 10.1056/NEJM199207303270504. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson WG, Khan H, Sager P et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–70. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 21.Gerstenfeld E, Sahakian A, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–82. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 22.Dibs SR, Ng J, Arora R et al. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: Multisite electrogram analysis and surface electrocardiographic correlations – a pilot study. Heart Rhythm. 2008;5:686–93. doi: 10.1016/j.hrthm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Lemola K, Ting M, Gupta P et al. Effects of two different catheter ablation techniques on spectral characteristics of atrial fibrillation. J Am Coll Cardiol. 2006;48:340–8. doi: 10.1016/j.jacc.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 24.Xi Q, Sahakian AV, Ng J, Swiryn S. Atrial fibrillatory wave characteristics on surface electrogram: ECG to ECG repeatability over twenty-four hours in clinically stable patients. J Cardiovasc Electrophysiol. 2004a;15:911–7. doi: 10.1046/j.1540-8167.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- 25.Nademanee K, McKenzie J, Kosar E et al. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004a;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 26.Sanders P, Berenfeld O, Hocini M et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 27.Hocini M, Nault I, Wright M et al. Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2010;55:1007–16. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haissaguerre M, Hocini M, Sanders P et al. Catheter ablation of long-lasting persistent atrial fibrillation: Clinical outcome and mechanisms of subsequent arrhythmias. J Cardio Electrophysiol. 2005b;16:1138–47. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 29.Allessie MA, de Groot NM, Houben RP. et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–15. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 30.Cox JL, Canavan TE, Schuessler RB et al. The surgical treatment of atrial fibrillation. Ii. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–26. [PubMed] [Google Scholar]
- 31.Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:402–5. [PubMed] [Google Scholar]
- 32.Weimar T, Bailey MS, Watanabe Y et al. The Cox-maze iv procedure for lone atrial fibrillation: A single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011;31(1):47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu T-J, Doshi RN, Huang H-LA et al. Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Cardiovasc Electrophysiol. 2002;13:571–7. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 34.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to identify rotors and focal beats in human atrial fibrillation. J Cardio Electrophysiol, 2012;23:447–54. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials as a precursor of atrial fibrillation. Circulation. 2002b;106:1968–73. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 36.Narayan SM, Franz MR. Quantifying fractionation and rate in human atrial fibrillation using monophasic action potentials: Implications for substrate mapping. Europace. 2007e;9:vi89–vi95. doi: 10.1093/europace/eum212. [DOI] [PubMed] [Google Scholar]
- 37.Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: A mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008c;52:1222–30. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan SM, Franz MR, Clopton P et al. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011b;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lalani G, Schricker A, Gibson M et al. Dynamic conduction slowing precedes human atrial fibrillation initiation: Insights from bi-atrial basket mapping on transitions to atrial fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayan SM, Krummen DE, Enyeart MW, Rappel W. Computational mapping approach identifies stable and long-lived electrical rotors and focal sources in human atrial fibrillation. 2012. in review. [DOI] [PMC free article] [PubMed]
- 41.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–54. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayan SM, Shivkumar K, Krummen DE Panoramic electrophysiological mapping but not individual electrogram morphology identifies sustaining sites for human atrial fibrillation (AF rotors and focal sources relate poorly to fractionated electrograms) 2012. in review. [DOI] [PMC free article] [PubMed]
- 43.Katritsis DG, Giazitzoglou E, Zografos T et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm. 2011;8:672–8. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 44.Scherlag BJ, Nakagawa H, Jackman WM et al. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13:37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 45.Narayan SM, Patel J, Mulpuru SK, Krummen DE. Focal impulse and rotor modulation (firm) of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up. Heart Rhythm. 2012;9 doi: 10.1016/j.hrthm.2012.03.055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haissaguerre M, Lim KT, Jacquemet V et al. Atrial fibrillatory cycle length: Computer simulation and potential clinical importance. Europace. 2007;9(Suppl. 6):vi64–70. doi: 10.1093/europace/eum208. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi Y, O’Neill MD, Hocini M. et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008;51:1003–10. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 48.Wazni OM, Marrouche NF, Martin DO et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA. 2005;293:2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 49.Wilber DJ, Pappone C, Neuzil P et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 50.Oral H, Chugh A, Good E et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007;115:2606–12. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 51.Oral H, Chugh A, Yoshida K et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782–9. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]